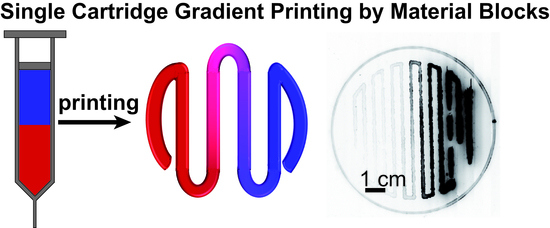
Flow Simulation and Gradient Printing of Fluorapatite- and Cell-Loaded Recombinant Spider Silk Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Flow Simulation
2.2. Bioprinting
2.3. Recombinant Spider Silk Protein Production, Protein Labeling, and Hydrogel Preparation
2.4. Fluorescence Imaging and Fluorescence Spectroscopy
2.5. Fluorapatite Particle Synthesis
2.6. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy
2.7. Microscopy
2.8. Rheology
2.9. Dynamic Light Scattering
2.10. Cell Culture
3. Results and Discussion
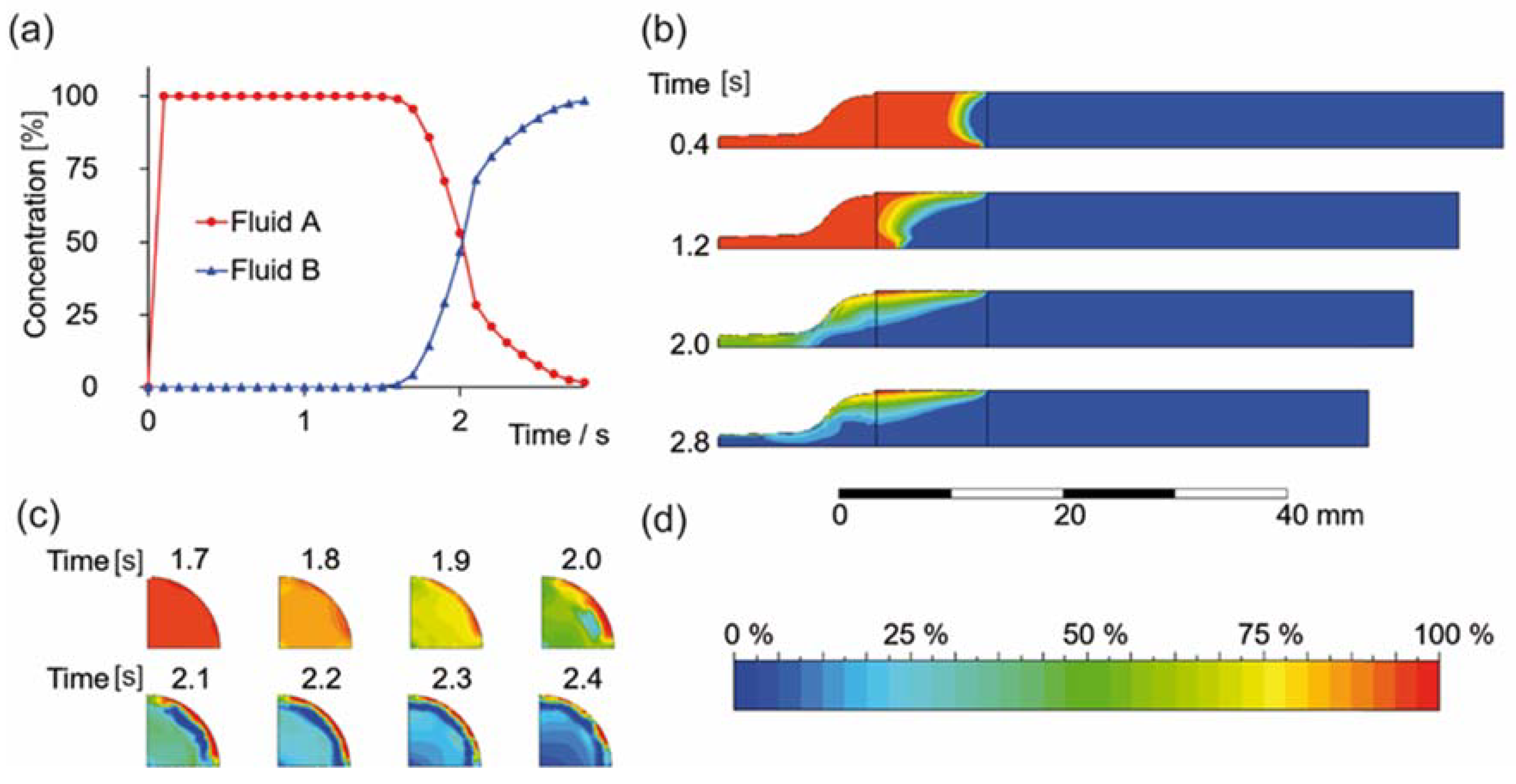
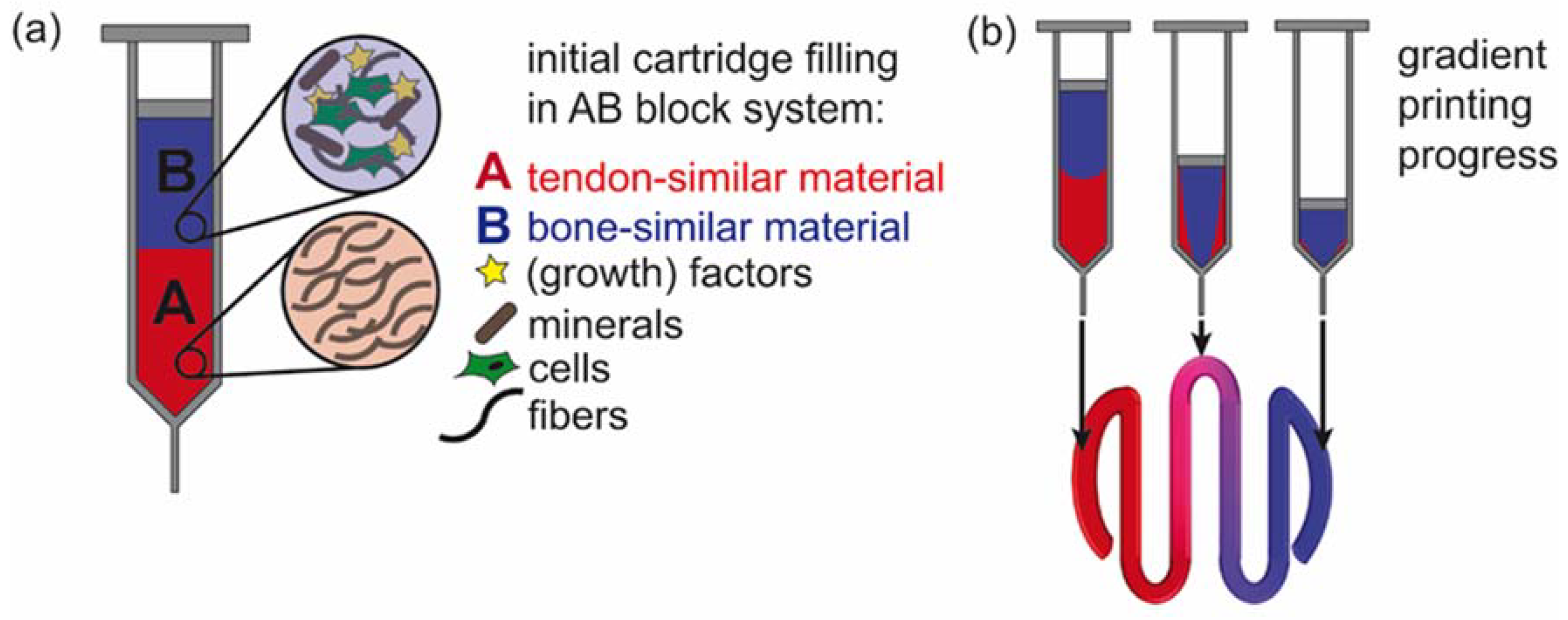
3.1. Flow Simulation of Gradient Material Printing
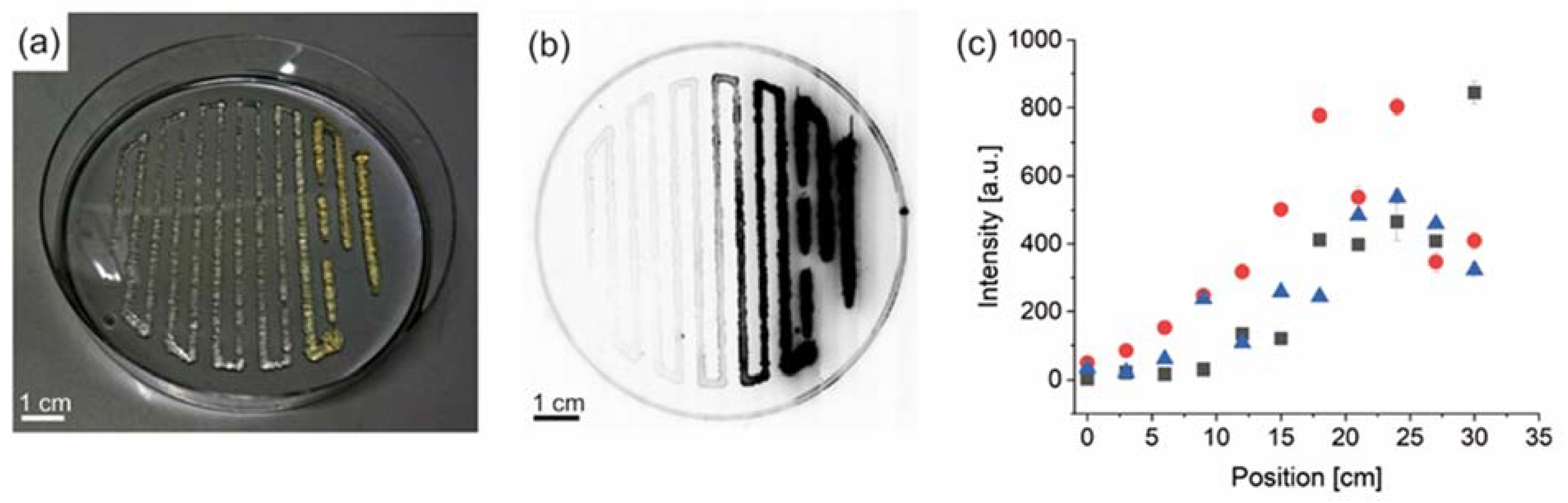
3.2. Experimental Confirmation of Gradient Formation after Bioprinting
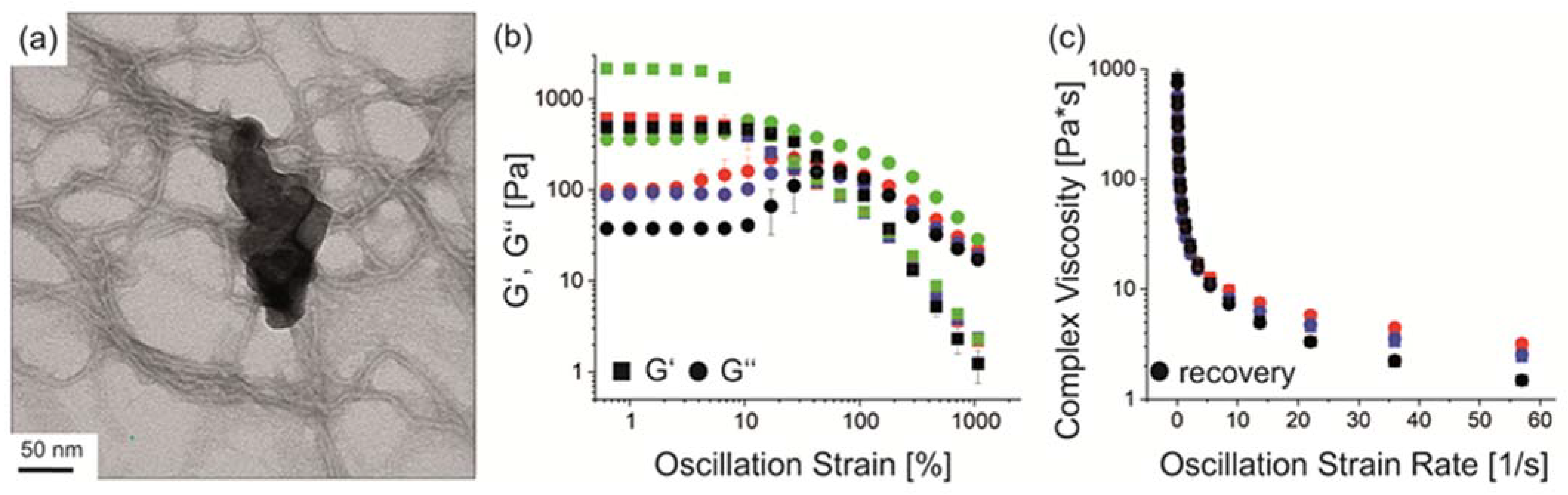
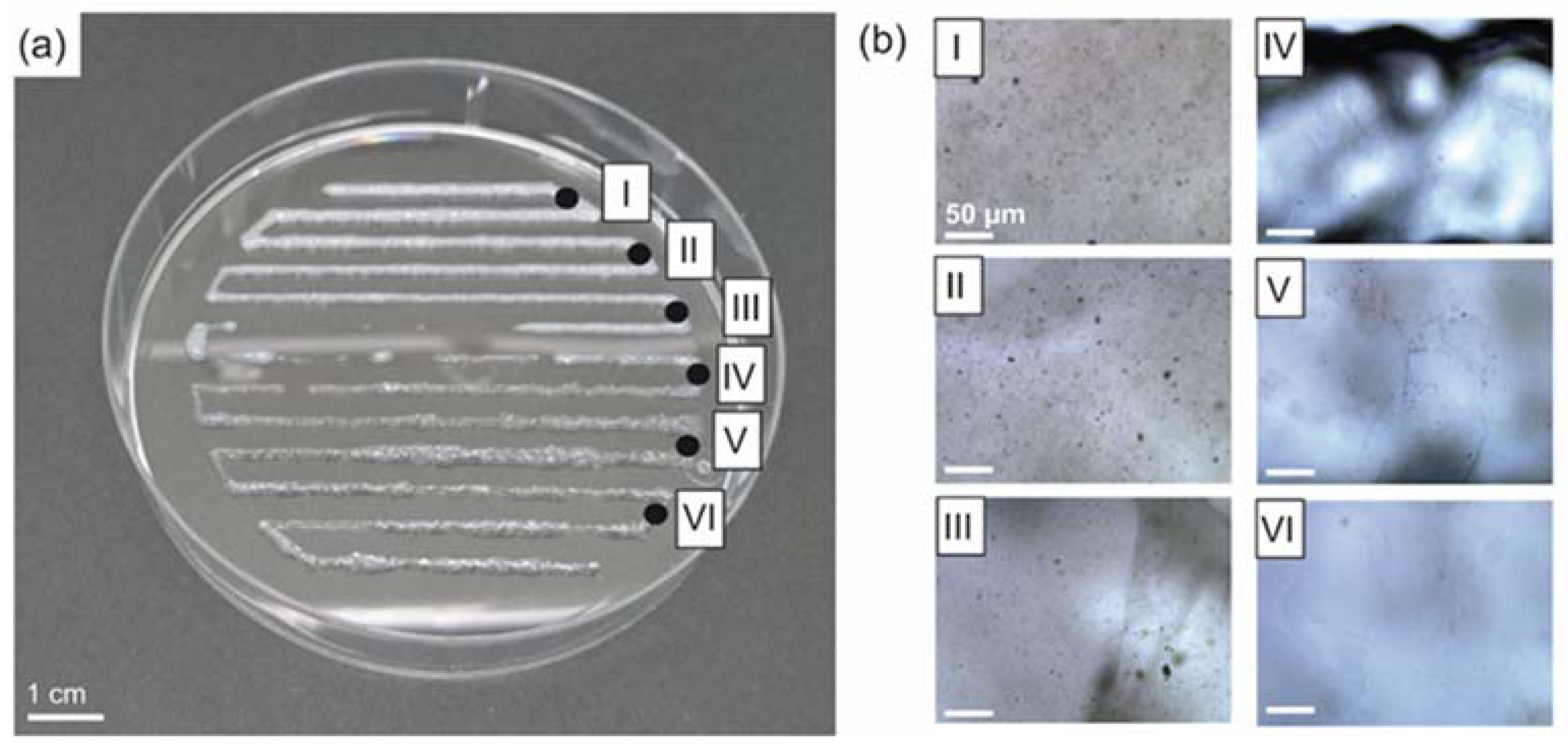
3.3. Spider Silk Fluorapatite Composite Hydrogels to Produce Bioactive Gradients
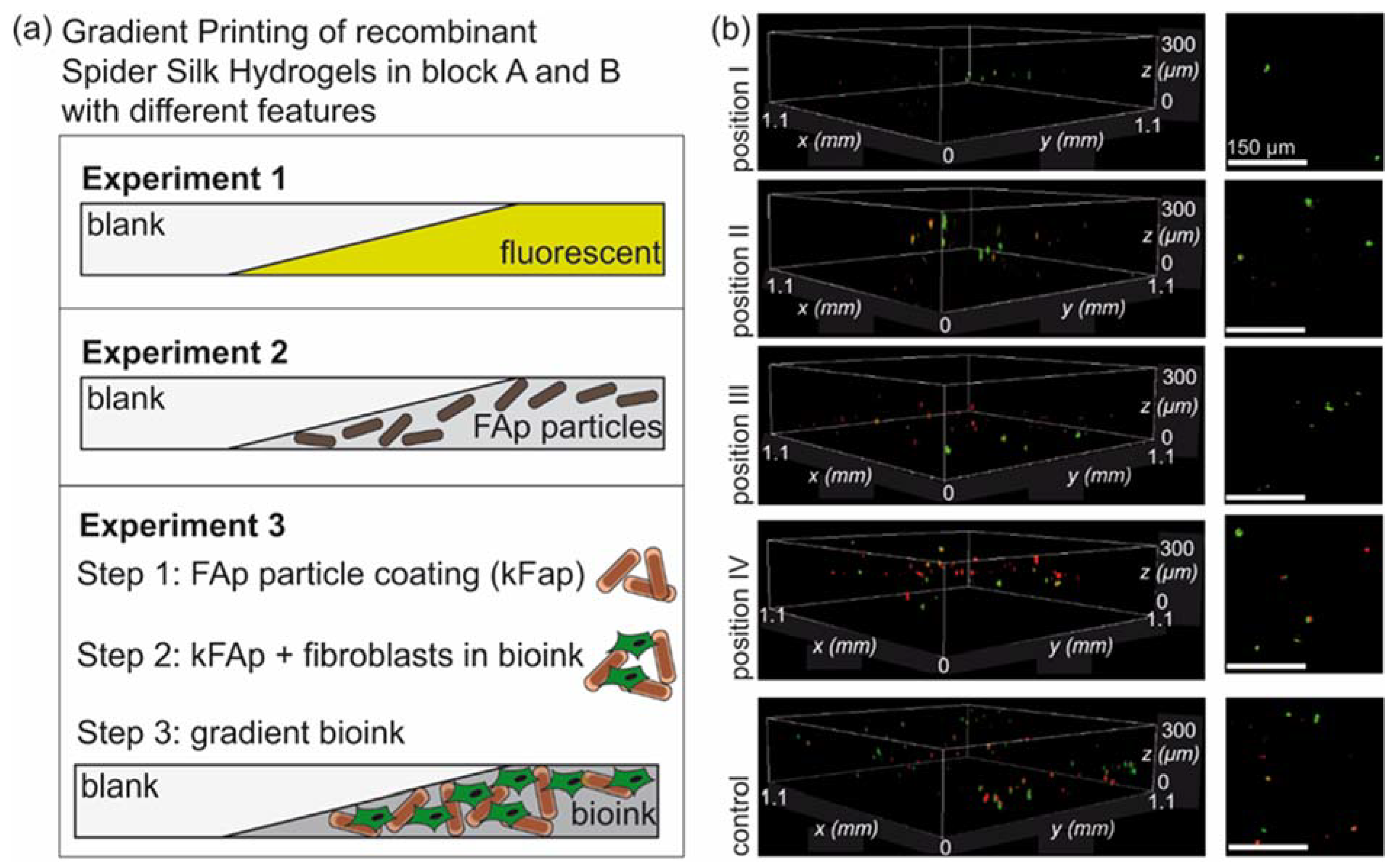
3.4. Biofabrication of Particle and Cell-Loaded Recombinant Spider Silk Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zylstra, U. Living things as hierarchically organized structures. Synthese 1992, 91, 111–133. [Google Scholar] [CrossRef]
- Claussen, K.U.; Scheibel, T.; Schmidt, H.W.; Giesa, R. Polymer Gradient Materials: Can Nature Teach Us New Tricks? Macromol. Mater. Eng. 2012, 297, 938–957. [Google Scholar] [CrossRef]
