Deletion of Growth Hormone Secretagogue Receptor in Kisspeptin Neurons in Female Mice Blocks Diet-Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Production of Kiss1Cre/EGFP;Ghsrfl/fl Mice
2.3. Chemicals
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Surgical Procedures
2.6. Food Intake, Body Weight, and Body Composition
2.7. Metabolic Monitoring
2.8. Glucose and Insulin Tolerance Tests
2.9. Behavioral Tests
2.9.1. Open Field Test (OFT)
2.9.2. Elevated Plus-Maze (EPM)
2.9.3. Light/Dark Box (LDB)
2.10. The Effects of Fasting on Meal Patterns
2.11. Core Body Temperature Recording
2.12. Statistical Analysis
3. Results
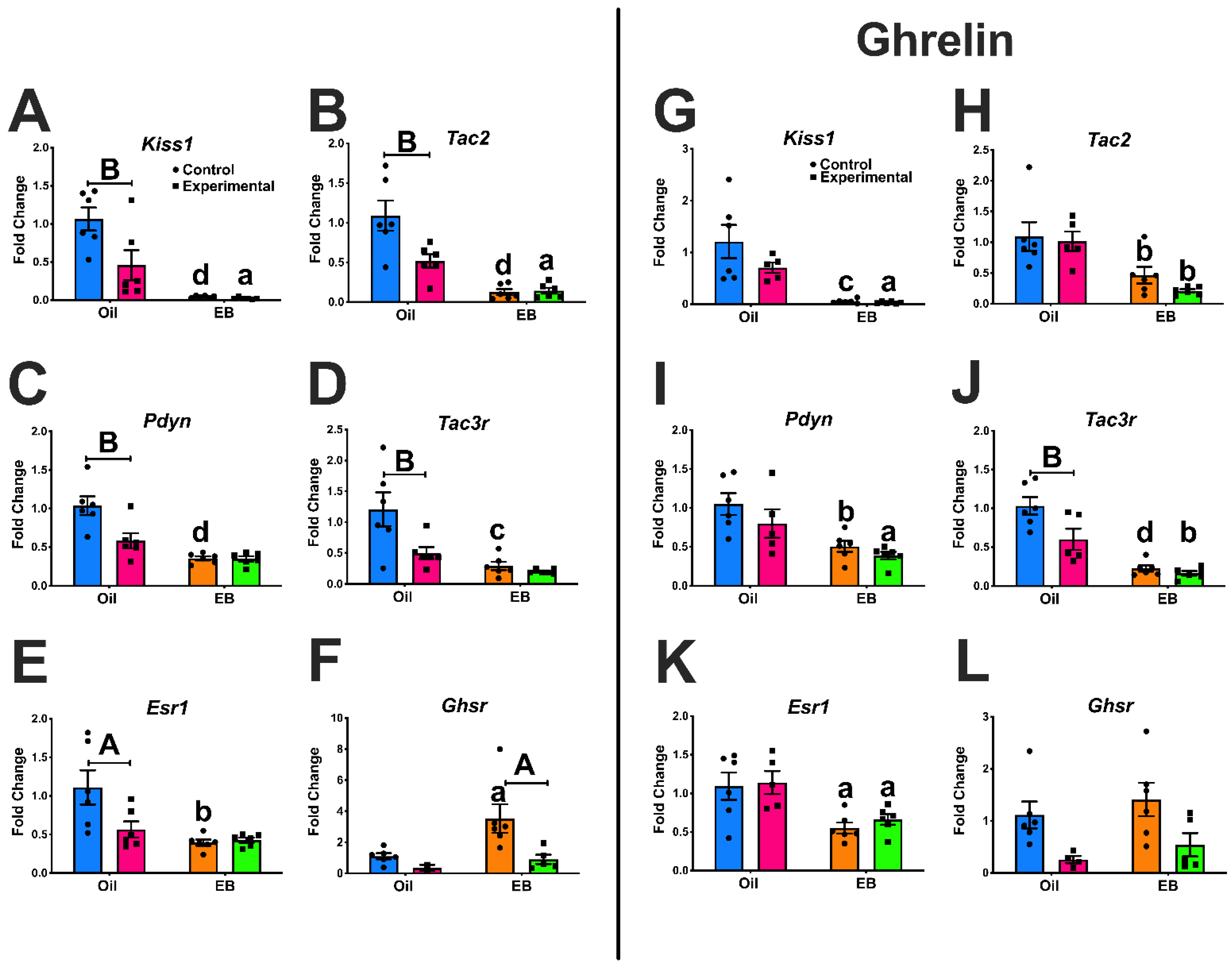
3.1. Experiment #1: Regulation of ARC Gene Expression
3.2. Fertility and LH Pulsatility
3.3. Experiment #2: Response to Ovariectomy
3.3.1. Body Weight Gain and Body Composition
3.3.2. Glucose and Insulin Tolerance Tests
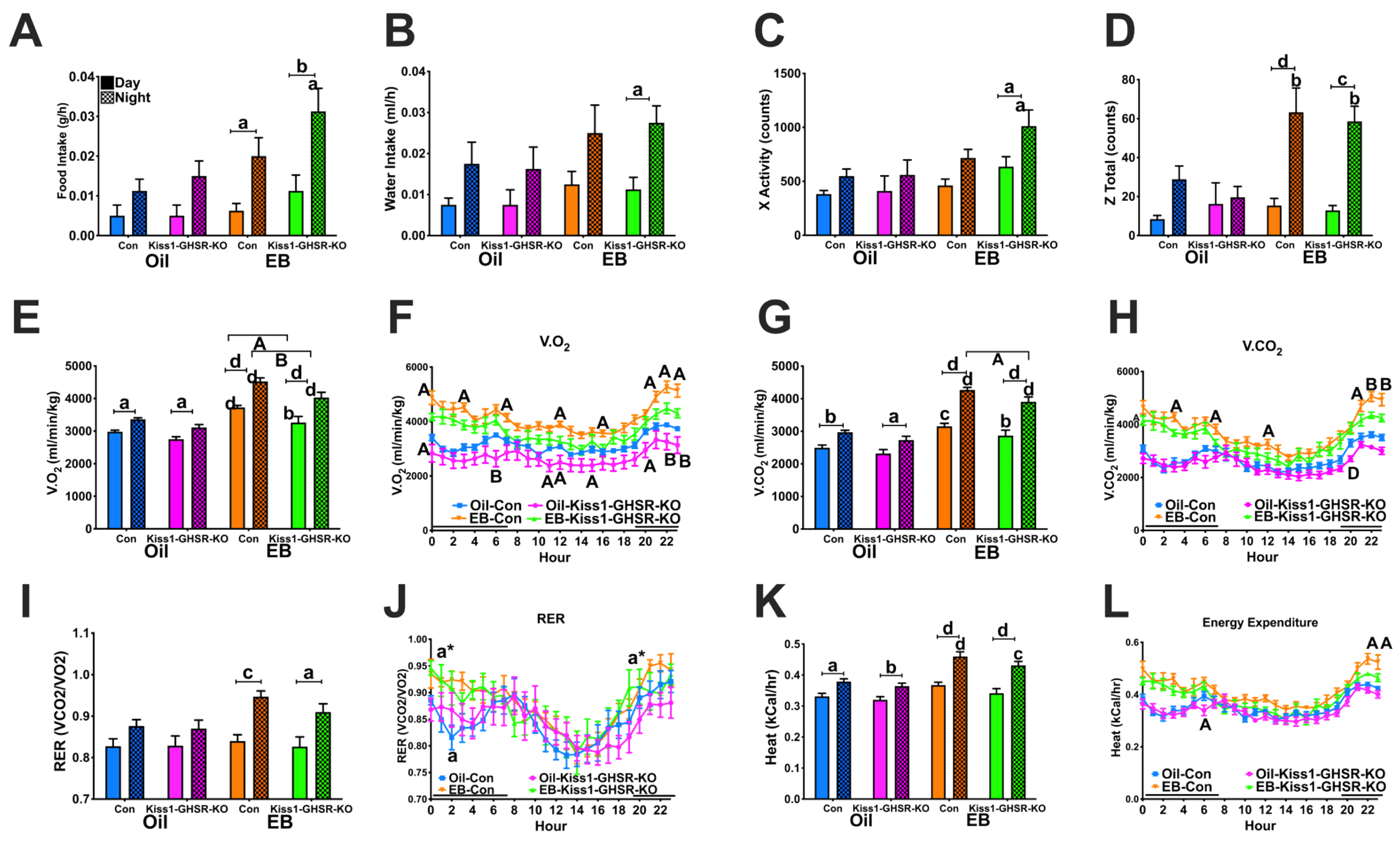
3.3.3. Feeding, Locomotor and Metabolic Behaviors in CLAMS
3.4. Experiment #3: Response to Diet Induced Obesity (DIO)
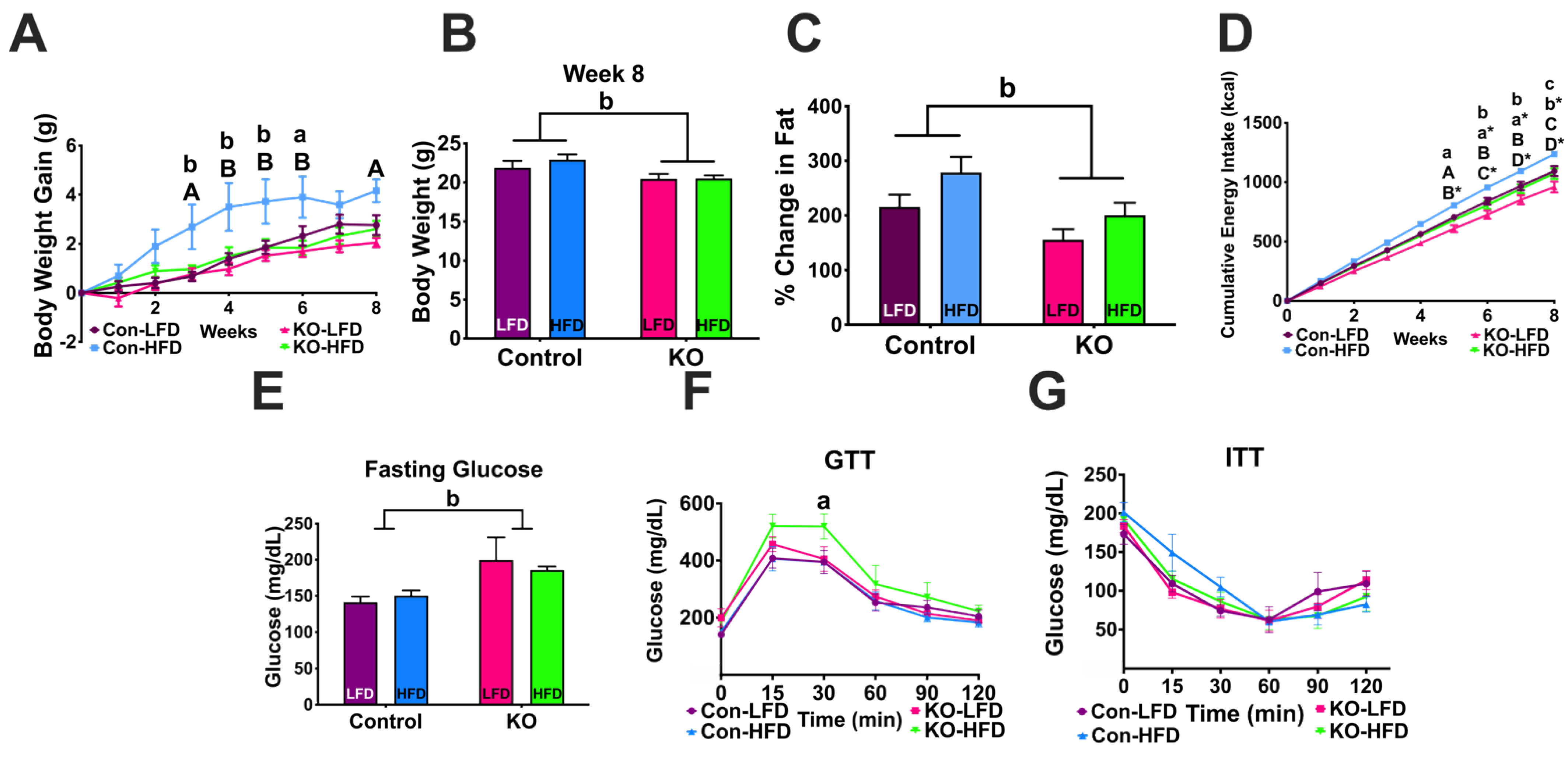
3.4.1. Body Weight Gain and Body Composition
3.4.2. Glucose and Insulin Tolerance Tests
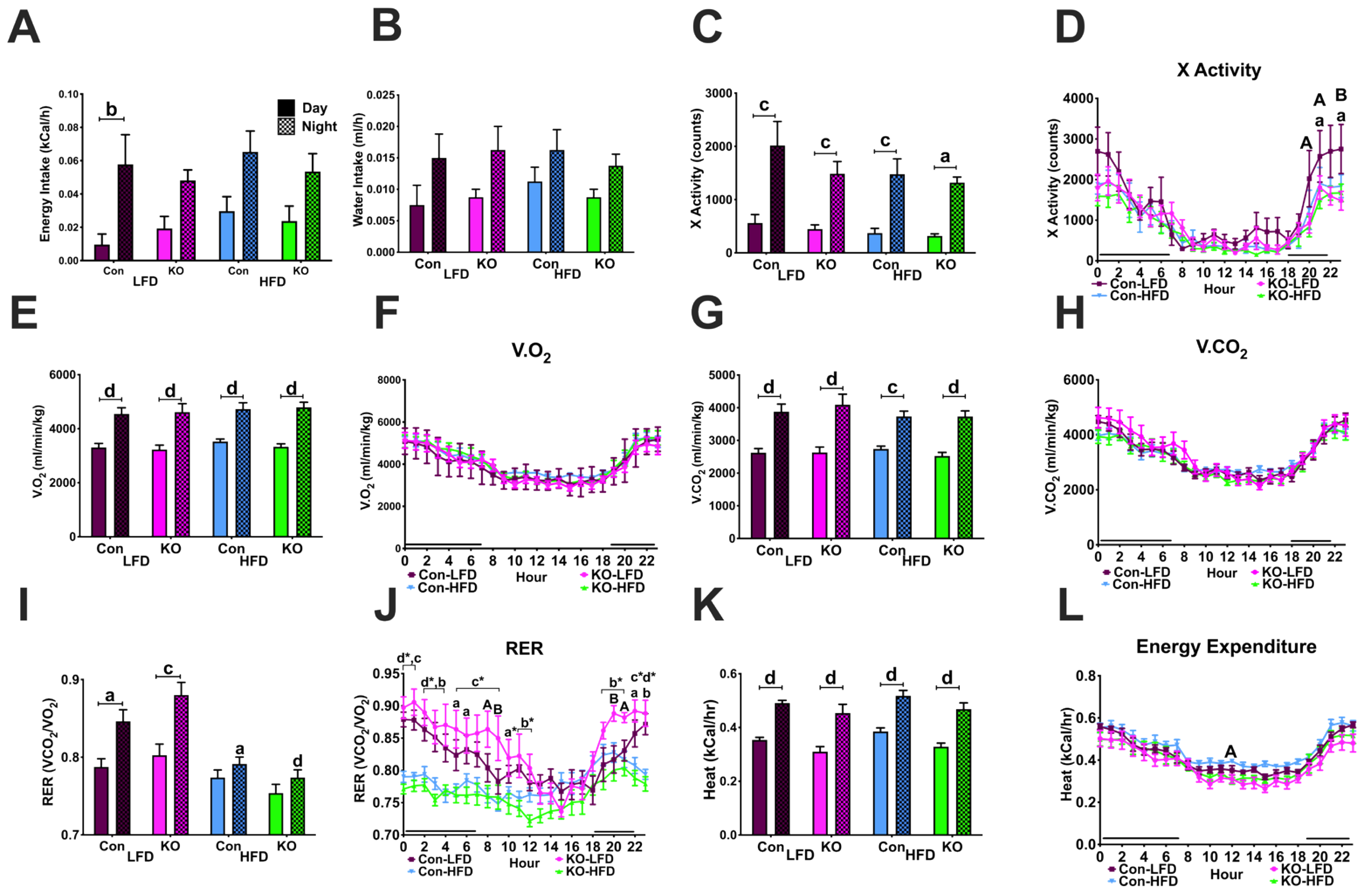
3.4.3. Locomotor Activity and Ingestive Behaviors in CLAMS
3.4.4. Metabolic Parameters in the CLAMS
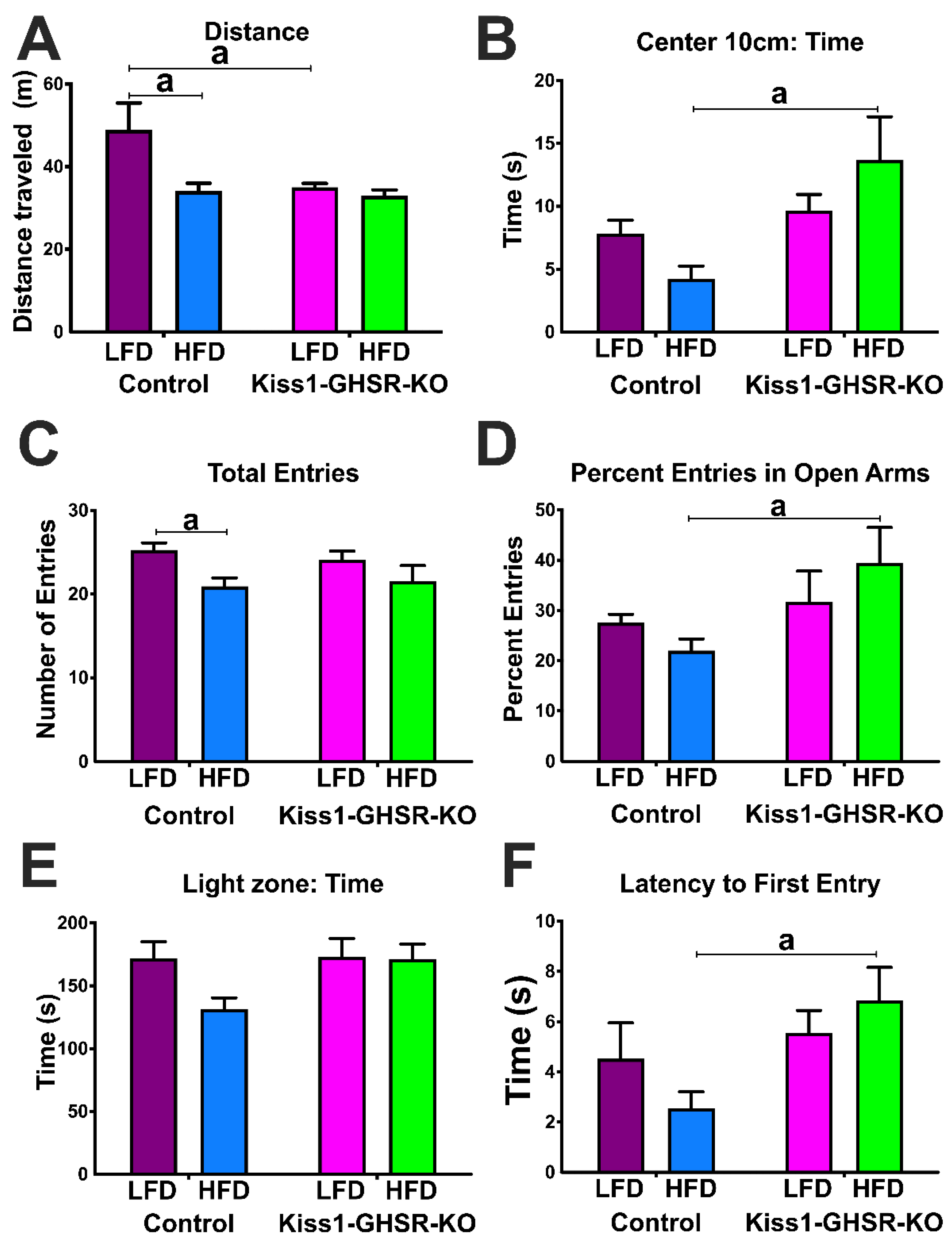
3.5. Experiment #4: Locomotor and Anxiety-like Behavior
3.5.1. Open Field Test
3.5.2. Elevated Plus Maze
3.5.3. Light Dark Box
3.6. Experiment #5: Fasting-Induced Refeeding
3.7. Experiment #6: Thermoregulation
3.8. Extra-Arcuate Gene Expression
3.9. Liver Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McAllan, B.M.; Geiser, F. Torpor during Reproduction in Mammals and Birds: Dealing with an Energetic Conundrum. Integr. Comp. Biol. 2014, 54, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Tena-Sempere, M. Interaction between Energy Homeostasis and Reproduction: Central Effects of Leptin and Ghrelin on the Reproductive Axis. Horm. Metab. Res. 2013, 45, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Tena-Sempere, M. Roles of Ghrelin and Leptin in the Control of Reproductive Function. Neuroendocrinology 2007, 86, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, M.L.; Tena-Sempere, M. Ghrelin and reproduction: A novel signal linking energy status and fertility? Mol. Cell Endocrinol. 2004, 226, 1–9. [Google Scholar] [CrossRef]
- Andrews, Z.B. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides 2011, 32, 2248–2255. [Google Scholar] [CrossRef]
- Cowley, M. The Distribution and Mechanism of Action of Ghrelin in the CNS Demonstrates a Novel Hypothalamic Circuit Regulating Energy Homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef]
