Abstract
Abdominal aortic aneurysm (AAA) is a life-threatening disease; however, there is no established treatment for patients with AAA. Fibrates are agonists of peroxisome proliferator-activated receptor alpha (PPARα) that are widely used as therapeutic agents to treat patients with hypertriglyceridemia. They can regulate the pathogenesis of AAA in multiple ways, for example, by exerting anti-inflammatory and anti-oxidative effects and suppressing the expression of matrix metalloproteinases. Previously, basic and clinical studies have evaluated the effects of fenofibrate on AAA. In this paper, we summarize the results of these studies and discuss the problems associated with using fenofibrate as a therapeutic agent for patients with AAA. In addition, we discuss a new perspective on the regulation of AAA by PPARα agonists.
1. Introduction
Abdominal aortic aneurysm (AAA) is characterized by the progressive dilation of the abdominal aorta that can lead to sudden death in a patient because of aortic rupture. Studies have reported that the primary pathogenesis underlying AAA development occurs as follows: (A) inflammatory cytokine production- and macrophage infiltration-associated inflammation [1,2], (B) oxidative stress [3,4], (C) vascular smooth muscle cell apoptosis accompanied by reduced elastin production [5,6], and (D) extracellular matrix degradation caused by activated matrix metalloproteinases (MMPs) in the vascular wall [7,8,9]. Although accumulated evidence has delineated the detailed pathophysiology of AAA development, an effective clinical treatment for patients with AAA remains to be established.
Fibrates are agonists of peroxisome proliferator-activated receptor alpha (PPARα), which is a transcription factor that is mainly expressed in hepatocytes. Binding of fibrates to PPARα either activates or inhibits the genes involved in lipid metabolism [10]. In contrast, activation of PPARα can regulate the expression of genes involved in inflammation and oxidative stress [11,12,13]. Notably, PPARα is expressed in almost all the cells of the body, including macrophages, vascular smooth muscle cells, and endothelial cells [12,14,15,16,17,18]. Previously, studies have demonstrated that fibrates decrease the production of inflammatory cytokines, infiltration of monocytes, and expression of MMP genes [19,20,21,22]. Fibrates have also been implicated in decreasing the production of antioxidative enzymes, including superoxide dismutase and catalase, in the aortic wall [23]. Since the mechanism of action of fibrates is counteractive to the mechanism of AAA pathogenesis, fibrates are potentially valuable therapeutic agents for AAA treatment (Figure 1).
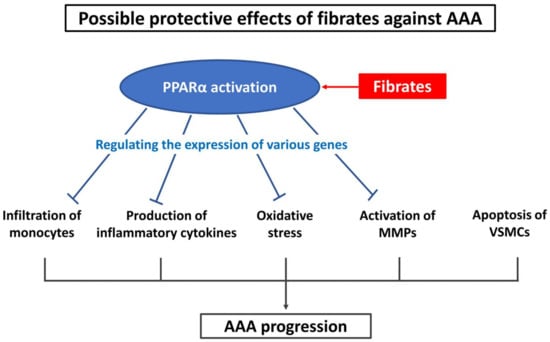
Figure 1.
Possible protective effects of fibrates against AAA. AAA—abdominal aortic aneurysm; MMPs—matrix metalloproteinases; PPARα—peroxisome proliferator-activated receptor alpha; VSMCs—vascular smooth muscle cells.
2. Discussion
Several basic and clinical studies have evaluated the protective effects of fenofibrate, the most common PPARα activator, on AAA. Golledge et al. (2010) reported that pre-administration of fenofibrate (100 mg/kg/day) to a hypercholesterolemic mouse model reduced angiotensin II (Ang II)-induced aortic expansion in the model. This reduction was associated with decreased expression of proinflammatory cytokine osteopontin (OPN) and macrophage infiltration in the aortic wall [24]. In addition, Krishna et al. (2012) reported that pre-administration of fenofibrate (100 mg/kg/day) significantly reduced suprarenal aortic dilatation induced by Ang II infusion in hypercholesterolemic mice; this was accompanied by a decrease in the abundance of macrophages, lymphocytes, and apoptotic cells in the aortic walls [25].