- Shaw, H.M.; Vázquez, O.T.; McGonagle, D.; Bydder, G.; Santer, R.M.; Benjamin, M. Development of the human Achilles tendon enthesis organ. J. Anat. 2008, 213, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Genin, G.M.; Kent, A.; Birman, V.; Wopenka, B.; Pasteris, J.D.; Marquez, P.J.; Thomopoulos, S. Functional Grading of Mineral and Collagen in the Attachment of Tendon to Bone. Biophys. J. 2009, 97, 976–985. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xie, J.; Lipner, J.; Yuan, X.; Thomopoulos, S.; Xia, Y. Nanofiber Scaffolds with Gradations in Mineral Content for Mimicking the Tendon-to-Bone Insertion Site. Nano. Lett. 2009, 9, 2763–2768. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.W.; Li, X.R.; Lipner, J.; Manning, C.N.; Schwartz, A.G.; Thomopoulos, S.; Xia, Y.N. “Aligned-to-random” nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site. Nanoscale 2010, 2, 923–926. [Google Scholar] [CrossRef] [Green Version]
- Libanori, R.; Erb, R.M.; Reiser, A.; Le Ferrand, H.; Suess, M.J.; Spolenak, R.; Studart, A.R. Stretchable heterogeneous composites with extreme mechanical gradients. Nat. Commun. 2012, 3, 1265. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C.; et al. Direct 3D Printing of High Strength Biohybrid Gradient Hydrogel Scaffolds for Efficient Repair of Osteochondral Defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef]
- Trachtenberg, J.E.; Placone, J.K.; Smith, B.T.; Fisher, J.P.; Mikos, A.G. Extrusion-based 3D printing of poly (propylene fumarate) scaffolds with hydroxyapatite gradients. J. Biomater. Sci. Polym. Ed. 2017, 28, 532–554. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Fon, D.; Li, X.; Tian, J.F.; Forsythe, J.; Garnier, G.; Shen, W. Biosurface engineering through ink jet printing. Colloids Surf. B 2010, 75, 441–447. [Google Scholar] [CrossRef]
