The Stability and Anti-Angiogenic Properties of Titanium Dioxide Nanoparticles (TiO2NPs) Using Caco-2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticle Characterization
2.2. Nanoparticle Preparation for Cell Culture
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. NO Assay
2.6. Innate Inflammatory Biomarkers IL-6 and IL-8
2.7. Protein Quantification
2.8. Cell Stress Biomarkers
2.9. Angiogenesis Proteome Profile
2.10. Statistical Analysis
3. Results
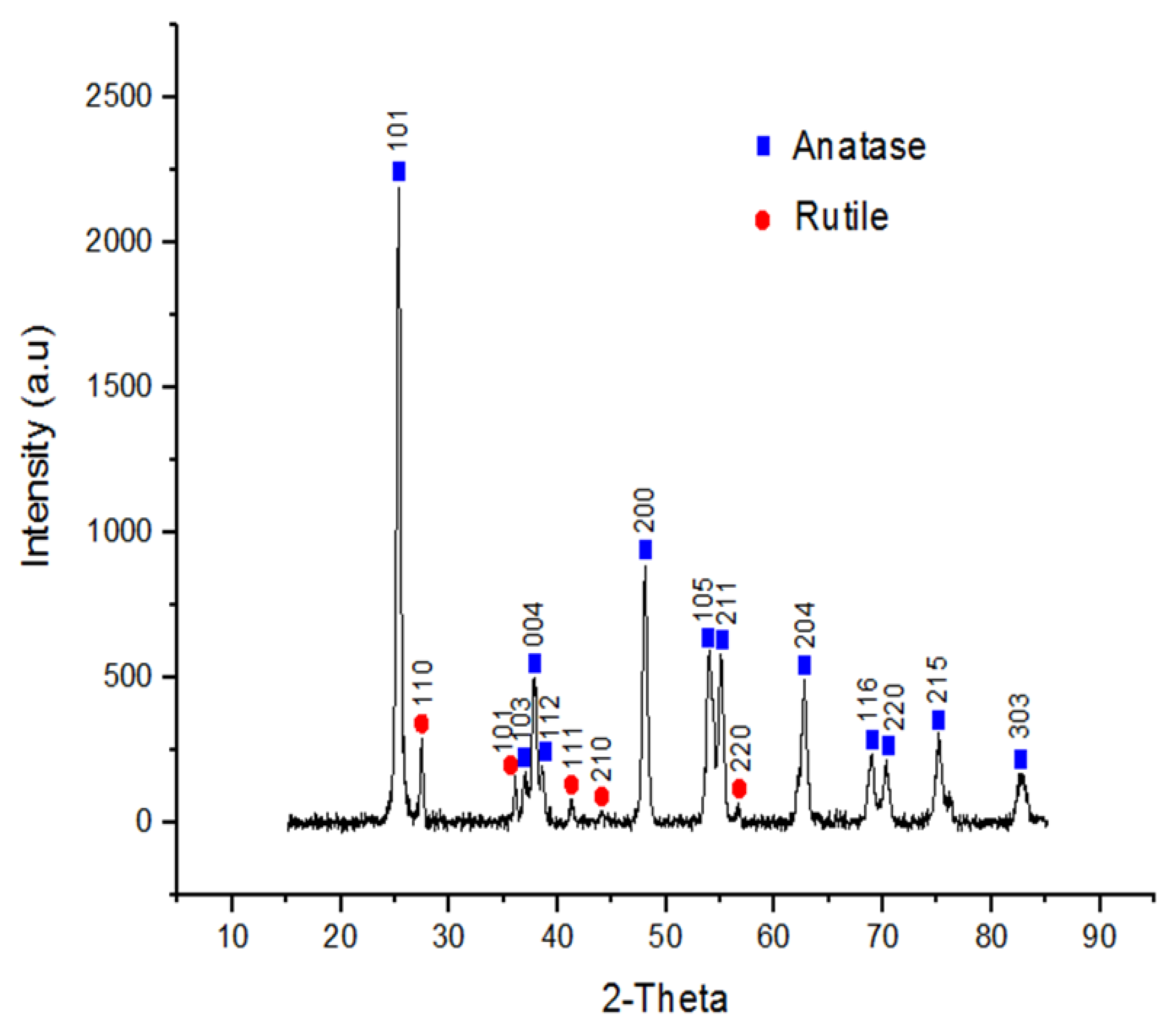
3.1. Nanoparticle Characterization
3.2. Nanoparticle Behaviour in Various Medias
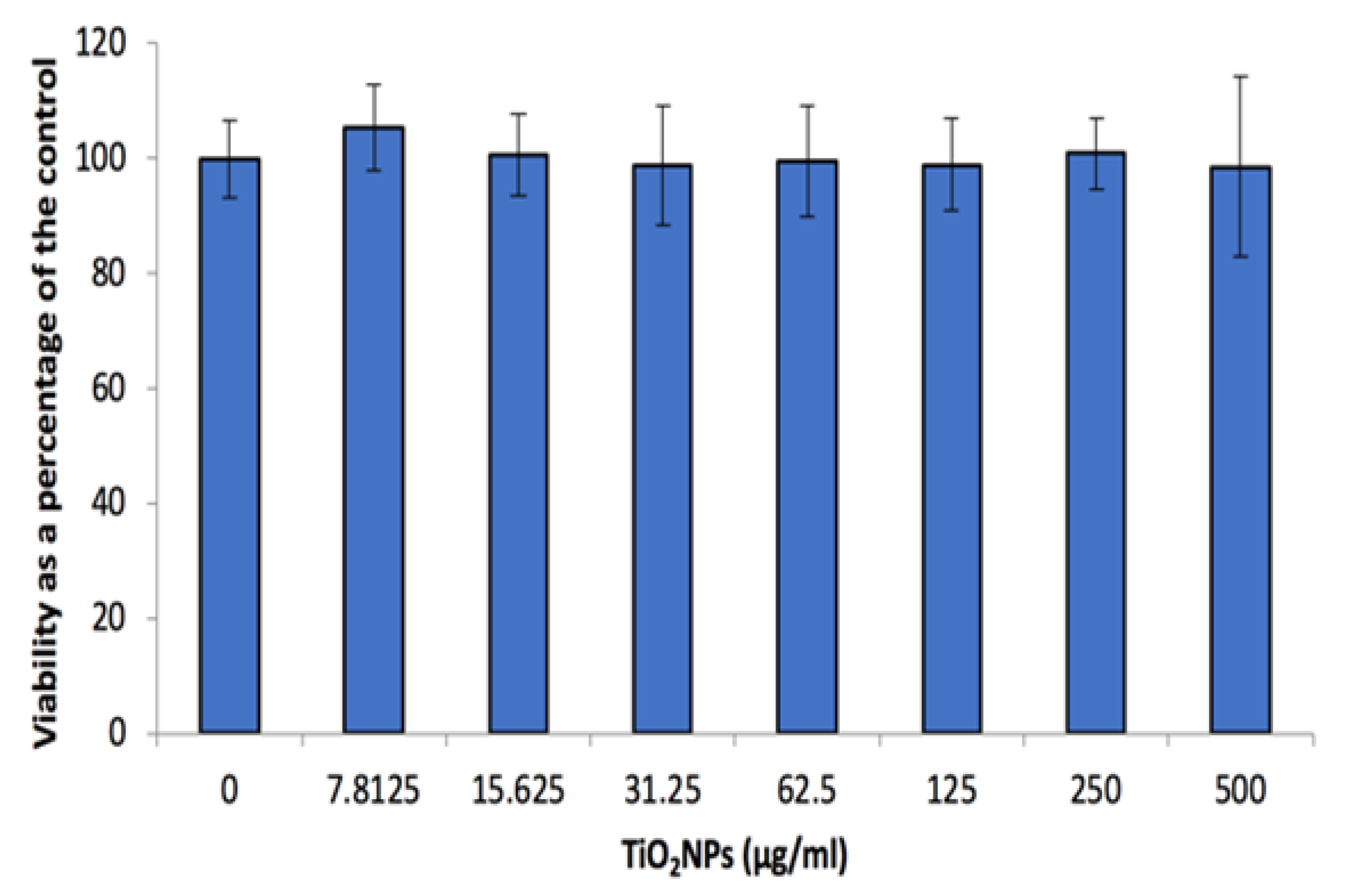
3.3. Cell Viability
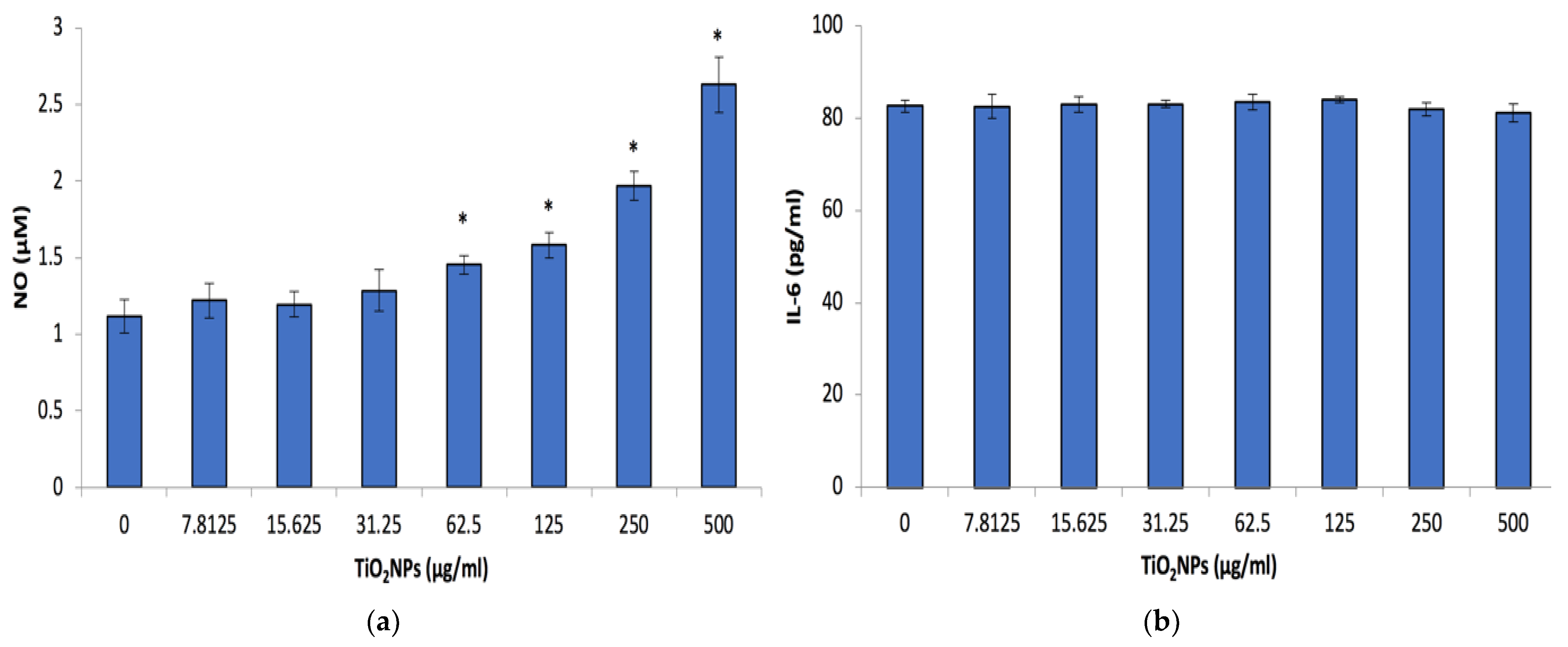
3.4. Inflammatory Biomarkers
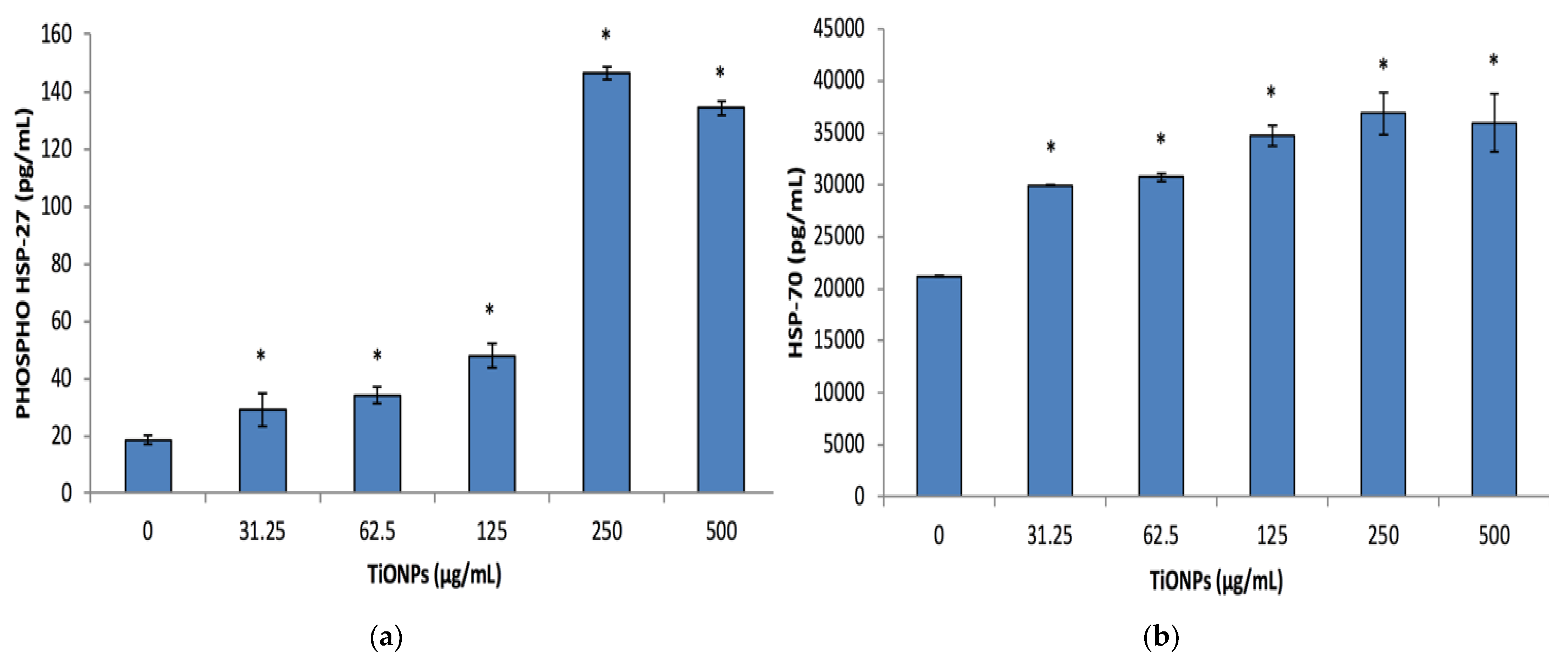
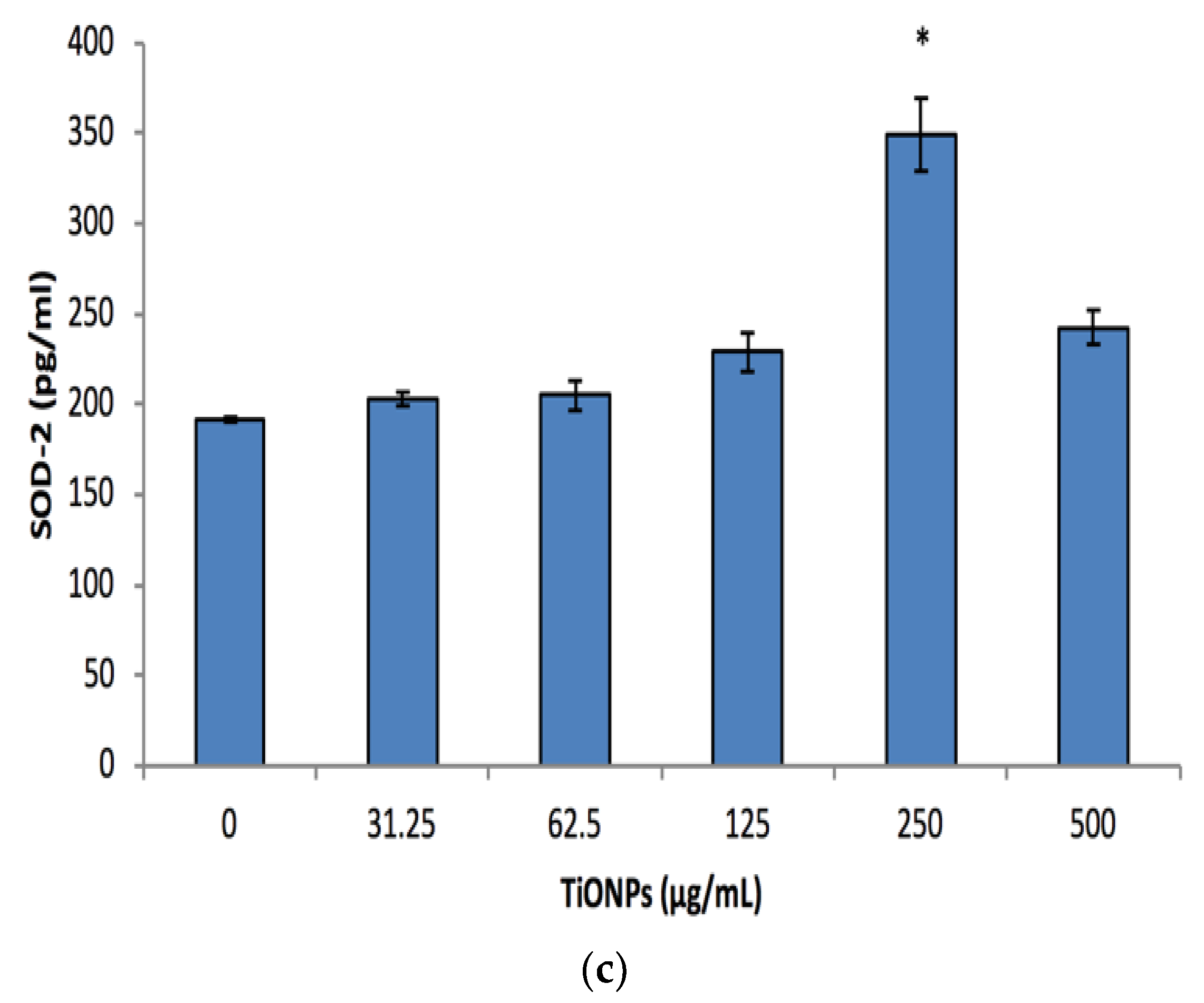
3.5. Cell Stress Biomarkers
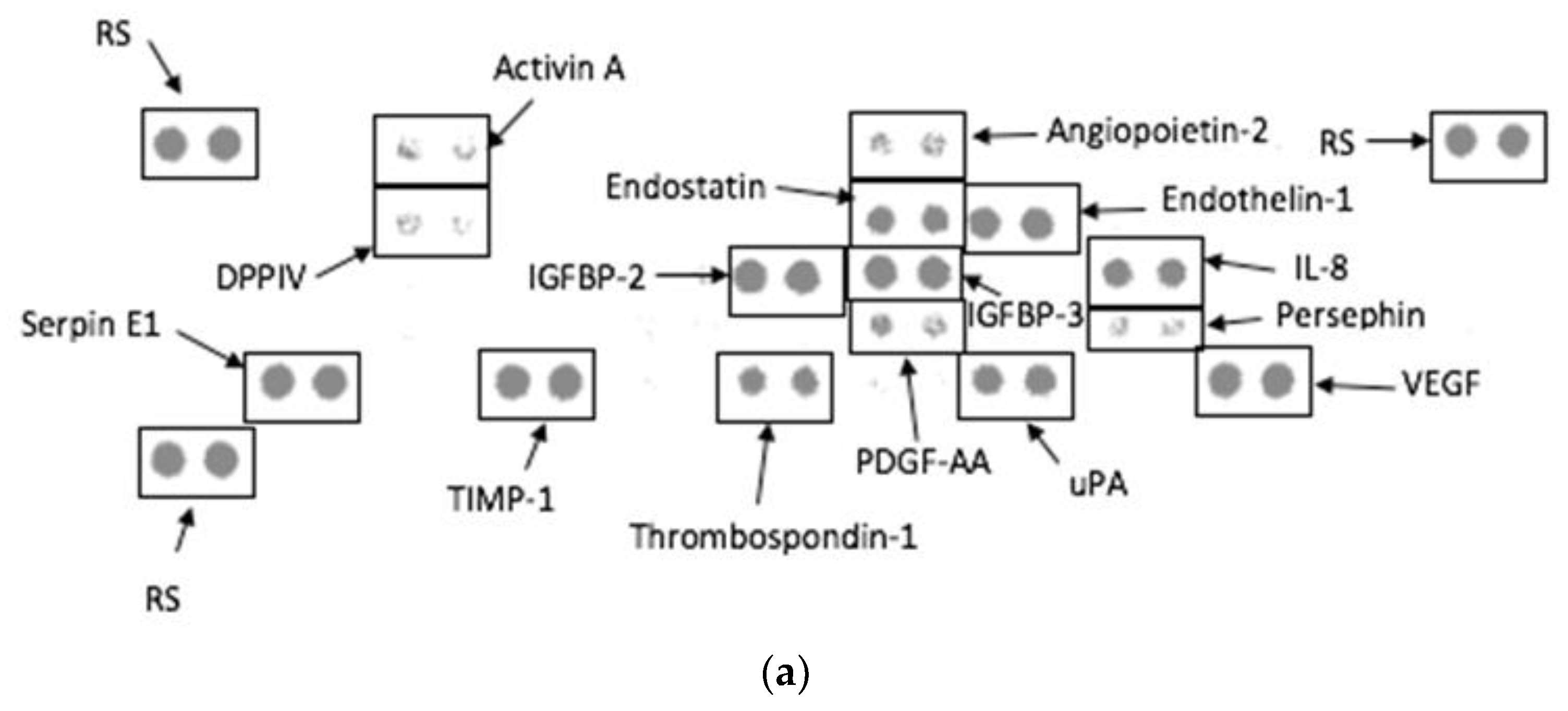
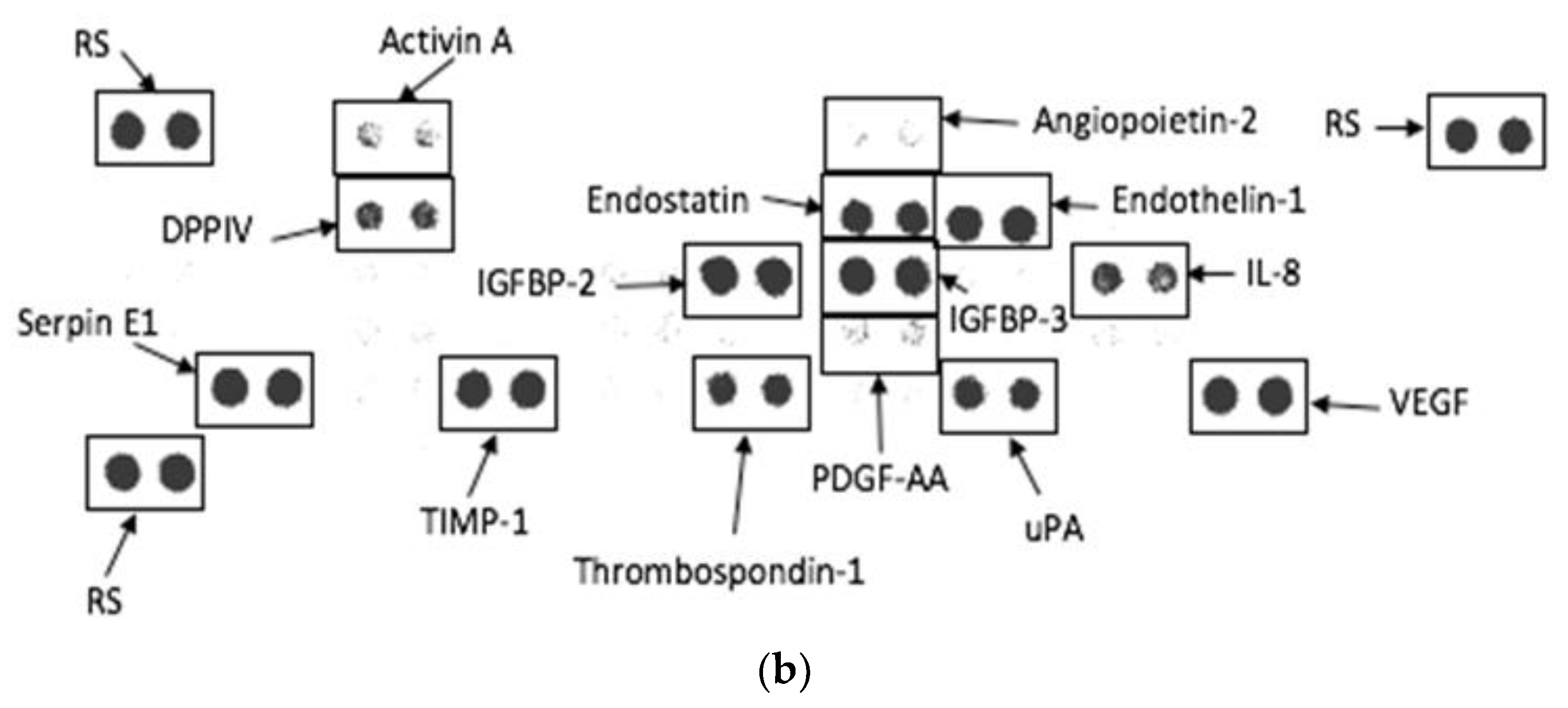
3.6. Angiogenesis Proteome Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Fontana, L.; Bergamaschi, A. Toxicological effects of titanium dioxide nanoparticles: A review of in vitro mammalian studies. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 481–508. [Google Scholar] [PubMed]
- Jain, A.; Jain, A.; Gulbake, A.; Shilpi, S.; Hurkat, P.; Jain, S.K. Peptide and protein delivery using new drug delivery systems. Critical Reviews™ in Therapeutic Drug Carrier Systems 2013, 30, 293–329. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Baranowska-Wojcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health-a Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, X.; Zhao, J.; Tang, G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J. Appl. Toxicol. 2009, 29, 330–337. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef] [PubMed]