- Fernández-Fernández, R.; Tena-Sempere, M.; Navarro, V.M.; Barreiro, M.L.; Castellano, J.M.; Aguilar, E.; Pinilla, L. Effects of Ghrelin upon Gonadotropin-Releasing Hormone and Gonadotropin Secretion in Adult Female Rats: In vivo and in vitro Studies. Neuroendocrinology 2005, 82, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, R.; Tena-Sempere, M.; Aguilar, E.; Pinilla, L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci. Lett. 2004, 362, 103–107. [Google Scholar] [CrossRef]
- Furuta, M.; Funabashi, T.; Kimura, F. Intracerebroventricular Administration of Ghrelin Rapidly Suppresses Pulsatile Luteinizing Hormone Secretion in Ovariectomized Rats. Biochem. Biophys. Res. Commun. 2001, 288, 780–785. [Google Scholar] [CrossRef]
- Forbes, S.; Li, X.F.; Kinsey-Jones, J.; O’Byrne, K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci. Lett. 2009, 460, 143–147. [Google Scholar] [CrossRef]
- Ogata, R.; Matsuzaki, T.; Iwasa, T.; Kiyokawa, M.; Tanaka, N.; Kuwahara, A.; Yasui, T.; Irahara, M. Hypothalamic Ghrelin Suppresses Pulsatile Secretion of Luteinizing Hormone via β-Endorphin in Ovariectomized Rats. Neuroendocrinology 2009, 90, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Nestor, C.C.; Zhang, C.; Padilla, S.L.; Palmiter, R.D.; Kelly, M.J.; Rønnekleiv, O.K. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife 2016, 5, e16246. [Google Scholar] [CrossRef]
- Brock, O.; Bakker, J. The Two Kisspeptin Neuronal Populations Are Differentially Organized and Activated by Estradiol in Mice. Endocrinology 2013, 154, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- de Croft, S.; Boehm, U.; Herbison, A.E. Neurokinin B Activates Arcuate Kisspeptin Neurons through Multiple Tachykinin Receptors in the Male Mouse. Endocrinology 2013, 154, 2750–2760. [Google Scholar] [CrossRef] [PubMed]
- Ruka, K.A.; Burger, L.L.; Moenter, S.M. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin b, and dynorphin by modulators of neurokinin 3 and k-opioid receptors in adult male mice. Endocrinology 2013, 154, 2761–2771. [Google Scholar] [CrossRef]
- Weems, P.W.; Witty, C.F.; Amstalden, M.; Coolen, L.; Goodman, R.L.; Lehman, M.N. κ-Opioid Receptor Is Colocalized in GnRH and KNDy Cells in the Female Ovine and Rat Brain. Endocrinology 2016, 157, 2367–2379. [Google Scholar] [CrossRef]
- Goodman, R.L.; Hileman, S.M.; Nestor, C.C.; Porter, K.L.; Connors, J.M.; Hardy, S.L.; Millar, R.P.; Cernea, M.; Coolen, L.M.; Lehman, M.N. Kisspeptin, Neurokinin B, and Dynorphin Act in the Arcuate Nucleus to Control Activity of the GnRH Pulse Generator in Ewes. Endocrinology 2013, 154, 4259–4269. [Google Scholar] [CrossRef]