- Miller, E.D.; Phillippi, J.A.; Fisher, G.W.; Campbell, P.G.; Walker, L.M.; Weiss, L.E. Inkjet Printing of Growth Factor Concentration Gradients and Combinatorial Arrays Immobilized on Biologically-Relevant Substrates. Comb. Chem. High. Throughput. Screen. 2009, 12, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Kokkinis, D.; Bouville, F.; Studart, A.R. 3D Printing of Materials with Tunable Failure via Bioinspired Mechanical Gradients. Adv. Mater. 2018, 30, 1705808. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, W.; Mille, L.S.; Ching, T.; Luo, Z.; Tang, G.; Garciamendez, C.E.; Lesha, A.; Hashimoto, M.; Zhang, Y.S. Digital Light Processing Based Bioprinting with Composable Gradients. Adv. Mater. 2022, 34, 2107038. [Google Scholar] [CrossRef]
- Mironov, V.; Prestwich, G.; Forgacs, G. Bioprinting living structures. J. Mater. Chem. 2007, 17, 2054–2060. [Google Scholar] [CrossRef]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironov, V.; Trusk, T.; Kasyanov, V.; Little, S.; Swaja, R.; Markwald, R. Biofabrication: A 21st century manufacturing paradigm. Biofabrication 2009, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Parker, S.T.; Syoji, D.; Wang, X.; Lewis, J.A.; Kaplan, D.L. Direct-Write Assembly of 3D Silk/Hydroxyapatite Scaffolds for Bone Co-Cultures. Adv. Healthc. Mater. 2012, 1, 729–735. [Google Scholar] [CrossRef]