- Musial, J.; Krakowiak, R.; Mlynarczyk, D.T.; Goslinski, T.; Stanisz, B.J. Titanium dioxide nanoparticles in food and personal care products—What do we know about their safety? Nanomaterials 2020, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-S.; Kang, B.-C.; Lee, J.K.; Jeong, J.; Che, J.-H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef]
- Janer, G.; Del Molino, E.M.; Fernández-Rosas, E.; Fernández, A.; Vázquez-Campos, S. Cell uptake and oral absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2014, 228, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, C.; Sun, J.; Zhong, G. Tissue distribution and excretion of intravenously administered titanium dioxide nanoparticles. Toxicol. Lett. 2011, 205, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef]
- Becker, K.; Schroecksnadel, S.; Geisler, S.; Carriere, M.; Gostner, J.M.; Schennach, H.; Herlin, N.; Fuchs, D. TiO(2) nanoparticles and bulk material stimulate human peripheral blood mononuclear cells. Food Chem. Toxicol. 2014, 65, 63–69. [Google Scholar] [CrossRef]
- Chen, E.Y.; Garnica, M.; Wang, Y.C.; Chen, C.S.; Chin, W.C. Mucin secretion induced by titanium dioxide nanoparticles. PLoS ONE 2011, 6, e16198. [Google Scholar] [CrossRef]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Saquib, Q.; Al-Khedhairy, A.A.; Siddiqui, M.A.; Abou-Tarboush, F.M.; Azam, A.; Musarrat, J. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicol. Vitr. 2012, 26, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Alinovi, R.; Goldoni, M.; Pinelli, S.; Campanini, M.; Aliatis, I.; Bersani, D.; Lottici, P.P.; Iavicoli, S.; Petyx, M.; Mozzoni, P. Oxidative and pro-inflammatory effects of cobalt and titanium oxide nanoparticles on aortic and venous endothelial cells. Toxicol. Vitr. 2015, 29, 426–437. [Google Scholar] [CrossRef]
- Huerta-Garcia, E.; Perez-Arizti, J.A.; Marquez-Ramirez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; Lopez-Marure, R. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic. Biol. Med. 2014, 73, 84–94. [Google Scholar] [CrossRef]
- Magdolenova, Z.; Bilaničová, D.; Pojana, G.; Fjellsbø, L.M.; Hudecova, A.; Hasplova, K.; Marcomini, A.; Dusinska, M. Impact of agglomeration and different dispersions of titanium dioxide nanoparticles on the human related in vitro cytotoxicity and genotoxicity. J. Environ. Monit. 2012, 14, 455–464. [Google Scholar] [CrossRef]
- Shukla, R.K.; Kumar, A.; Gurbani, D.; Pandey, A.K.; Singh, S.; Dhawan, A. TiO(2) nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology 2013, 7, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Fatisson, J.; Quevedo, I.R.; Wilkinson, K.J.; Tufenkji, N. Physicochemical characterization of engineered nanoparticles under physiological conditions: Effect of culture media components and particle surface coating. Colloids Surf. B Biointerfaces 2012, 91, 198–204. [Google Scholar] [CrossRef]