However, two randomized controlled trials that assessed the effects of fenofibrate on AAA in humans revealed differing results. In the FAME (Fenofibrate in the management of AbdoMinal aortic anEurysm) trial, patients scheduled to undergo open AAA repair (n = 43) were treated with fenofibrate (145 mg/day) or a placebo for at least 2 weeks before their surgeries [26]. In this trial, although the serum triglyceride (TG) levels were significantly reduced by the fenofibrate treatment, the concentration of OPN or the number of macrophages in the aortic tissue was not significantly different between the two groups. In the FAME-2 trial, 140 patients with AAA were enrolled and treated with fenofibrate (145 mg/day) or a placebo for 24 weeks. However, the fenofibrate treatment did not significantly reduce the serum concentration of OPN or the rate of AAA progression [27].
There are several possibilities as to why fenofibrate exhibits inconsistent protective effects against AAA in basic and clinical studies (Table 1).

Table 1.
Difference in the progression and treatment of abdominal aortic aneurysm following fenofibrate administration in mice and humans.
First, this discrepancy may be because the human pathology during the development of AAA is difficult to mimic in animal models. In humans, this development is affected by various factors, including genetics, ethnicity, age, sex, smoking habit, alcohol consumption, hypertension, hyperlipidemia, and renal function [28,29,30,31,32,33,34,35,36]. Therefore, it is extremely difficult to reproduce an environment that takes into account all these factors that contribute to AAA development in mouse models. Furthermore, small AAAs have a slower rate of progression than large AAAs. For instance, a meta-analysis reported that a 3.5 cm AAA takes approximately 6 years to grow to 5.5 cm [38]. Another meta-analysis demonstrated that to maintain the risk of an AAA rupture in male patients to under 1%, follow-ups are required at an estimated interval of 8.5 years for a 3.0 cm AAA and 17 months for a 5.0 cm AAA [39]. On the contrary, a preliminary study using ultrasonography demonstrated that AAAs in hypercholesterolemic mice progress rapidly within 1 week and usually rupture within 2 weeks following AAA induction [37]. Therefore, it is highly possible that the effects of fenofibrate on the rapidly progressing AAAs in mice cannot reflect the effects in humans. In addition, drug treatments in basic studies are usually provided either before or during the administration of the stimulants that induce AAA. However, these protocols only evaluate the favorable effects of the treatments during the acute phase of AAA formation. For example, previous studies that have assessed the impact of doxycycline on AAA development in murine models reported varying results for doxycycline administration before and after AAA induction [41,42,43]. Notably, mice were administered fenofibrate before AAA development, induced by Ang II, in both the basic studies we discussed previously [24,25]. Therefore, it is possible that fenofibrate does not regulate oxidative stress, inflammation, or MMP-mediated extracellular matrix degradation in vascular walls of already-formed AAA.
Another possibility is that fenofibrate does not effectively regulate the pathogenesis of AAA in humans. This may be because the efficacy of a fibrate is clinically limited because of its dose-dependent side effects, such as liver damage and increased serum creatinine levels [44,45,46]. In addition, fenofibrate needs to be administered at a higher concentration to activate human PPARα than that needed to activate mouse PPARα [40]. This is a critical weakness of fenofibrate as a PPARα agonist. On the contrary, pemafibrate is a recently developed selective modulator of PPARα [47,48]. It has the potential to enhance PPARα activation even at low effective concentrations, and it has lower off-target side effects than fenofibrate [49,50]. A clinical study has demonstrated that pemafibrate (0.2 and 0.4 mg/day) is significantly more effective in lowering the serum TG levels than fenofibrate (106.6 mg/day). It also lowers the rates of adverse drug reactions in patients with elevated serum TG levels and decreases the serum levels of high-density lipoprotein cholesterol [51]. Furthermore, in basic research, pemafibrate (1 mg/kg/day) exhibited stronger effects in reducing the expression levels of vascular cell adhesion molecule 1, macrophage marker F4/80, monocyte chemoattractant protein 1, and interleukin-6. Notably, it ameliorated the development of plaque formation in the aortic walls of hypercholesterolemic mouse models compared to fenofibrate (250 mg/kg/day) [52]. Future studies are warranted to evaluate the efficacy of pemafibrate on AAA.
