Role of Prostaglandins in Nitric Oxide-Induced Glial Cell-Mediated Vasodilation in Rat Retina
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
2.3. Surgical Protocols
2.4. Fundus Photography and Measurement of the Retinal Arteriole Diameter
2.5. Experimental Protocols
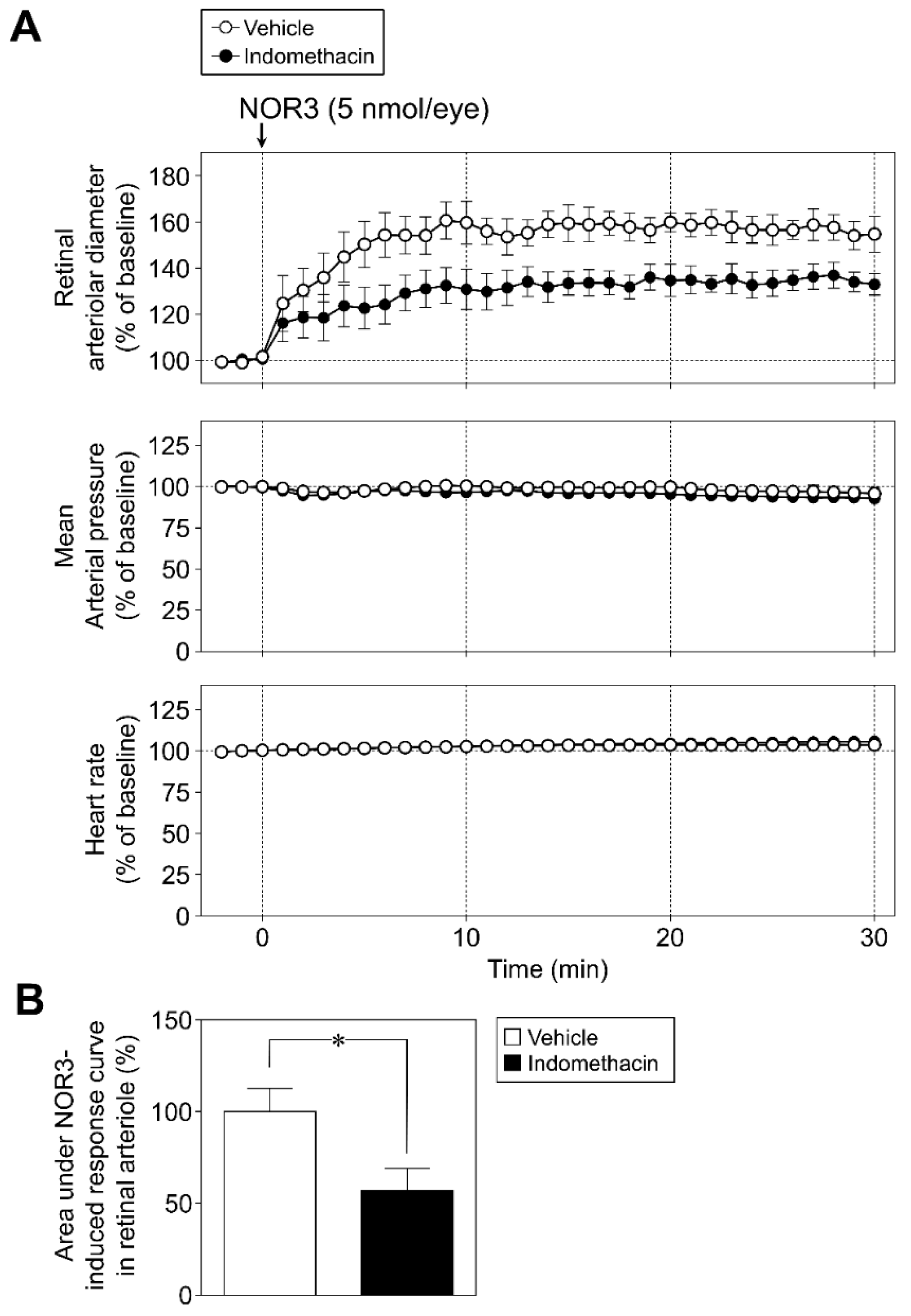
2.5.1. Protocol 1: Role of Prostaglandins in Retinal Vasodilator Responses to NOR3
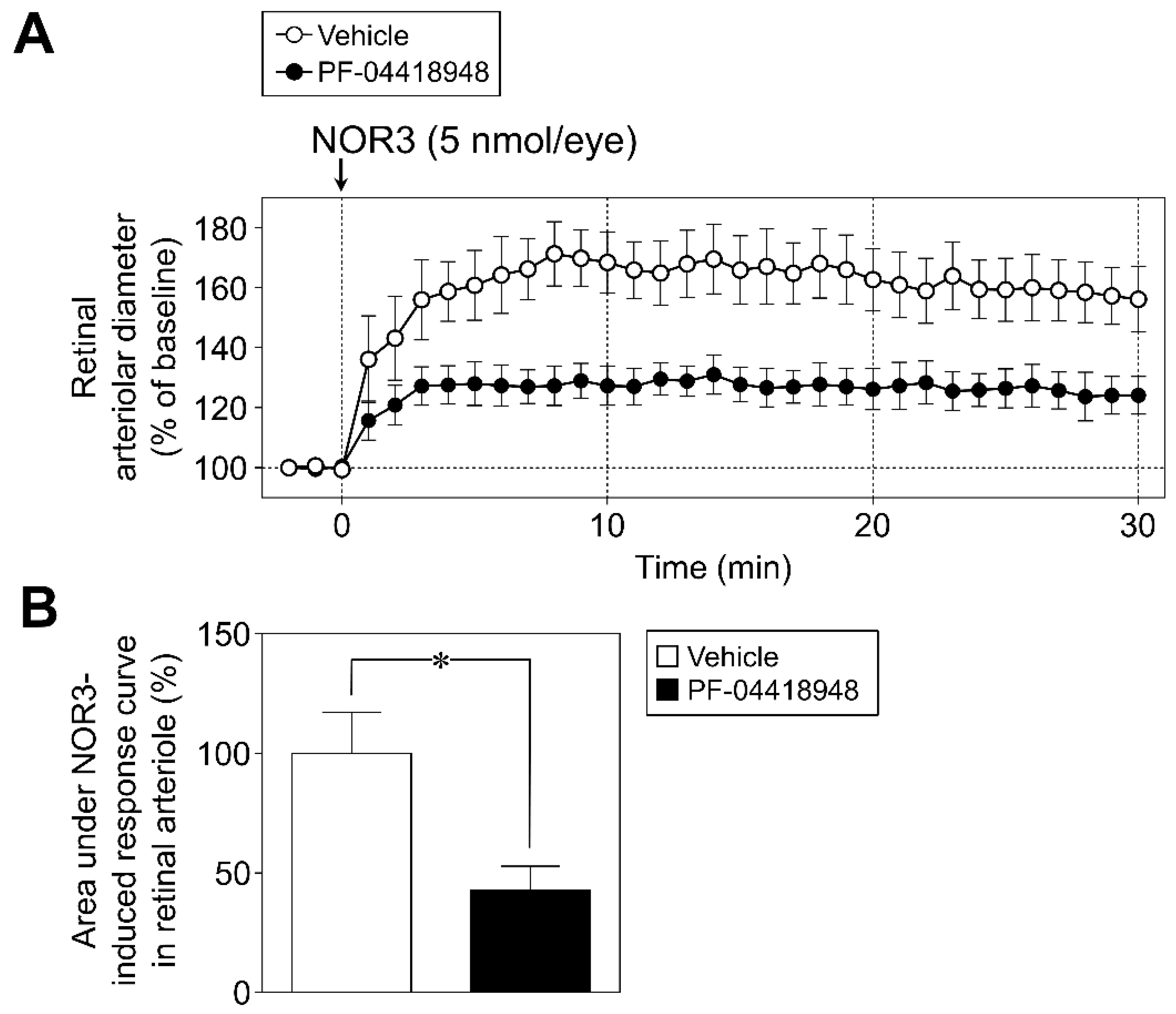
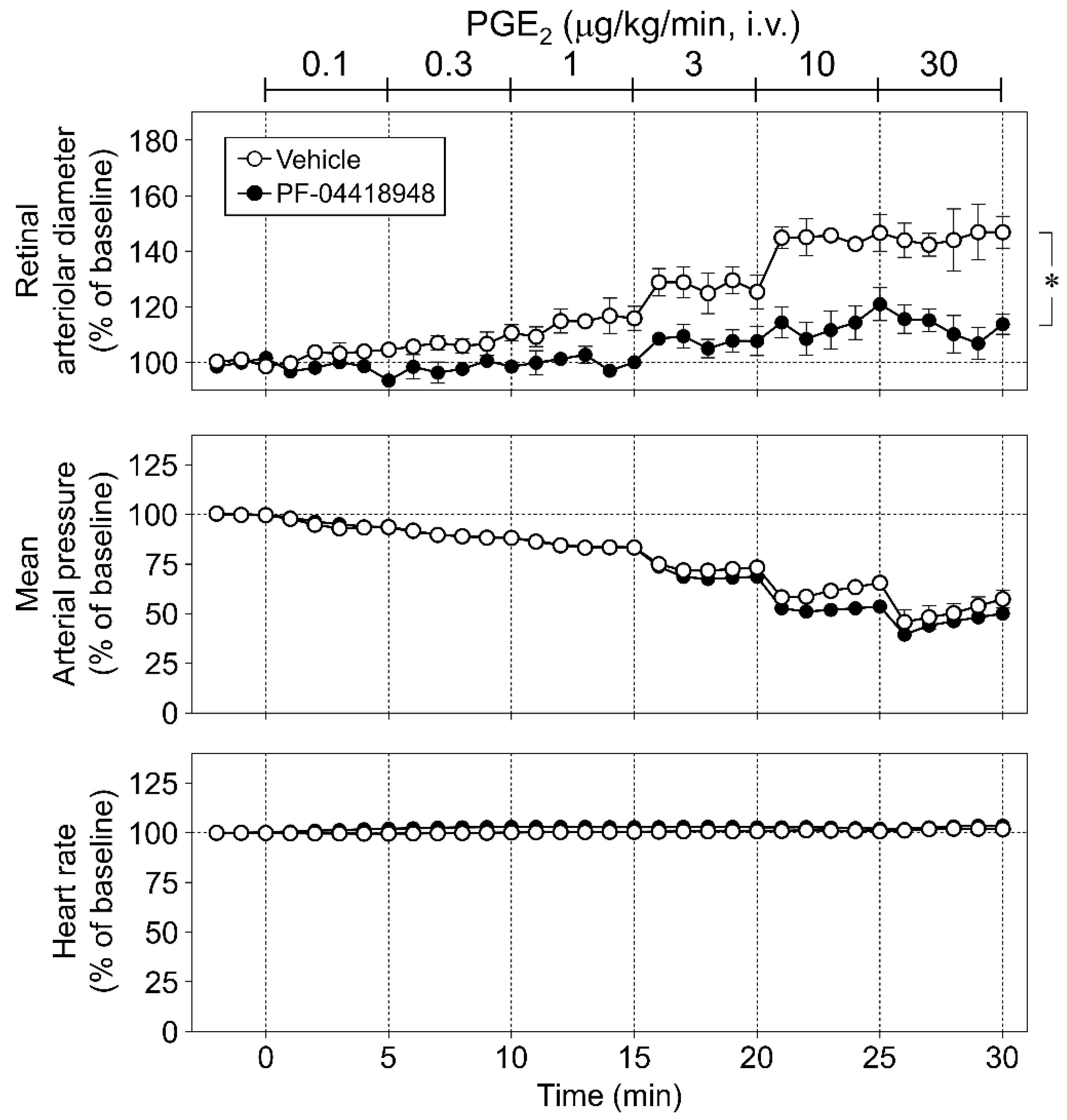
2.5.2. Protocol 2: Role of Prostanoid EP2 Receptor in Retinal Vasodilator Responses to NOR3
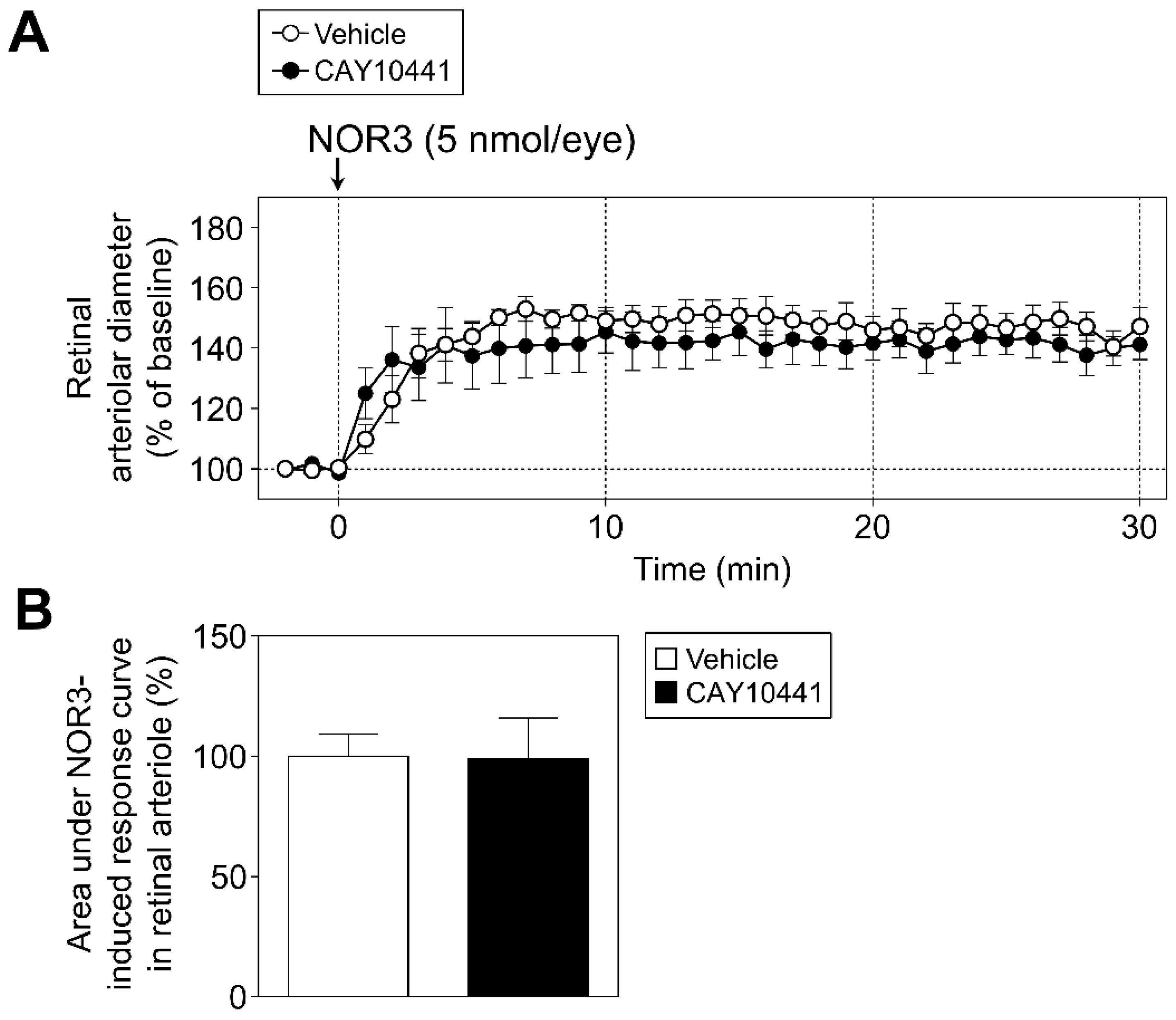
2.5.3. Protocol 3: Role of Prostanoid IP Receptor in Retinal Vasodilator Responses to NOR3
2.6. Data Analysis
3. Results
3.1. The Baseline Value of Retinal Arteriolar Diameter, Mean Arterial Pressure, and Heart Rate
3.2. Role of Prostaglandins in Retinal Vasodilator Responses to NOR3
3.3. Role of Prostanoid EP2 Receptor in Retinal Vasodilator Responses to NOR3