- Dinesh, P.; Yadav, C.S.; Kannadasan, S.; Rasool, M. Cytotoxicity and immunomodulatory effects of sol-gel combustion based titanium dioxide (TiO2) particles of large surface area on RAW 264.7 macrophages. Toxicol. Vitr. 2017, 43, 92–103. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages; InTech: Sydney, Australia, 2018; Volume 2. [Google Scholar]
- Granger, D.L.; Taintor, R.R.; Boockvar, K.S.; Hibbs, J.B., Jr. Measurement of Nitrate and Nitrite in Biological Samples Using Nitrate Reductase and Griess Reaction. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 268, pp. 142–151. [Google Scholar]
- Chen, Z.; Wang, Y.; Ba, T.; Li, Y.; Pu, J.; Chen, T.; Song, Y.; Gu, Y.; Qian, Q.; Yang, J. Genotoxic evaluation of titanium dioxide nanoparticles in vivo and in vitro. Toxicol. Lett. 2014, 226, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An interplay of oxidative stress, inflammation and cell death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.B.; Fidalgo de Cortalezzi, M.M. An experimental study on the aggregation of TiO2 nanoparticles under environmentally relevant conditions. Water Res. 2013, 47, 3887–3898. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Jin, X.; George, S.; Xia, T.; Meng, H.; Wang, X.; Suarez, E.; Zhang, H.; Hoek, E.M.; Godwin, H. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol. 2010, 44, 7309–7314. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Park, M.V.