3. Conclusions
In this article, we describe the problems of reproducing the therapeutic effects of fibrates against AAA, from basic studies to clinical research. This is because fenofibrate can only be administered at limited concentrations in human subjects to activate PPARα; high concentrations of fenofibrate increase the risk of off-target effects. Moreover, it does not significantly attenuate the inflammation and dilatation of AAA. Thus, future basic and clinical studies focusing on the impact of pemafibrate to treat AAA are required. These studies will help investigate the possibility of using PPARα agonists as treatment options for AAA.
Author Contributions
Conceptualization, N.A.; writing—original draft preparation, N.A.; writing—review and editing, N.A. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Grant-in-Aid for Scientific Research (C) to T.M.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Middleton, R.K.; Lloyd, G.M.; Bown, M.J.; Cooper, N.J.; London, N.J.; Sayers, R.D. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: A protein array study. J. Vasc. Surg. 2007, 45, 574–580. [Google Scholar] [CrossRef]
- Li, H.; Bai, S.; Ao, Q.; Wang, X.; Tian, X.; Li, X.; Tong, H.; Hou, W.; Fan, J. Modulation of immune-inflammatory responses in abdominal aortic aneurysm: Emerging molecular targets. J. Immunol. Res. 2018, 2018, 7213760. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.J.; Sharp, W.J.; Fang, X.; Oberley, L.W.; Oberley, T.D.; Weintraub, N.L. Oxidative stress in human abdominal aortic aneurysms: A potential mediator of aneurysmal remodeling. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 560–565. [Google Scholar] [CrossRef]
- McCormick, M.L.; Gavrila, D.; Weintraub, N.L. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.L.; Geng, Y.J.; Sukhova, G.K.; Whittemore, A.D.; Knox, J.; Libby, P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation 1999, 99, 96–104. [Google Scholar] [CrossRef]
- Rowe, V.L.; Stevens, S.L.; Reddick, T.T.; Freeman, M.B.; Donnell, R.; Carroll, R.C.; Goldman, M.H. Vascular smooth muscle cell apoptosis in aneurysmal, occlusive, and normal human aortas. J. Vasc. Surg. 2000, 31, 567–576. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Delvenne, P.; Nusgens, B.V.; Limet, R.; Lapière, C.M. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J. Vasc. Surg. 1996, 24, 127–133. [Google Scholar] [CrossRef]
- Davis, V.; Persidskaia, R.; Baca-Regen, L.; Itoh, Y.; Nagase, H.; Persidsky, Y.; Ghorpade, A.; Baxter, B.T. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef]
- Minnich, A.; Tian, N.; Byan, L.; Bilder, G. A potent PPARalpha agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E270–E279. [Google Scholar] [CrossRef]
- Inoue, I.; Noji, S.; Awata, T.; Takahashi, K.; Nakajima, T.; Sonoda, M.; Komoda, T.; Katayama, S. Bezafibrate has an antioxidant effect: Peroxisome proliferator-activated receptor alpha is associated with Cu2+, Zn2+-superoxide dismutase in the liver. Life Sci. 1998, 63, 135–144. [Google Scholar] [CrossRef]
- Inoue, I.; Goto, S.I.; Matsunaga, T.; Nakajima, T.; Awata, T.; Hokari, S.; Komoda, T.; Katayama, S. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+, Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metab.-Clin. Exp. 2001, 50, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 2010, 612089. [Google Scholar] [CrossRef]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauça, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Auboeuf, D.; Rieusset, J.; Fajas, L.; Vallier, P.; Frering, V.; Riou, J.P.; Staels, B.; Auwerx, J.; Laville, M.; Vidal, H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: No alteration in adipose tissue of obese and NIDDM patients. Diabetes 1997, 46, 1319–1327. [Google Scholar] [CrossRef]