- Ding, Z.; Han, H.; Fan, Z.; Lu, H.; Sang, Y.; Yao, Y.; Cheng, Q.; Lu, Q.; Kaplan, D.L. Nanoscale Silk–Hydroxyapatite Hydrogels for Injectable Bone Biomaterials. ACS Appl. Mater. Inter. 2017, 9, 16913–16921. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Jalilifiroozinezhad, S.; Mozafari, M.; Rabiee, M. Synthesis and solubility of calcium fluoride/hydroxy-fluorapatite nanocrystals for dental applications. Ceram. Int. 2011, 37, 2007–2014. [Google Scholar] [CrossRef]
- Liu, J.; Jin, T.C.; Chang, S.; Czajka-Jakubowska, A.; Clarkson, B.H. Adhesion and growth of dental pulp stem cells on enamel-like fluorapatite surfaces. J. Biomed. Mater. Res. Part A 2011, 96, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawska, A.; Hochrein, O.; Brickmann, J.; Kniep, R.; Zahn, D. The Nucleation Mechanism of Fluorapatite–Collagen Composites: Ion Association and Motif Control by Collagen Proteins. Angew. Chem.-Int. Edit. 2008, 47, 4982–4985. [Google Scholar] [CrossRef]
- Tlatlik, H.; Simon, P.; Kawska, A.; Zahn, D.; Kniep, R. Biomimetic Fluorapatite–Gelatine Nanocomposites: Pre-Structuring of Gelatine Matrices by Ion Impregnation and Its Effect on Form Development. Angew. Chem.-Int. Edit. 2006, 45, 1905–1910. [Google Scholar] [CrossRef]
- Michael, F.M.; Khalid, M.; Ratnam, C.T.; Chee, C.Y.; Rashmi, W.; Hoque, M.E. Sono-synthesis of nanohydroxyapatite: Effects of process parameters. Ceram. Int. 2016, 42, 6263–6272. [Google Scholar] [CrossRef]