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Zaikova, T.; Baldock, B.L.; Balik-Meisner, M.; To, K.; Reif, D.M.; Kennedy, Z.C.; Hutchison, J.E.; Tanguay, R.L. Systematic determination of the relationship between nanoparticle core diameter and toxicity for a series of structurally analogous gold nanoparticles in zebrafish. Nanotoxicology 2019, 13, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, A.; Lanceleur, R.; Mourot, A.; Lavault, M.-T.; Casterou, G.; Jarry, G.; Hogeveen, K.; Fessard, V. Toxicity, genotoxicity and proinflammatory effects of amorphous nanosilica in the human intestinal Caco-2 cell line. Toxicol. Vitr. 2015, 29, 398–407. [Google Scholar]
- Koeneman, B.A.; Zhang, Y.; Westerhoff, P.; Chen, Y.; Crittenden, J.C.; Capco, D.G. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol. Toxicol. 2010, 26, 225–238. [Google Scholar] [PubMed]
- Peters, K.; Unger, R.E.; Kirkpatrick, C.J.; Gatti, A.M.; Monari, E. Effects of nano-scaled particles on endothelial cell function in vitro: Studies on viability, proliferation and inflammation. J. Mater. Sci. Mater. Med. 2004, 15, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-P.; Sun, Y.-P. Photocatalytic killing effect of TiO2 nanoparticles on Ls-174-t human colon carcinoma cells. World J. Gastroenterol. WJG 2004, 10, 3191. [Google Scholar]
- Asakura, H.; Kitahora, T. Antioxidants and Polyphenols in Inflammatory Bowel Disease: Ulcerative Colitis and Crohn Disease. In Polyphenols: Prevention and Treatment of Human Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–292. [Google Scholar]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88. [Google Scholar]
- Miller, D.J.; Fort, P.E. Heat shock proteins regulatory role in neurodevelopment. Front. Neurosci. 2018, 12, 821. [Google Scholar]