- Bosch, M.A.; Tonsfeldt, K.J.; Rønnekleiv, O.K. mRNA expression of ion channels in GnRH neurons: Subtype-specific regulation by 17β-estradiol. Mol. Cell. Endocrinol. 2013, 367, 85–97. [Google Scholar] [CrossRef]
- Oakley, A.E.; Clifton, D.K.; Steiner, R.A. Kisspeptin Signaling in the Brain. Endocr. Rev. 2009, 30, 713–743. [Google Scholar] [CrossRef]
- Roepke, T.A. Oestrogen Modulates Hypothalamic Control of Energy Homeostasis through Multiple Mechanisms. J. Neuroendocr. 2009, 21, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Frazao, R.; Lemko, H.M.D.; da Silva, R.P.; Ratra, D.V.; Lee, C.E.; Williams, K.W.; Elias, C.F. Estradiol modulates Kiss1 neuronal response to ghrelin. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E606. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M. Metabolic regulation of kisspeptin—The link between energy balance and reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Mittelman-Smith, M.A.; Williams, H.; Krajewski-Hall, S.J.; Lai, J.; Ciofi, P.; McMullen, N.T.; Rance, N.E. Arcuate Kisspeptin/Neurokinin B/Dynorphin (KNDy) Neurons Mediate the Estrogen Suppression of Gonadotropin Secretion and Body Weight. Endocrinology 2012, 153, 2800–2812. [Google Scholar] [CrossRef] [PubMed]
- Nestor, C.; Qiu, J.; Padilla, S.L.; Zhang, C.; Bosch, M.A.; Fan, W.; Aicher, S.A.; Palmiter, R.D.; Rønnekleiv, O.K.; Kelly, M.J. Optogenetic Stimulation of Arcuate Nucleus Kiss1 Neurons Reveals a Steroid-Dependent Glutamatergic Input to POMC and AgRP Neurons in Male Mice. Mol. Endocrinol. 2016, 30, 630–644. [Google Scholar] [CrossRef]
- Li-Ying, F.; van den Pol, A.N. Kisspeptin Directly Excites Anorexigenic Proopiomelanocortin Neurons but Inhibits Orexigenic Neuropeptide Y Cells by an Indirect Synaptic Mechanism. J. Neurosci. 2010, 30, 10205–10219. [Google Scholar]
- Padilla, S.L.; Johnson, C.W.; Barker, F.D.; Patterson, M.A.; Palmiter, R.D. A Neural Circuit Underlying the Generation of Hot Flushes. Cell Rep. 2018, 24, 271–277. [Google Scholar] [CrossRef]
- Rance, N.E.; Dacks, P.A.; Mittelman-Smith, M.A.; Romanovsky, A.A.; Krajewski-Hall, S.J. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: A novel hypothesis on the mechanism of hot flushes. Front. Neuroendocr. 2013, 34, 211–227. [Google Scholar] [CrossRef]
- Krajewski-Hall, S.J.; Blackmore, E.M.; Mcminn, J.R.; Rance, N.E. Estradiol alters body temperature regulation in the female mouse. Temperature 2018, 5, 56–69. [Google Scholar] [CrossRef]
- Nestor, C.C.; Kelly, M.J.; Rønnekleiv, O.K. Cross-talk between reproduction and energy homeostasis: Central impact of estrogens, leptin and kisspeptin signaling. Horm. Mol. Biol. Clin. Investig. 2014, 17, 109–128. [Google Scholar] [CrossRef]