- Chinetti, G.; Griglio, S.; Antonucci, M.; Torra, I.P.; Delerive, P.; Majd, Z.; Fruchart, J.C.; Chapman, J.; Najib, J.; Staels, B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J. Biol. Chem. 1998, 273, 25573–25580. [Google Scholar] [CrossRef]
- Inoue, I.; Shino, K.; Noji, S.; Awata, T.; Katayama, S. Expression of peroxisome proliferator-activated receptor alpha (PPAR alpha) in primary cultures of human vascular endothelial cells. Biochem. Biophys. Res. Commun. 1998, 246, 370–374. [Google Scholar] [CrossRef]
- Staels, B.; Koenig, W.; Habib, A.; Merval, R.; Lebret, M.; Torra, I.P.; Delerive, P.; Fadel, A.; Chinetti, G.; Fruchart, J.C.; et al. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature 1998, 393, 790–793. [Google Scholar] [CrossRef]
- Delerive, P.; De Bosscher, K.; Besnard, S.; Vanden Berghe, W.; Peters, J.M.; Gonzalez, F.J.; Fruchart, J.C.; Tedgui, A.; Haegeman, G.; Staels, B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J. Biol. Chem. 1999, 274, 32048–32054. [Google Scholar] [CrossRef]
- Kooistra, T.; Verschuren, L.; de Vries-van der Weij, J.; Koenig, W.; Toet, K.; Princen, H.M.G.; Kleemann, R. Fenofibrate reduces atherogenesis in ApoE*3Leiden mice: Evidence for multiple antiatherogenic effects besides lowering plasma cholesterol. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2322–2330. [Google Scholar] [CrossRef]
- Calkin, A.C.; Cooper, M.E.; Jandeleit-Dahm, K.A.; Allen, T.J. Gemfibrozil decreases atherosclerosis in experimental diabetes in association with a reduction in oxidative stress and inflammation. Diabetologia 2006, 49, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.Y.; Liu, J.T.; Liu, N.; Wang, Z.D.; Liu, C.H. PPARalpha activator fenofibrate modulates angiotensin II-induced inflammatory responses in vascular smooth muscle cells via the TLR4-dependent signaling pathway. Biochem. Pharmacol. 2009, 78, 1186–1197. [Google Scholar] [CrossRef]
- Xu, N.; Wang, Q.; Jiang, S.; Wang, Q.; Hu, W.; Zhou, S.; Zhao, L.; Xie, L.; Chen, J.; Wellstein, A.; et al. Fenofibrate improves vascular endothelial function and contractility in diabetic mice. Redox Biol. 2019, 20, 87–97. [Google Scholar] [CrossRef]
- Golledge, J.; Cullen, B.; Rush, C.; Moran, C.S.; Secomb, E.; Wood, F.; Daugherty, A.; Campbell, J.H.; Norman, P.E. Peroxisome proliferator-activated receptor ligands reduce aortic dilatation in a mouse model of aortic aneurysm. Atherosclerosis 2010, 210, 51–56. [Google Scholar] [CrossRef]
- Krishna, S.M.; Seto, S.W.; Moxon, J.V.; Rush, C.; Walker, P.J.; Norman, P.E.; Golledge, J. Fenofibrate increases high-density lipoprotein and sphingosine 1 phosphate concentrations limiting abdominal aortic aneurysm progression in a mouse model. Am. J. Pathol. 2012, 181, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Moxon, J.V.; Rowbotham, S.E.; Pinchbeck, J.L.; Lazzaroni, S.M.; Morton, S.K.; Moran, C.S.; Quigley, F.; Jenkins, J.S.; Reid, C.M.; Cavaye, D.; et al. A randomised controlled trial assessing the effects of peri-operative fenofibrate administration on abdominal aortic aneurysm pathology: Outcomes from the FAME trial. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 452–460. [Google Scholar] [CrossRef]