- Lindquist, S.; Craig, E. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Bigagli, E.; Lodovici, M. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxidative Med. Cell. Longev. 2019, 2019, 5953685. [Google Scholar] [CrossRef]
- Krüger, K.; Cossais, F.; Neve, H.; Klempt, M. Titanium dioxide nanoparticles activate IL8-related inflammatory pathways in human colonic epithelial Caco-2 cells. J. Nanopart. Res. 2014, 16, 2402. [Google Scholar] [CrossRef]
- De Angelis, I.; Barone, F.; Zijno, A.; Bizzarri, L.; Russo, M.T.; Pozzi, R.; Franchini, F.; Giudetti, G.; Uboldi, C.; Ponti, J. Comparative study of ZnO and TiO2 nanoparticles: Physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology 2013, 7, 1361–1372. [Google Scholar] [CrossRef]
- Sharma, V.K. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—A review. J. Environ. Sci. Health Part A 2009, 44, 1485–1495. [Google Scholar] [CrossRef]
- Nogueira, C.M.; de Azevedo, W.M.; Dagli, M.L.Z.; Toma, S.H.; de Arruda Leite, A.Z.; Lordello, M.L.; Nishitokukado, I.; Ortiz-Agostinho, C.L.; Duarte, M.I.S.; Ferreira, M.A. Titanium dioxide induced inflammation in the small intestine. World J. Gastroenterol. WJG 2012, 18, 4729. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Lee, K.-J.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef]
- Linares, P.M.; Chaparro, M.; Gisbert, J.P. Angiopoietins in inflammation and their implication in the development of inflammatory bowel disease. A review. J. Crohn’s Colitis 2014, 8, 183–190. [Google Scholar] [CrossRef]
