Establishment of a Drug Screening Model for Cardiac Complications of Acute Renal Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance
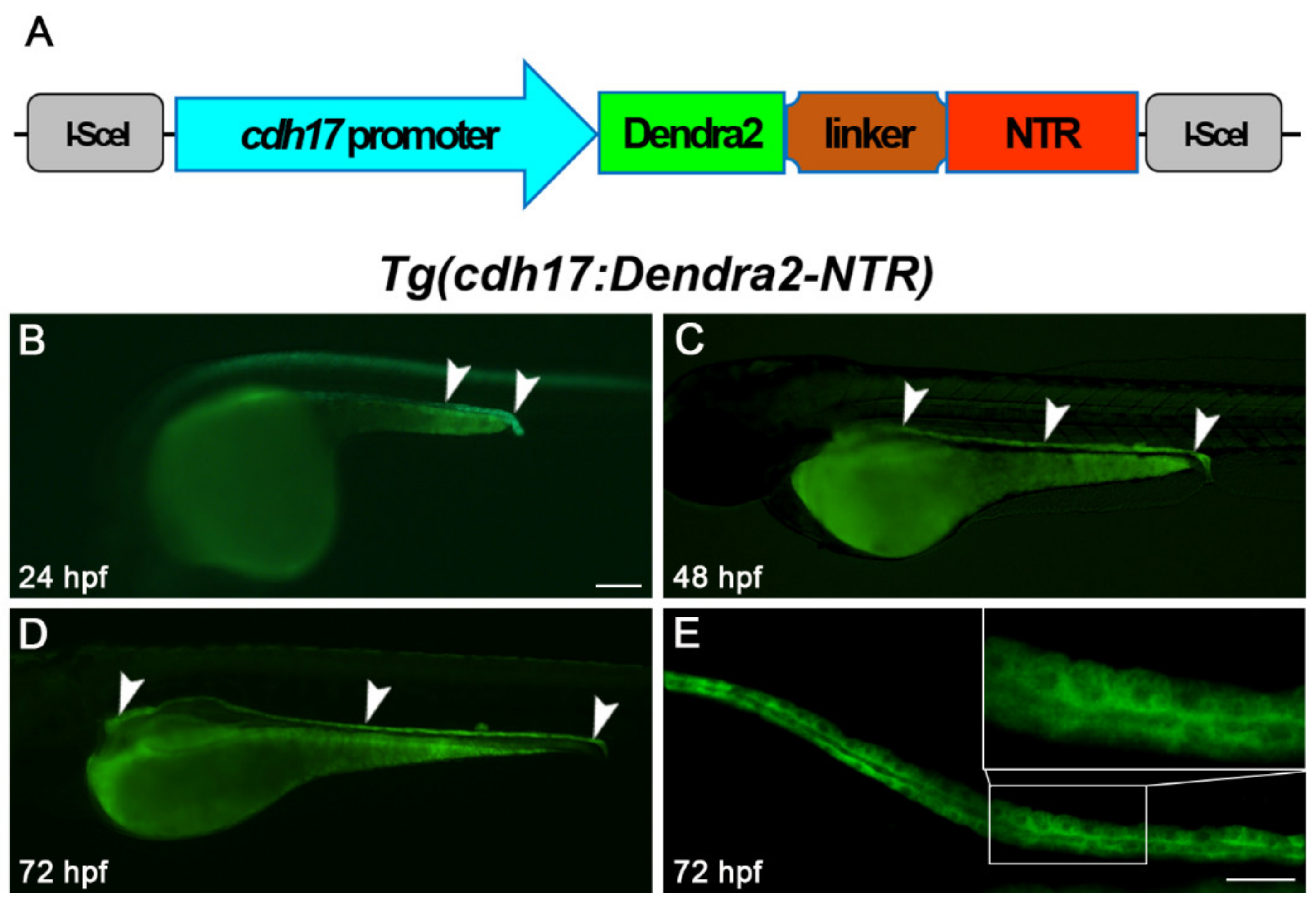
2.2. Generation of Tg(cdh17-Dendra2-NTR) Transgenic Line
2.3. NTR/MTZ Induced Renal Tubular Epithelial Cells Ablation
2.4. FITC-Inulin Clearance Assay
2.5. Determination of Uric Acid and Urea Nitrogen
2.6. Apoptosis Detection
2.7. Drug Treatment
2.8. ROS Detection
2.9. Acquisition Phenotype Data
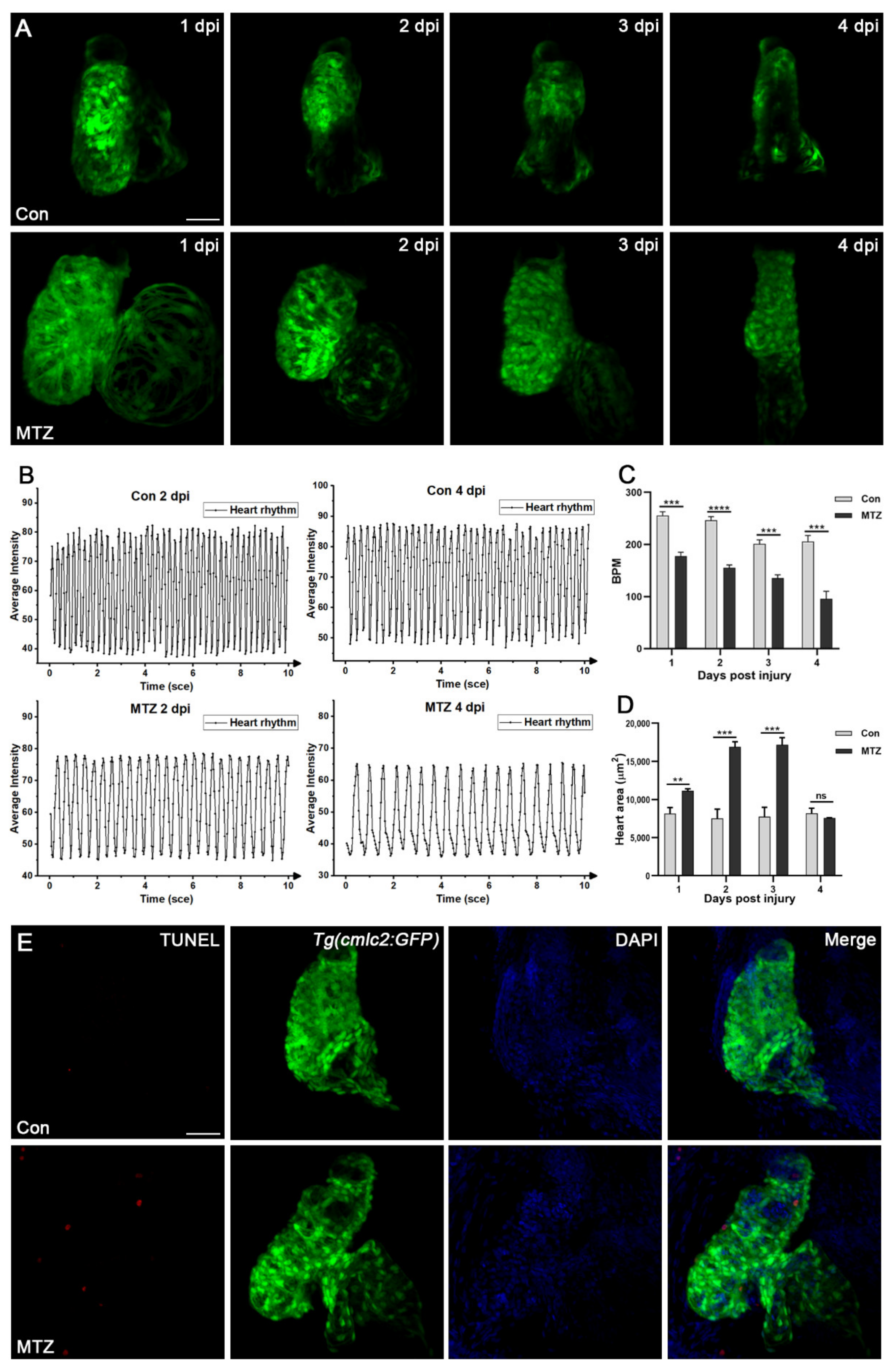
2.10. Assessment of Heart Rate and Blood Flow
3. Results
3.1. Generation of Tg(cdh17:Dendra2-NTR) Transgenic Line
3.2. Establishment of Zebrafish Larvae ARF Model
3.3. Renal Tubules Injury Mediated the Renal Function Loss
3.4. Zebrafish ARF Model Can Simulate Human CRS-3 Complications
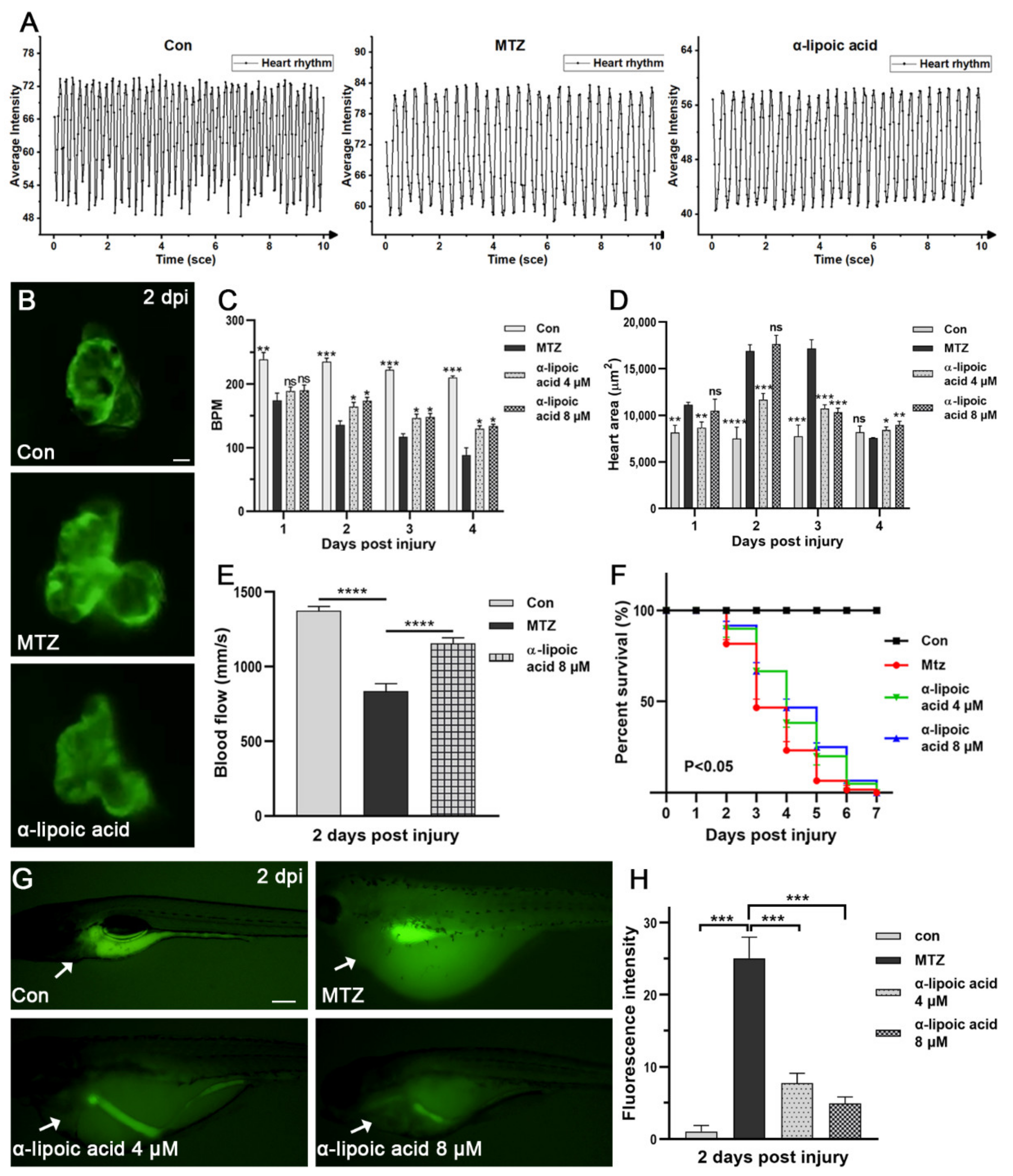
3.5. Evaluation the Efficacy of Approved Therapeutic Drugs on the CRS-3 Model
3.6. Screening Potential Drugs and Exploring Possible Mechanisms of Drugs Effect on CRS-3 Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schaubroeck, H.A.; Gevaert, S.; Bagshaw, S.M.; Kellum, J.A.; Hoste, E.A. Acute cardiorenal syndrome in acute heart failure: Focus on renal replacement therapy. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Nair, N.; Chakraborty, R.; Nemer, L.; Dasgupta, R.; Varian, K. An Update on the pathophysiology and treatment of cardiorenal syndrome. Cardiol. Res. 2020, 11, 76. [Google Scholar] [CrossRef]
- Sepahi, A.; Kraus, A.; Casadei, E.; Johnston, C.A.; Galindo-Villegas, J.; Kelly, C.; García-Moreno, D.; Muñoz, P.; Mulero, V.; Huertas, M. Olfactory sensory neurons mediate ultrarapid antiviral immune responses in a TrkA-dependent manner. Proc. Natl. Acad. Sci. USA 2019, 116, 12428–12436. [Google Scholar] [CrossRef]