- Pinchbeck, J.L.; Moxon, J.V.; Rowbotham, S.E.; Bourke, M.; Lazzaroni, S.; Morton, S.K.; Matthews, E.O.; Hendy, K.; Jones, R.E.; Bourke, B.; et al. Randomized placebo-controlled trial assessing the effect of 24-week fenofibrate therapy on circulating markers of abdominal aortic aneurysm: Outcomes from the FAME-2 trial. J. Am. Heart Assoc. 2018, 7, e009866. [Google Scholar] [CrossRef]
- Helgadottir, A.; Thorleifsson, G.; Magnusson, K.P.; Grétarsdottir, S.; Steinthorsdottir, V.; Manolescu, A.; Jones, G.T.; Rinkel, G.J.E.; Blankensteijn, J.D.; Ronkainen, A.; et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat. Genet. 2008, 40, 217–224. [Google Scholar] [CrossRef]
- Elmore, J.R.; Obmann, M.A.; Kuivaniemi, H.; Tromp, G.; Gerhard, G.S.; Franklin, D.P.; Boddy, A.M.; Carey, D.J. Identification of a genetic variant associated with abdominal aortic aneurysms on chromosome 3p12.3 by genome wide association. J. Vasc. Surg. 2009, 49, 1525–1531. [Google Scholar] [CrossRef]
- Gretarsdottir, S.; Baas, A.F.; Thorleifsson, G.; Holm, H.; den Heijer, M.; de Vries, J.P.P.M.; Kranendonk, S.E.; Zeebregts, C.J.A.M.; van Sterkenburg, S.M.; Geelkerken, R.H.; et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat. Genet. 2010, 42, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Bown, M.J.; Jones, G.T.; Harrison, S.C.; Wright, B.J.; Bumpstead, S.; Baas, A.F.; Gretarsdottir, S.; Badger, S.A.; Bradley, D.T.; Burnand, K.; et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am. J. Hum. Genet. 2011, 89, 619–627. [Google Scholar] [CrossRef]
- Van De Luijtgaarden, K.M.; Rouwet, E.V.; Hoeks, S.E.; Stolker, R.J.; Verhagen, H.J.M.; Majoor-Krakauer, D. Risk of abdominal aortic aneurysm (AAA) among male and female relatives of AAA patients. Vasc. Med. 2017, 22, 112–118. [Google Scholar] [CrossRef]
- Rodin, M.B.; Daviglus, M.L.; Wong, G.C.; Liu, K.; Garside, D.B.; Greenland, P.; Stamler, J. Middle age cardiovascular risk factors and abdominal aortic aneurysm in older age. Hypertension 2003, 42, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.R.; Willett, W.C.; Rimm, E.B. Smoking, hypertension, alcohol consumption, and risk of abdominal aortic aneurysm in men. Am. J. Epidemiol. 2007, 165, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Darbinian, J.A.; Go, A.S.; Fireman, B.H.; Lee, C.D.; Grey, D.P. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: The Kaiser multiphasic health checkup cohort study. Ann. Epidemiol. 2007, 17, 669–678. [Google Scholar] [CrossRef]
- Forsdahl, S.H.; Singh, K.; Solberg, S.; Jacobsen, B.K. Risk factors for abdominal aortic aneurysms: A 7-year prospective study: The Tromsø Study, 1994–2001. Circulation 2009, 119, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Barisione, C.; Charnigo, R.; Howatt, D.A.; Moorleghen, J.J.; Rateri, D.L.; Daugherty, A. Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography. J. Vasc. Surg. 2006, 44, 372–376. [Google Scholar] [CrossRef]
- Powell, J.T.; Sweeting, M.J.; Brown, L.C.; Gotensparre, S.M.; Fowkes, F.G.; Thompson, S.G. Systematic review and meta-analysis of growth rates of small abdominal aortic aneurysms. Br. J. Surg. 2011, 98, 609–618. [Google Scholar] [CrossRef]