- Herbison, A.E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol. 2016, 12, 452–466. [Google Scholar] [CrossRef]
- Yang, J.A.; Yasrebi, A.; Snyder, M.; Roepke, T.A. The interaction of fasting, caloric restriction, and diet-induced obesity with 17beta-estradiol on the expression of KNDy neuropeptides and their receptors in the female mouse. Mol. Cell Endocrinol. 2016, 437, 35–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde, K.; Roepke, T.A. 17β-Estradiol Increases Arcuate KNDy Neuronal Sensitivity to Ghrelin Inhibition of the M-Current in Female Mice. Neuroendocrinology 2019, 110, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Wade, G.N.; Gray, J.M.; Bartness, T.J. Gonadal influences on adiposity. Int. J. Obes. 1985, 9 (Suppl. S1), 83–92. [Google Scholar] [PubMed]
- Santollo, J.; Eckel, L.A. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav. Brain Res. 2008, 191, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Krause, W.C.; Rodriguez, R.; Gegenhuber, B.; Matharu, N.; Rodriguez, A.N.; Padilla, A.M.; Ingraham, H.A. Estrogen Drives Melanocortin Neurons To Reduce Sedentary Behavior. bioRxiv 2019, 794792. [Google Scholar] [CrossRef]
- Stincic, T.L.; Grachev, P.; Bosch, M.A.; Rønnekleiv, O.K.; Kelly, M.J. Estradiol Drives the Anorexigenic Activity of Proopiomelanocortin Neurons in Female Mice. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Qiu, J.; Rivera, H.M.; Bosch, M.A.; Padilla, S.L.; Stincic, T.L.; Palmiter, R.D.; Kelly, M.J.; Rønnekleiv, O.K. Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. eLife 2018, 7, e35656. [Google Scholar] [CrossRef]
- Santollo, J.; Eckel, L.A. The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiol. Behav. 2008, 93, 842–850. [Google Scholar] [CrossRef]
- Navarro, V.M.; Bosch, M.A.; León, S.; Simavli, S.; True, C.; Pinilla, L.; Carroll, R.S.; Seminara, S.B.; Tena-Sempere, M.; Rønnekleiv, O.K.; et al. The Integrated Hypothalamic Tachykinin-Kisspeptin System as a Central Coordinator for Reproduction. Endocrinology 2015, 156, 627–637. [Google Scholar] [CrossRef]
- Roa, J.; Barroso, A.; Ruiz-Pino, F.; Vázquez, M.J.; Seoane-Collazo, P.; Martínez-Sanchez, N.; García-Galiano, D.; Ilhan, T.; Pineda, R.; León, S.; et al. Metabolic regulation of female puberty via hypothalamic AMPK–kisspeptin signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E10758–E10767. [Google Scholar] [CrossRef]