- Galindo-Villegas, J. The Zebrafish Disease and Drug Screening Model: A Strong Ally Against Covid-19. Front. Pharmacol. 2020, 11, 680. [Google Scholar] [CrossRef]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Curado, S.; Stainier, D.Y.; Anderson, R.M. Nitroreductase-mediated cell/tissue ablation in zebrafish: A spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat. Protoc. 2008, 3, 948–954. [Google Scholar] [CrossRef]
- Huang, J.; Mckee, M.; Huang, H.D.; Xiang, A.; Davidson, A.J.; Lu, H.A. A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int. 2013, 83, 1193–1200. [Google Scholar] [CrossRef]
- Pourghadamyari, H.; Rezaei, M.; Basiri, M.; Tahamtani, Y.; Asgari, B.; Hassani, S.-N.; Meshkani, R.; Golmohammadi, T.; Baharvand, H. Generation of a transgenic zebrafish model for pancreatic beta cell regeneration. Galen Med. J. 2019, 8, 1056. [Google Scholar] [CrossRef]
- Zhang, R.; Han, P.; Yang, H.; Ouyang, K.; Lee, D.; Lin, Y.-F.; Ocorr, K.; Kang, G.; Chen, J.; Stainier, D.Y. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature 2013, 498, 497–501. [Google Scholar] [CrossRef]
- Choi, T.Y.; Ninov, N.; Stainier, D.Y.; Shin, D. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology 2014, 146, 776–788. [Google Scholar] [CrossRef]
- He, J.; Lu, H.; Zou, Q.; Luo, L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 2014, 146, 789–800.e8. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Ni, R.; Yang, Q.; Zhang, Y.; Luo, L. Cerebrovascular injuries induce lymphatic invasion into brain parenchyma to guide vascular regeneration in zebrafish. Dev. Cell 2019, 49, 697–710.e5. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, M.; Huang, C.; Enyedi, B.; Niethammer, P. Live imaging of leukocyte recruitment in a zebrafish model of chemical liver injury. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Plessis, A.; Perrin, A.; Haber, J.E.; Dujon, B. Site-specific recombination determined by I-SceI, a mitochondrial group I intron-encoded endonuclease expressed in the yeast nucleus. Genetics 1992, 130, 451–460. [Google Scholar] [CrossRef]
- Rider, S.A.; Tucker, C.S.; del-Pozo, J.; Rose, K.N.; MacRae, C.A.; Bailey, M.A.; Mullins, J.J. Techniques for the in vivo assessment of cardio-renal function in zebrafish (Danio rerio) larvae. J. Physiol. 2012, 590, 1803–1809. [Google Scholar] [CrossRef]
- Sampurna, B.P.; Audira, G.; Juniardi, S.; Lai, Y.-H.; Hsiao, C.-D. A simple imagej-based method to measure cardiac rhythm in zebrafish embryos. Inventions 2018, 3, 21. [Google Scholar] [CrossRef]
- Matrone, G.; Taylor, J.M.; Wilson, K.S.; Baily, J.; Love, G.D.; Girkin, J.M.; Mullins, J.J.; Tucker, C.S.; Denvir, M.A. Laser-targeted ablation of the zebrafish embryonic ventricle: A novel model of cardiac injury and repair. Int. J. Cardiol. 2013, 168, 3913–3919. [Google Scholar] [CrossRef] [PubMed]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Dev. Biol. 2001, 230, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S. Cardiotoxicity of uremic toxins: A driver of cardiorenal syndrome. Toxins 2018, 10, 352. [Google Scholar] [CrossRef]
- Di Lullo, L.; Reeves, P.B.; Bellasi, A.; Ronco, C. Cardiorenal syndrome in acute kidney injury. In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Testani, J.M.; Brisco, M.A.; Tang, W.W.; Kimmel, S.E.; Tiku-Owens, A.; Forfia, P.R.; Coca, S.G. Potential effects of digoxin on long-term renal and clinical outcomes in chronic heart failure. J. Card. Fail. 2013, 19, 295–302. [Google Scholar] [CrossRef][Green Version]