- RESCAN Collaborators; Bown, M.J.; Sweeting, M.J.; Brown, L.C.; Powell, J.T.; Thompson, S.G. Surveillance intervals for small abdominal aortic aneurysms: A meta-analysis. JAMA 2013, 309, 806–813. [Google Scholar] [CrossRef][Green Version]
- Willson, T.M.; Brown, P.J.; Sternbach, D.D.; Henke, B.R. The PPARs: From orphan receptors to drug discovery. J. Med. Chem. 2000, 43, 527–550. [Google Scholar] [CrossRef]
- Prall, A.K.; Longo, G.M.; Mayhan, W.G.; Waltke, E.A.; Fleckten, B.; Thompson, R.W.; Baxter, B.T. Doxycycline in patients with abdominal aortic aneurysms and in mice: Comparison of serum levels and effect on aneurysm growth in mice. J. Vasc. Surg. 2002, 35, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.W.; Cassis, L.A.; Daugherty, A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lu, H.; Moorleghen, J.J.; Howatt, D.A.; Rateri, D.L.; Cassis, L.A.; Daugherty, A. Doxycycline does not influence established abdominal aortic aneurysms in angiotensin II-infused mice. PLoS ONE 2012, 7, e46411. [Google Scholar] [CrossRef]
- Ahmad, J.; Odin, J.A.; Hayashi, P.H.; Chalasani, N.; Fontana, R.J.; Barnhart, H.; Cirulli, E.T.; Kleiner, D.E.; Hoofnagle, J.H. Identification and characterization of fenofibrate-induced liver injury. Dig. Dis. Sci. 2017, 62, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Mychaleckyj, J.C.; Craven, T.; Nayak, U.; Buse, J.; Crouse, J.R.; Elam, M.; Kirchner, K.; Lorber, D.; Marcovina, S.; Sivitz, W.; et al. Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care 2012, 35, 1008–1014. [Google Scholar] [CrossRef]
- Ncube, V.; Starkey, B.; Wang, T. Effect of fenofibrate treatment for hyperlipidaemia on serum creatinine and cystatin C. Ann. Clin. Biochem. 2012, 49, 491–493. [Google Scholar] [CrossRef]
- Raza-Iqbal, S.; Tanaka, T.; Anai, M.; Inagaki, T.; Matsumura, Y.; Ikeda, K.; Taguchi, A.; Gonzalez, F.J.; Sakai, J.; Kodama, T. Transcriptome analysis of K-877 (a novel selective PPARα modulator (SPPARMα))-regulated genes in primary human hepatocytes and the mouse liver. J. Atheroscler. Thromb. 2015, 22, 754–772. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Takei, K.; Arulmozhiraja, S.; Sladek, V.; Matsuo, N.; Han, S.-I.; Matsuzaka, T.; Sekiya, M.; Tokiwa, T.; Shoji, M.; et al. Molecular association model of PPARα and its new specific and efficient ligand, pemafibrate: Structural basis for SPPARMα. Biochem. Biophys. Res. Commun. 2018, 499, 239–245. [Google Scholar] [CrossRef]
- Fruchart, J.C. Selective peroxisome proliferator-activated receptorα modulators (SPPARMα): The next generation of peroxisome proliferator-activated receptor α-agonists. Cardiovasc. Diabetol. 2013, 12, 82. [Google Scholar] [CrossRef]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: A multicenter, placebo-controlled, double-blind, randomized trial. J. Atheroscler. Thromb. 2018, 25, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Arai, H.; Yokote, K.; Araki, E.; Suganami, H.; Yamashita, S.; K-877 Study Group. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: Results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J. Clin. Lipidol. 2018, 12, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Hennuyer, N.; Duplan, I.; Paquet, C.; Vanhoutte, J.; Woitrain, E.; Touche, V.; Colin, S.; Vallez, E.; Lestavel, S.; Lefebvre, P.; et al. The novel selective PPARα modulator (SPPARMα) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis 2016, 249, 200–208. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).