- Gottsch, M.L.; Popa, S.M.; Lawhorn, J.K.; Qiu, J.; Tonsfeldt, K.; Bosch, M.A.; Kelly, M.J.; Rønnekleiv, O.K.; Sanz, E.; McKnight, G.S.; et al. Molecular Properties of Kiss1 Neurons in the Arcuate Nucleus of the Mouse. Endocrinology 2011, 152, 4298–4309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bosch, M.A.; Qiu, J.; Rønnekleiv, O.K.; Kelly, M.J. 17β-Estradiol Increases Persistent Na+ Current and Excitability of AVPV/PeN Kiss1 Neurons in Female Mice. Mol. Endocrinol. 2015, 29, 518–527. [Google Scholar] [CrossRef]
- True, C.; Verma, S.; Grove, K.L.; Smith, M.S. Cocaine- and Amphetamine-Regulated Transcript Is a Potent Stimulator of GnRH and Kisspeptin Cells and May Contribute to Negative Energy Balance-induced Reproductive Inhibition in Females. Endocrinology 2013, 154, 2821–2832. [Google Scholar] [CrossRef]
- Padilla, S.L.; Qiu, J.; Nestor, C.; Zhang, C.; Smith, A.W.; Whiddon, B.B.; Rønnekleiv, O.K.; Kelly, M.J.; Palmiter, R.D. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc. Natl. Acad. Sci. USA 2017, 114, 2413–2418. [Google Scholar] [CrossRef]
- Sanz, E.; Quintana, A.; Deem, J.D.; Steiner, R.A.; Palmiter, R.D.; McKnight, G.S. Fertility-regulating Kiss1 neurons arise from hypothalamic POMC-expressing progenitors. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 5549–5556. [Google Scholar] [CrossRef] [PubMed]
- Roepke, T.A.; Qiu, J.; Smith, A.W.; Rønnekleiv, O.K.; Kelly, M.J. Fasting and 17β-estradiol differentially modulate the M-current in NPY neurons. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 11825–11835. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Wiersielis, K.; Yasrebi, A.; Conde, K.; Armstrong, L.; Guo, G.L.; Roepke, T.A. Sex- and age-dependent effects of maternal organophosphate flame-retardant exposure on neonatal hypothalamic and hepatic gene expression. Reprod. Toxicol. 2020, 94, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Krumm, E.A.; Patel, V.J.; Tillery, T.S.; Yasrebi, A.; Shen, J.; Guo, G.L.; Marco, S.M.; Buckley, B.T.; Roepke, T.A. Organophosphate Flame-Retardants Alter Adult Mouse Homeostasis and Gene Expression in a Sex-Dependent Manner Potentially Through Interactions with ERα. Toxicol. Sci. 2017, 162, 212–224. [Google Scholar] [CrossRef]
- Yasrebi, A.; Hsieh, A.; Mamounis, K.J.; Krumm, E.A.; Yang, J.A.; Magby, J.; Roepke, T.A. Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17beta-estradiol. Mol. Cell Endocrinol. 2016, 422, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Keith, F. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates; Compact, (3rd ed.): The Coronal Plates and Diagrams; Elsevier Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Wiersielis, K.R.; Adams, S.; Yasrebi, A.; Conde, K.; Roepke, T. Maternal exposure to organophosphate flame retardants alters locomotor and anxiety-like behavior in male and female adult offspring. Horm. Behav. 