- Gomez, H.J.; Cirillo, V.J.; Irvin, J.D. Enalapril: A review of human pharmacology. Drugs 1985, 30, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free. Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Tibullo, D.; Volti, G.L.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Gyurászová, M.; Kovalčíková, A.G.; Renczés, E.; Kmeťová, K.; Celec, P.; Bábíčková, J.; Tóthová, Ľ. Oxidative stress in animal models of acute and chronic renal failure. Dis. Markers 2019, 2019, 8690805. [Google Scholar] [CrossRef]
- Park, S.; Karunakaran, U.; Ho Jeoung, N.; Jeon, J.-H.; Lee, I.-K. Physiological effect and therapeutic application of alpha lipoic acid. Curr. Med. Chem. 2014, 21, 3636–3645. [Google Scholar] [CrossRef]
- Xie, J.; Yin, H.; Nichols, T.D.; Yoder, J.A.; Horowitz, J.M. Sp2 Is a Maternally Inherited Transcription Factor Required for Embryonic Development. J. Biol. Chem. 2010, 285, 4153–4164. [Google Scholar] [CrossRef]
- Diep, C.Q.; Ma, D.; Deo, R.C.; Holm, T.M.; Naylor, R.W.; Arora, N.; Wingert, R.A.; Bollig, F.; Djordjevic, G.; Lichman, B. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 2011, 470, 95–100. [Google Scholar] [CrossRef]
- Chen, Z.; Luciani, A.; Mateos, J.M.; Barmettler, G.; Giles, R.H.; Neuhauss, S.; Devuyst, O. Transgenic zebrafish modeling low-molecular-weight proteinuria and lysosomal storage diseases. Kidney Int. 2020, 97, 1150–1163. [Google Scholar] [CrossRef]
- Paatero, I.; Casals, E.; Niemi, R.; Ozliseli, E.; Rosenholm, J.M.; Sahlgren, C. Analyses in zebrafish embryos reveal that nanotoxicity profiles are dependent on surface-functionalization controlled penetrance of biological membranes. Sci. Rep. 2017, 7, 8423. [Google Scholar] [CrossRef] [PubMed]
- Teijeiro-Valino, C.; Yebra-Pimentel, E.; Guerra-Varela, J.; Csaba, N.; Alonso, M.J.; Sanchez, L. Assessment of the permeability and toxicity of polymeric nanocapsules using the zebrafish model. Nanomedicine 2017, 12, 2069–2082. [Google Scholar] [CrossRef]