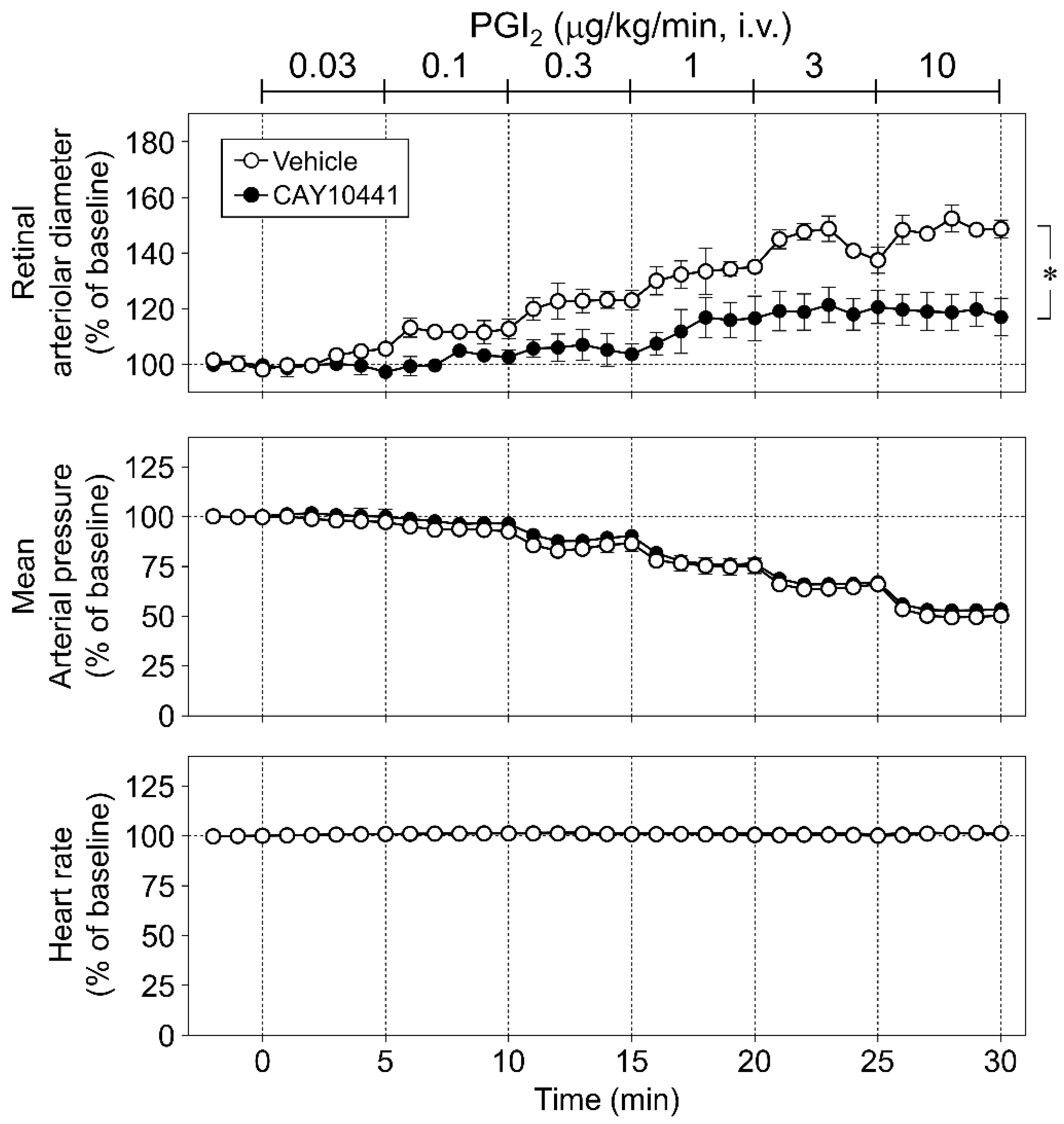
3.4. Role of Prostanoid IP Receptor in Retinal Vasodilator Responses to NOR3
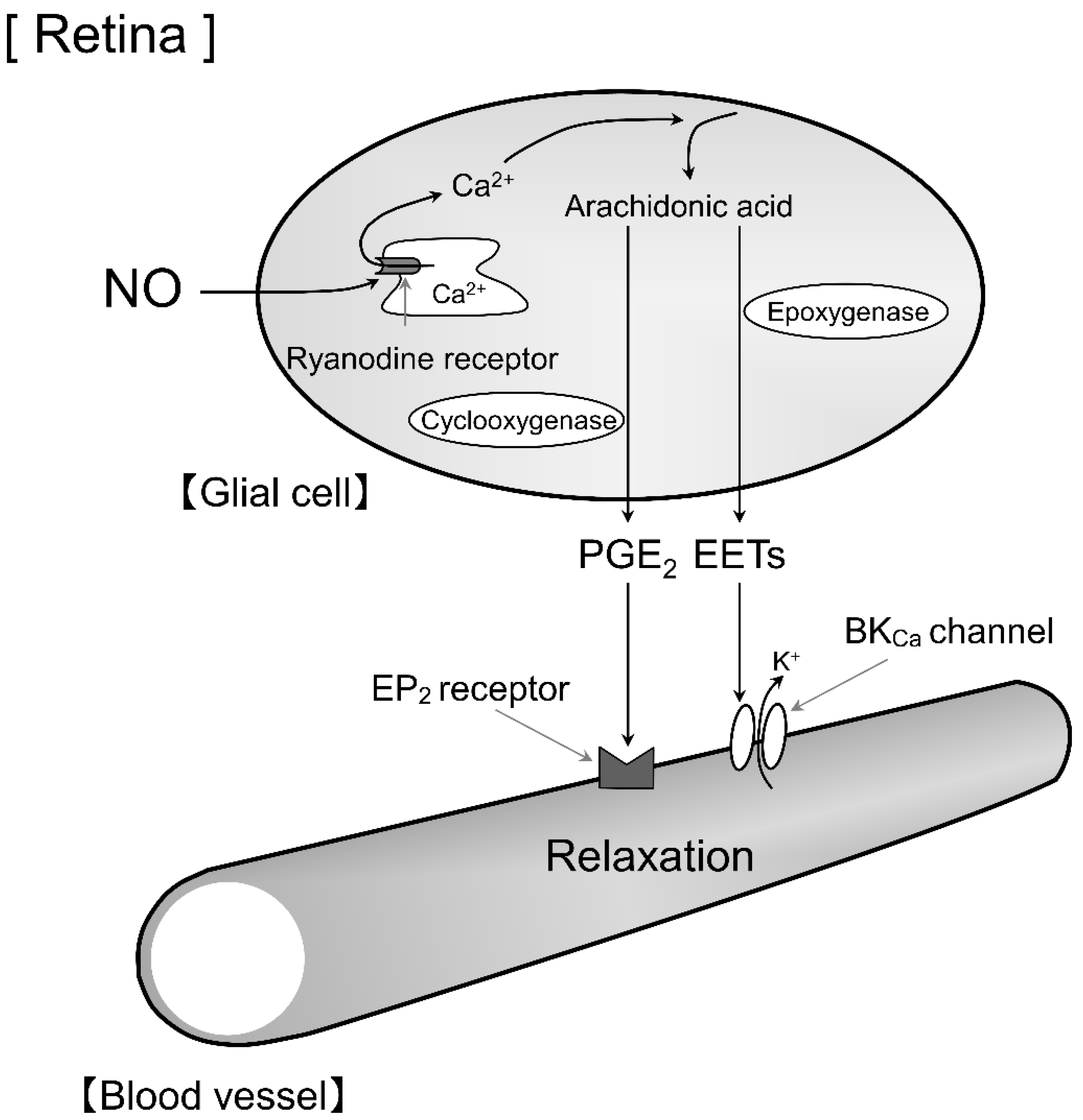
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Zonta, M.; Angulo, M.C.; Gobbo, S.; Rosengarten, B.; Hossmann, K.-A.; Pozzan, T.; Carmignoto, P. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003, 6, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Vecino, E.; Rodriguez, F.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia–neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Kur, J.; Newman, E.A.; Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012, 31, 377–406. [Google Scholar] [CrossRef]
- Someya, E.; Akagawa, M.; Mori, A.; Morita, A.; Yui, N.; Asano, D.; Sakamoto, K.; Nakahara, T. Role of Neuron–Glia Signaling in Regulation of Retinal Vascular Tone in Rats. Int. J. Mol. Sci. 2019, 20, 1952. [Google Scholar] [CrossRef]