2020, 122, 104759. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test, in Mood and Anxiety Related Phenotypes in Mice; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Roepke, T.; Bosch, M.A.; Rick, E.A.; Lee, B.; Wagner, E.J.; Seidlova-Wuttke, D.; Wuttke, W.; Scanlan, T.S.; Rønnekleiv, O.K.; Kelly, M.J. Contribution of a Membrane Estrogen Receptor to the Estrogenic Regulation of Body Temperature and Energy Homeostasis. Endocrinology 2010, 151, 4926–4937. [Google Scholar] [CrossRef]
- Goto, M.; Arima, H.; Watanabe, M.; Hayashi, M.; Banno, R.; Sato, I.; Nagasaki, H.; Oiso, Y. Ghrelin Increases Neuropeptide Y and Agouti-Related Peptide Gene Expression in the Arcuate Nucleus in Rat Hypothalamic Organotypic Cultures. Endocrinology 2006, 147, 5102–5109. [Google Scholar] [CrossRef]
- Sun, Y.; Garcia, J.M.; Smith, R.G. Ghrelin and Growth Hormone Secretagogue Receptor Expression in Mice during Aging. Endocrinology 2007, 148, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.-C.; Sakata, I.; Kohno, D.; Perello, M.; Osborne-Lawrence, S.; Repa, J.J.; Zigman, J.M. Ghrelin Directly Stimulates Glucagon Secretion from Pancreatic α-Cells. Mol. Endocrinol. 2011, 25, 1600–1611. [Google Scholar] [CrossRef]
- Islam, M.N.; Mita, Y.; Maruyama, K.; Tanida, R.; Zhang, W.; Sakoda, H.; Nakazato, M. Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J. Endocrinol. 2020, 244, 13–23. [Google Scholar] [CrossRef]
- Luo, Q.; Hu, J.; Yang, G.; Yuan, X.; Chen, Z.; Wang, D.; Lu, Y.; Zhu, L.; Wang, G. Fasting Increases Iron Export by Modulating Ferroportin 1 Expression through the Ghrelin/GHSR1α/MAPK Pathway in the Liver. Biol. Trace Element Res. 2020, 199, 267–277. [Google Scholar] [CrossRef]
- Popa, S.M.; Moriyama, R.M.; Caligioni, C.S.; Yang, J.J.; Cho, C.M.; Concepcion, T.L.; Oakley, A.E.; Lee, I.H.; Sanz, E.; Amieux, P.S.; et al. Redundancy in Kiss1 Expression Safeguards Reproduction in the Mouse. Endocrinology 2013, 154, 2784–2794. [Google Scholar] [CrossRef]
- Sun, Y.; Asnicar, M.; Saha, P.K.; Chan, L.; Smith, R.G. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006, 3, 379–386. [Google Scholar] [CrossRef]
- Sun, Y.; Butte, N.F.; Garcia, J.M.; Smith, R.G. Characterization of Adult Ghrelin and Ghrelin Receptor Knockout Mice under Positive and Negative Energy Balance. Endocrinology 2008, 149, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.V.; Plum, L.; Ono, H.; Gutiérrez-Juárez, R.; Shanabrough, M.; Borok, E.; Horvath, T.L.; Rossetti, L.; Accili, D. Divergent Regulation of Energy Expenditure and Hepatic Glucose Production by Insulin Receptor in Agouti-Related Protein and POMC Neurons. Diabetes 2010, 59, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.; Joung, H.-Y.; Liu, S.-M.; Chua, S.C.; Schwartz, G.J.; Jo, Y.-H. Optogenetic stimulation of the liver-projecting melanocortinergic pathway promotes hepatic glucose production. Nat. Commun. 2020, 11, 6295. [Google Scholar] [CrossRef] [PubMed]