- Risau, W.; Drexler, H.; Mironov, V.; Smits, A.; Siegbahn, A.; Funa, K.; Heldin, C.-H. Platelet-derived growth factor is angiogenic in vivo. Growth Factors 1992, 7, 261–266. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Son, J.G.; Song, N.W.; Kim, Y.-I.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Anti-angiogenic effect of bare titanium dioxide nanoparticles on pathologic neovascularization without unbearable toxicity. Nanomed. Nanotechnol. Biol. Med. 2014, 10, e1109–e1117. [Google Scholar] [CrossRef]
- Walia, A.; Yang, J.F.; Huang, Y.-h.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 2422–2438. [Google Scholar] [CrossRef]
- Kaneda, H.; Arao, T.; Matsumoto, K.; De Velasco, M.; Tamura, D.; Aomatsu, K.; Kudo, K.; Sakai, K.; Nagai, T.; Fujita, Y. Activin A inhibits vascular endothelial cell growth and suppresses tumour angiogenesis in gastric cancer. Br. J. Cancer 2011, 105, 1210–1217. [Google Scholar] [CrossRef]
- Kitlinska, J.; Lee, E.W.; Li, L.; Pons, J.; Estes, L.; Zukowska, Z. Dual role of dipeptidyl peptidase IV (DPP IV) in angiogenesis and vascular remodeling. In Dipeptidyl Aminopeptidases in Health and Disease; Springer: Berlin, Germany, 2004; pp. 215–222. [Google Scholar]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213. [Google Scholar] [CrossRef]


| MEDIA | Number of Days | |||
|---|---|---|---|---|
| 0 | 1 | 7 | 14 | |
| 150 Mm NaCl | 297.33 ± 43.55 | 431.87 ± 59.81 | 347.94 ± 72.48 | 647.53 ± 56.18 * |
| 1× PBS | 787.33 ± 65.24 | 787.33 ± 65.24 | 915.3 ± 113.18 | 1055.8 ± 86.95 **** |
| DMEM | 787.33 ± 65.24 | 962 ± 140.63 | 1442.35 ± 491.99 **** | 685.04 ± 121.19 |