- Someya, E.; Mori, A.; Asano, D.; Morita, A.; Sakamoto, K.; Nakahara, T. Role of Glial Cells in μ-Opioid Receptor-Mediated Vasodilation in the Rat Retina. Curr. Eye Res. 2018, 43, 350–356. [Google Scholar] [CrossRef]
- Mori, A.; Saito, M.; Sakamoto, K.; Narita, M.; Nakahara, T.; Ishii, K. Stimulation of prostanoid IP and EP2 receptors dilates retinal arterioles and increases retinal and choroidal blood flow in rats. Eur. J. Pharmacol. 2007, 570, 135–141. [Google Scholar] [CrossRef]
- Ogawa, N.; Mori, A.; Hasebe, M.; Hoshino, M.; Saito, M.; Sakamoto, K.; Nakahara, T.; Ishii, K. Nitric oxide dilates rat retinal blood vessels by cyclooxygenase-dependent mechanisms. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R968–R977. [Google Scholar] [CrossRef]
- Mori, A.; Namekawa, R.; Hasebe, M.; Saito, M.; Sakamoto, K.; Nakahara, T.; Ishii, K. Involvement of prostaglandin I2 in nitric oxide-induced vasodilation of retinal arterioles in rats. Eur. J. Pharmacol. 2015, 764, 249–255. [Google Scholar] [CrossRef]