- Alsina, R.; Trotta, M.; Bumaschny, V.F. Hypothalamic Proopiomelanocortin Is Necessary for Normal Glucose Homeostasis in Female Mice. Front. Endocrinol. 2018, 9, 554. [Google Scholar] [CrossRef]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Verhulst, P.-J.; Depoortere, I. Ghrelin’s second life: From appetite stimulator to glucose regulator. World J. Gastroenterol. 2012, 18, 3183–3195. [Google Scholar]
- Park, S.; Jiang, H.; Zhang, H.; Smith, R.G. Modification of ghrelin receptor signaling by somatostatin receptor-5 regulates insulin release. Proc. Natl. Acad. Sci. USA 2012, 109, 19003–19008. [Google Scholar] [CrossRef]
- Licholai, J.A.; Nguyen, K.P.; Fobbs, W.C.; Schuster, C.J.; Ali, M.A.; Kravitz, A.V. Why Do Mice Overeat High-Fat Diets? How High-Fat Diet Alters the Regulation of Daily Caloric Intake in Mice. Obesity 2018, 26, 1026–1033. [Google Scholar] [CrossRef]
- Ibos, K.; Bodnár, É.; Bagosi, Z.; Bozsó, Z.; Tóth, G.; Szabó, G.; Csabafi, K. Kisspeptin-8 Induces Anxiety-Like Behavior and Hypolocomotion by Activating the HPA Axis and Increasing GABA Release in the Nucleus Accumbens in Rats. Biomedicines 2021, 9, 112. [Google Scholar] [CrossRef]
- Wang, P.; Loh, K.H.; Wu, M.; Morgan, D.A.; Schneeberger, M.; Yu, X.; Chi, J.; Kosse, C.; Kim, D.; Rahmouni, K.; et al. A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 2020, 583, 839–844. [Google Scholar] [CrossRef]
- Uchida, Y.; Nagashima, K.; Yuri, K. Fasting or systemic des-acyl ghrelin administration to rats facilitates thermoregulatory behavior in a cold environment. Brain Res. 2018, 1696, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Tokizawa, K.; Onoue, Y.; Uchida, Y.; Nagashima, K. Ghrelin Induces Time-Dependent Modulation of Thermoregulation in the Cold. Chrono-Int. 2012, 29, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Steyn, F.J.; Wan, Y.; Clarkson, J.; Veldhuis, J.D.; Herbison, A.E.; Chen, C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology 2013, 154, 4939–4945. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Yang, J.A.; Hughes, J.K.; Parra, R.A.; Volk, K.M.; Kauffman, A.S. Stress rapidly suppresses in vivo LH pulses and increases activation of RFRP-3 neurons in male mice. J. Endocrinol. 2018, 239, 339–350. [Google Scholar] [CrossRef]