- Cao, L.-Y.; Zhang, C.-B.; Huang, J.-F. Synthesis of hydroxyapatite nanoparticles in ultrasonic precipitation. Ceram. Int. 2005, 31, 1041–1044. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Jin, Q.; Jin, T.; Chang, S.; Zhang, Z.; Czajka-Jakubowska, A.; Giannobile, W.V.; Nör, J.E.; Clarkson, B.H. The stimulation of adipose-derived stem cell differentiation and mineralization by ordered rod-like fluorapatite coatings. Biomaterials 2012, 33, 5036–5046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.; Cao, L.; Wei, J.; Ma, Y.; Song, S.; Weng, W.; Li, H.; Liu, C.; Su, J. Development of a bioactive composite of nanofluorapatite and poly (butylene succinate) for bonetissue regeneration. J. Mat. Chem. B 2014, 2, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Leal-Egana, A.; Scheibel, T. Silk-based materials for biomedical applications. Biotechnol. Appl. Biochem. 2010, 55, 155–167. [Google Scholar] [CrossRef]
- Schacht, K.; Scheibel, T. Controlled Hydrogel Formation of a Recombinant Spider Silk Protein. Biomacromolecules 2011, 12, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, V.J.; Trossmann, V.T.; Jacobi, S.; Döbl, A.; Scheibel, T. Recombinant Spider Silk Gels Derived from Aqueous–Organic Solvents as Depots for Drugs. Angew. Chem.-Int. Edit. 2021, 60, 11847–11851. [Google Scholar] [CrossRef] [PubMed]
- Schacht, K.; Juengst, T.; Schweinlin, M.; Ewald, A.; Groll, J.; Scheibel, T. Biofabrication of Cell-Loaded 3D Spider Silk Constructs. Angew. Chem. Int. Ed. 2015, 54, 2816–2820. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Trossmann, V.T.; Scheibel, T. Impact of Cell Loading of Recombinant Spider Silk Based Bioinks on Gelation and Printability. Macromol. Biosci. 2022, 22, 2100390. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Bargel, H.; Anby, M.U.; Lafargue, D.; Scheibel, T. Recombinant Spider Silk Hydrogels for Sustained Release of Biologicals. ACS Biomater. Sci. Eng. 2018, 4, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Bargel, H.; Scheibel, T. Recombinant Spider Silk-Silica Hybrid Scaffolds with Drug-Releasing Properties for Tissue Engineering Applications. Macromol. Rapid Commun. 2020, 41, 1900426. [Google Scholar] [CrossRef] [Green Version]
- DeSimone, E.; Schacht, K.; Pellert, A.; Scheibel, T. Recombinant spider silk-based bioinks. Biofabrication 2017, 9, 044104. [Google Scholar] [CrossRef]
- Malalasekera, W.; Versteeg, H.K. An Introduction to Computational Fluid Dynamics-The Finite Volume Method, 2nd ed.; Pearson Education Limited: Essex, UK, 2007. [Google Scholar]
- Morrison, F.A. Understanding Rheology; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- ANSYS, Inc. ANSYS CFX-Solver Theory Guide, 16.2 ed.; ANSYS, Inc.: Canonsburg, PA, USA, 2015. [Google Scholar]
- Huemmerich, D.; Helsen, C.W.; Quedzuweit, S.; Oschmann, J.; Rudolph, R.; Scheibel, T. Primary Structure Elements of Spider Dragline Silks and Their Contribution to Protein Solubility. Biochemistry 2004, 43, 13604–13612. [Google Scholar] [CrossRef]
- Doblhofer, E.; Scheibel, T. Engineering of Recombinant Spider Silk Proteins Allows Defined Uptake and Release of Substances. J. Pharm. Sci. 2015, 104, 988–994. [Google Scholar] [CrossRef]