| DMEM (10% FBS) | 787.33 ± 65.24 | 911.67 ± 75.06 | 1121.26 ± 216.26 | 1821.8 ± 450.9 ** |
| MEDIA | Number of Days | |||
|---|---|---|---|---|
| 0 | 1 | 7 | 14 | |
| 150 Mm NaCl | −23.3 ± 0.66 | −23.82 ± 1.49 | −20.06 ± 5.84 | −12.43 ± 0.45 *** |
| 1× PBS | −26.03 ± 2.29 | −16.05 ± 2.77 | −27.87 ± 1.97 | −16.48 ± 1.29 * |
| DMEM | −14.1 ± 0.87 | −13.76 ± 1.66 | −8.29 ± 1.9 **** | −12.62 ± 2.41 |
| DMEM (10% FBS) | 13.03 ± 0.85 | −13.4 ± 1.35 | −13.7 ± 1.18 | −16.45 ± 1.1 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, O.M.; Lategan, K.L.; de Cortalezzi, M.F.; Pool, E.J. The Stability and Anti-Angiogenic Properties of Titanium Dioxide Nanoparticles (TiO2NPs) Using Caco-2 Cells. Biomolecules 2022, 12, 1334. https://doi.org/10.3390/biom12101334
David OM, Lategan KL, de Cortalezzi MF, Pool EJ. The Stability and Anti-Angiogenic Properties of Titanium Dioxide Nanoparticles (TiO2NPs) Using Caco-2 Cells. Biomolecules. 2022; 12(10):1334. https://doi.org/10.3390/biom12101334
Chicago/Turabian StyleDavid, Oladipupo Moyinoluwa, Kim Leigh Lategan, Maria Fidalgo de Cortalezzi, and Edmund John Pool. 2022. "The Stability and Anti-Angiogenic Properties of Titanium Dioxide Nanoparticles (TiO2NPs) Using Caco-2 Cells" Biomolecules 12, no. 10: 1334. https://doi.org/10.3390/biom12101334
APA StyleDavid, O. M., Lategan, K. L., de Cortalezzi, M. F., & Pool, E. J. (2022). The Stability and Anti-Angiogenic Properties of Titanium Dioxide Nanoparticles (TiO2NPs) Using Caco-2 Cells. Biomolecules, 12(10), 1334. https://doi.org/10.3390/biom12101334