- Negrón, A.L.; Radovick, S. High-Fat Diet Alters LH Secretion and Pulse Frequency in Female Mice in an Es-trous Cycle-Dependent Manner. Endocrinology 2020, 161, bqaa146. [Google Scholar] [CrossRef]
- Qiu, J.; Bosch, M.A.; Zhang, C.; Rønnekleiv, O.K.; Kelly, M.J. Estradiol Protects Neuropeptide Y/Agouti-Related Peptide Neurons against Insulin Resistance in Females. Neuroendocrinology 2020, 110, 105–118. [Google Scholar] [CrossRef]
- Roepke, T.A.; Zhang, C.; Rønnekleiv, O.K.; Kelly, M.J. Gonadotropin-releasing hormone (GnRH) activates the m-current in GnRH neurons: An autoregulatory negative feedback mechanism? Endocrinology 2008, 149, 2459–2466. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Gene | Accession Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| Agrp | NM_007427.2 | CTCCACTGAAGGGCATCAGAA | ATCTAGCACCTCCGCCAAA |
| Cart | NM_013732 | GCTCAAGAGTAAACGCATTCC | GTCCCTTCACAAGCACTTCAA |
| Esr1 | NM_007956 | GCGCAAGTGTTACGAAGTG | TTCGGCCTTCCAAGTCATC |
| Gapdh | NM_008084.2 | TGACGTGCCGCCTGGAGAAA | AGTGTAGCCCAAGATGCCCTTCAG |
| Ghsr | NM_177330 | CAGGGACCAGAACCACAAAC | AGCCAGGCTCGAAAGACT |
| Hprt | NM_013556 | GCTTGCTGGTGAAAAGGACCTCTCGAAG | CCCTGAAGTACTCATTATAGTCAAGGGCAT |
| Kiss1 | NM_178260 | TGATCTCAATGGCTTCTTGGCAGC | CTCTCTGCATACCGCGATTCCTTT |
| Npy | NM_023456 | ACTGACCCTCGCTCTATCTC | TCTCAGGGCTGGATCTCTTG |
| Pdyn | NM_018863 | AGCTTGCCTCCTCGTGATG | GGCACTCCAGGGAGCAAAT |
| Pomc | NM_008895 | GGAAGATGCCGAGATTCTGC | TCCGTTGCCAGGAAACAC |
| Tac2 | NM_001199971 | CGTGACATGCACGACTTC | CCAACAGGAGGACCTTAC |
| Tac3r | NM_021382 | TACACCATCGTTGGAATTAC | ATGTCACCACCACAATAATC |
| Tac2 (Single Cell) | NM_021382 | TCTGGAAGGATTGCTGAAAGTG | GTAGGGAAGGGAGCCAACAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conde, K.; Kulyk, D.; Vanschaik, A.; Daisey, S.; Rojas, C.; Wiersielis, K.; Yasrebi, A.; Degroat, T.J.; Sun, Y.; Roepke, T.A. Deletion of Growth Hormone Secretagogue Receptor in Kisspeptin Neurons in Female Mice Blocks Diet-Induced Obesity. Biomolecules 2022, 12, 1370. https://doi.org/10.3390/biom12101370
Conde K, Kulyk D, Vanschaik A, Daisey S, Rojas C, Wiersielis K, Yasrebi A, Degroat TJ, Sun Y, Roepke TA. Deletion of Growth Hormone Secretagogue Receptor in Kisspeptin Neurons in Female Mice Blocks Diet-Induced Obesity. Biomolecules. 2022; 12(10):1370. https://doi.org/10.3390/biom12101370
Chicago/Turabian StyleConde, Kristie, Danielle Kulyk, Allison Vanschaik, Sierra Daisey, Catherine Rojas, Kimberly Wiersielis, Ali Yasrebi, Thomas J. Degroat, Yuxiang Sun, and Troy A. Roepke. 2022. "Deletion of Growth Hormone Secretagogue Receptor in Kisspeptin Neurons in Female Mice Blocks Diet-Induced Obesity" Biomolecules 12, no. 10: 1370. https://doi.org/10.3390/biom12101370