- Willigeroth, S.F. Strategien zur Synthese Kolloidaler Fluorapatitpartikel und Nanokomposite-Dissertation. PhD Thesis, Christian-Albrechts-Universität Kiel, Kiel, Germany, 2002; pp. 33–37. [Google Scholar]
- Smoluchowski, M.V. Experiments on a mathematical theory of kinetic coagulation of coloid solutions. Z. Phys. Chem. 1917, 92, 129–168. [Google Scholar]
- Hausmann, M.K.; Rühs, P.A.; Siqueira, G.; Läuger, J.; Libanori, R.; Zimmermann, T.; Studart, A.R. Dynamics of Cellulose Nanocrystal Alignment during 3D Printing. ACS Nano 2018, 12, 6926–6937. [Google Scholar] [CrossRef] [PubMed]
- Kumar Panigrahi, S.; Kumar Mishra, A. Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. J. Photochem. Photobiol. C 2019, 41, 100318. [Google Scholar] [CrossRef]
- Baddiel, C.B.; Berry, E.E. Spectra structure correlations in hydroxy and fluorapatite. Spectrochim. Acta 1966, 22, 1407–1416. [Google Scholar] [CrossRef]
- Lucke, M.; Mottas, I.; Herbst, T.; Hotz, C.; Römer, L.; Schierling, M.; Herold, H.M.; Slotta, U.; Spinetti, T.; Scheibel, T.; et al. Engineered hybrid spider silk particles as delivery system for peptide vaccines. Biomaterials 2018, 172, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Steiner, D.; Winkler, S.; Heltmann-Meyer, S.; Trossmann, V.T.; Fey, T.; Scheibel, T.; Horch, R.E.; Arkudas, A. Enhanced vascularization and de novo tissue formation in hydrogels made of engineered RGD-tagged spider silk proteins in the arteriovenous loop model. Biofabrication 2021, 13, 045003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.L.; Tan, T.T.Y.; Ng, K.W.; Loo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef]