- Newman, E.A. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J. Neurosci. 2005, 25, 5502–5510. [Google Scholar] [CrossRef]
- Metea, M.R. Glial Cells Dilate and Constrict Blood Vessels: A Mechanism of Neurovascular Coupling. J. Neurosci. 2006, 26, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140195. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.J.; MacVicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Yano, E.; Nishikiori, M.; Fujino, S.; Nakahara, T. N-Methyl-D-Aspartic Acid Receptor-Mediated Vasodilation Is Attenuated in the Retinas of Diabetic Rats. Curr. Eye Res. 2022, 47, 1193–1199. [Google Scholar] [CrossRef]
- Mishra, A.; Newman, E.A. Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia 2010, 58, 1996–2004. [Google Scholar] [CrossRef]
- Rinaldi, C.; Donato, L.; Alibrandi, S.; Scimone, C.; D’Angelo, R.; Sidoti, A. Oxidative Stress and the Neurovascular Unit. Life 2021, 11, 767. [Google Scholar] [CrossRef]
- Ihara, M.; Yamamoto, Y. Emerging Evidence for Pathogenesis of Sporadic Cerebral Small Vessel Disease. Stroke 2016, 47, 554–560. [Google Scholar] [CrossRef]
- Sng, C.C.; Ang, M.; Barton, K. Uveitis and glaucoma: New insights in the pathogenesis and treatment. Prog Brain Res. 2015, 221, 243–269. [Google Scholar] [CrossRef]
- Player, J.K.; Riordan, S.M.; Duncan, R.S.; Koulen, P. Analysis of Glaucoma Associated Genes in Response to Inflammation, an Examination of a Public Data Set Derived from Peripheral Blood from Patients with Hepatitis C. Clin. Ophthalmol. 2022, 16, 2093–2103. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Minchenko, A.G.; Marinescu, V.; Fathallah, L.; Kennedy, A.; Stockert, C.M.; Frank, R.N.; Stevens, M.J. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia 2001, 44, 1102–1110. [Google Scholar] [CrossRef]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, R.; Hassouneh, R.; Hébert, R.L. PGE2, Kidney Disease, and Cardiovascular Risk: Beyond Hypertension and Diabetes. J. Am. Soc. Nephrol. 