- Zhou, T.; Gui, L.; Liu, M.; Li, W.; Hu, P.; Duarte, D.; Niu, H.; Chen, L. Transcriptomic responses to low temperature stress in the Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2019, 84, 1145–1156. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Yu, X.; Guo, L.; Zhang, X.; Xia, D. The flavonoid-rich fraction from rhizomes of Smilax glabra Roxb. ameliorates renal oxidative stress and inflammation in uric acid nephropathy rats through promoting uric acid excretion. Biomed. Pharmacother. 2019, 111, 162–168. [Google Scholar] [CrossRef]
- Liu, J. Evaluation of serum cystatin C for diagnosis of acute rejection after renal transplantation. Transpl. Proc. 2012, 44, 1250–1253. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; Yang, W.; Yu, T.; Dai, L.; Liu, X.; Zhang, J.; Zhao, J.; Liu, C. Establishment of a Drug Screening Model for Cardiac Complications of Acute Renal Failure. Biomolecules 2021, 11, 1370. https://doi.org/10.3390/biom11091370
Liao S, Yang W, Yu T, Dai L, Liu X, Zhang J, Zhao J, Liu C. Establishment of a Drug Screening Model for Cardiac Complications of Acute Renal Failure. Biomolecules. 2021; 11(9):1370. https://doi.org/10.3390/biom11091370
Chicago/Turabian StyleLiao, Shuyi, Wenmin Yang, Ting Yu, Lu Dai, Xiaoliang Liu, Jiangping Zhang, Jinghong Zhao, and Chi Liu. 2021. "Establishment of a Drug Screening Model for Cardiac Complications of Acute Renal Failure" Biomolecules 11, no. 9: 1370. https://doi.org/10.3390/biom11091370
APA StyleLiao, S., Yang, W., Yu, T., Dai, L., Liu, X., Zhang, J., Zhao, J., & Liu, C. (2021). Establishment of a Drug Screening Model for Cardiac Complications of Acute Renal Failure. Biomolecules, 11(9), 1370. https://doi.org/10.3390/biom11091370