- Neubauer, V.J.; Döbl, A.; Scheibel, T. Silk-Based Materials for Hard Tissue Engineering. Materials 2021, 14, 674. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neubauer, V.J.; Hüter, F.; Wittmann, J.; Trossmann, V.T.; Kleinschrodt, C.; Alber-Laukant, B.; Rieg, F.; Scheibel, T. Flow Simulation and Gradient Printing of Fluorapatite- and Cell-Loaded Recombinant Spider Silk Hydrogels. Biomolecules 2022, 12, 1413. https://doi.org/10.3390/biom12101413
Neubauer VJ, Hüter F, Wittmann J, Trossmann VT, Kleinschrodt C, Alber-Laukant B, Rieg F, Scheibel T. Flow Simulation and Gradient Printing of Fluorapatite- and Cell-Loaded Recombinant Spider Silk Hydrogels. Biomolecules. 2022; 12(10):1413. https://doi.org/10.3390/biom12101413
Chicago/Turabian StyleNeubauer, Vanessa J., Florian Hüter, Johannes Wittmann, Vanessa T. Trossmann, Claudia Kleinschrodt, Bettina Alber-Laukant, Frank Rieg, and Thomas Scheibel. 2022. "Flow Simulation and Gradient Printing of Fluorapatite- and Cell-Loaded Recombinant Spider Silk Hydrogels" Biomolecules 12, no. 10: 1413. https://doi.org/10.3390/biom12101413
APA StyleNeubauer, V. J., Hüter, F., Wittmann, J., Trossmann, V. T., Kleinschrodt, C., Alber-Laukant, B., Rieg, F., & Scheibel, T. (2022). Flow Simulation and Gradient Printing of Fluorapatite- and Cell-Loaded Recombinant Spider Silk Hydrogels. Biomolecules, 12(10), 1413. https://doi.org/10.3390/biom12101413