2016, 27, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.-P.; Liu, S.-Q.; Wang, S. Intravitreal conbercept improves outcome of proliferative diabetic retinopathy through inhibiting inflammation and oxidative stress. Life Sci. 2021, 265, 118795. [Google Scholar] [CrossRef] [PubMed]
- Yanni, S.E.; Barnett, J.M.; Clark, M.L.; Penn, J.S. The Role of PGE2Receptor EP4in Pathologic Ocular Angiogenesis. Investig. Opthalmology Vis. Sci. 2009, 50, 5479–5486. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, G.; Carreiro, S.; Anderson, S.; Gukasyan, H.; Sartnurak, S.; Younis, H.; Gale, D.; Xiang, C.; Wells, P.; Dinh, D.; et al. Effect of PF-04217329 a prodrug of a selective prostaglandin EP2 agonist on intraocular pressure in preclinical models of glaucoma. Exp. Eye Res. 2011, 93, 256–264. [Google Scholar] [CrossRef]
- Mori, A.; Ishii, T.; Kuroki, T.; Shigeta, N.; Sakamoto, K.; Nakahara, T.; Ishii, K. The prostanoid EP2 receptor agonist ONO-AE1-259-01 protects against glutamate-induced neurotoxicity in rat retina. Eur. J. Pharmacol. 2009, 616, 64–67. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Alibrandi, S.; Esposito, T.; Alafaci, C.; D’Angelo, R.; Sidoti, A. Transcriptome analysis provides new molecular signatures in sporadic Cerebral Cavernous Malformation endothelial cells. Biochim. Et Biophys. Acta Mol. Basis Dis. 2020, 1866, 165956. [Google Scholar] [CrossRef]
| Treatments | Retinal Arteriolar Diameter (µm) | Mean Arterial Pressure (mmHg) | Heart Rate (Beats/min) |
|---|---|---|---|
| Protocol 1 | |||
| Vehicle (n = 5) | 44 ± 2 | 112 ± 2 | 371 ± 17 |
| Indomethacin (n = 5) | 45 ± 2 | 112 ± 1 | 371 ± 3 |
| Protocol 2 | |||
| Vehicle (n = 8) | 44 ± 3 | 110 ± 2 | 399 ± 8 |
| PF-04418948 (n = 9) | 46 ± 3 | 113 ± 2 | 381 ± 8 |
| Protocol 3 | |||
| Vehicle (n = 8) | 43 ± 2 | 111 ± 2 | 379 ± 8 |
| CAY10441 (n = 9) | 46 ± 1 | 112 ± 1 | 372 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, A.; Seki, H.; Mizukoshi, S.; Uezono, T.; Sakamoto, K. Role of Prostaglandins in Nitric Oxide-Induced Glial Cell-Mediated Vasodilation in Rat Retina. Biomolecules 2022, 12, 1403. https://doi.org/10.3390/biom12101403
Mori A, Seki H, Mizukoshi S, Uezono T, Sakamoto K. Role of Prostaglandins in Nitric Oxide-Induced Glial Cell-Mediated Vasodilation in Rat Retina. Biomolecules. 2022; 12(10):1403. https://doi.org/10.3390/biom12101403
Chicago/Turabian StyleMori, Asami, Haruka Seki, Satoru Mizukoshi, Takashi Uezono, and Kenji Sakamoto. 2022. "Role of Prostaglandins in Nitric Oxide-Induced Glial Cell-Mediated Vasodilation in Rat Retina" Biomolecules 12, no. 10: 1403. https://doi.org/10.3390/biom12101403
APA StyleMori, A., Seki, H., Mizukoshi, S., Uezono, T., & Sakamoto, K. (2022). Role of Prostaglandins in Nitric Oxide-Induced Glial Cell-Mediated Vasodilation in Rat Retina. Biomolecules, 12(10), 1403. https://doi.org/10.3390/biom12101403






