Local Long-Term Inner Ear Drug Delivery in Normal Hearing Guinea Pig—An Animal Model to Develop Preventive Treatment for Noise-Induced Hearing Loss
Abstract
:1. Introduction
2. Materials and Methods
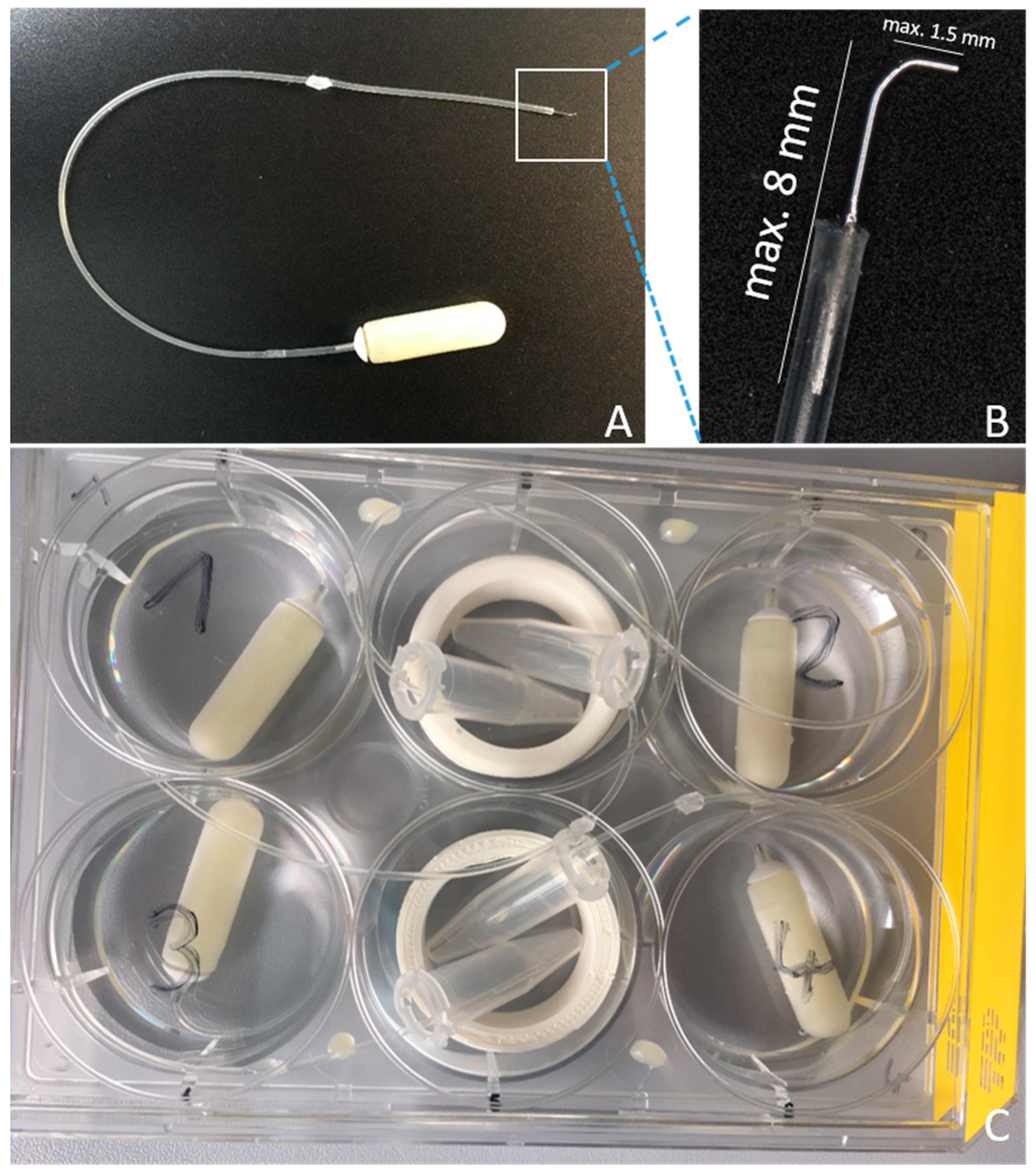
2.1. Pump Preparation
2.2. Animals and Experimental Timeline
2.3. Acoustically Evoked Auditory Brainstem Response (ABR) Measurement
2.4. HDD Implantation
2.5. Noise Trauma
2.6. µCT
2.7. Statistical Analysis
3. Results
3.1. Qualitiy Control of the Implant
3.2. ABR Measurements
3.2.1. Comparison of the Click Thresholds at Specific Time Points and Click Thresholds over Time
3.2.2. Effect of HDD Implantation on Frequency-Specific ABR Thresholds
3.2.3. Effect of Noise Exposure on Frequency-Specific ABR Thresholds
3.2.4. Effect of HDD Implantation and Noise Exposure on Frequency-Specific ABR Thresholds
3.2.5. Effect of HDD Implantation on Noise Trauma
3.3. µCT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Spoendlin, H. Degeneration Behaviour of the Cochlear Nerve. Arch. Klin. Exp. Ohren. Nasen. Kehlkopfheilkd. 1971, 200, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Dodson, H.C.; Mohuiddin, A. Response of Spiral Ganglion Neurones to Cochlear Hair Cell Destruction in the Guinea Pig. J. Neurocytol. 2000, 29, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Ylikoski, J. Correlative Studies on the Cochlear Pathology and Hearing Loss in Guinea-Pigs after Intoxication with Ototoxic Antibiotics. Acta Otolaryngol. 1974, 326, 1–62. [Google Scholar]
- Wu, P.Z.; O’Malley, J.T.; de Gruttola, V.; Liberman, M.C. Primary Neural Degeneration in Noise-Exposed Human Cochleas: Correlations with Outer Hair Cell Loss and Word-Discrimination Scores. J. Neurosci. 2021, 41, 4439–4447. [Google Scholar] [CrossRef]
- Makary, C.A.; Shin, J.; Kujawa, S.G.; Liberman, M.C.; Merchant, S.N. Age-Related Primary Cochlear Neuronal Degeneration in Human Temporal Bones. JARO-J. Assoc. Res. Otolaryngol. 2011, 12, 711–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felix, H.; Pollak, A.; Gleeson, M.; Johnsson, L.G. Degeneration Pattern of Human First-Order Cochlear Neurons. Adv. Otorhinolaryngol. 2002, 59, 116–123. [Google Scholar] [CrossRef]
- Johnsson, L.G.; Felix, H.; Gleeson, M.; Pollak, A. Observations on the Pattern of Sensorineural Degeneration in the Human Cochlea. Acta Otolaryngol. 1989, 108, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Synaptopathy in the Noise-Exposed and Aging Cochlea: Primary Neural Degeneration in Acquired Sensorineural Hearing Loss. Hear. Res. 2015, 330, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Green, S.H. Functional Role of Neurotrophin-3 in Synapse Regeneration by Spiral Ganglion Neurons on Inner Hair Cells after Excitotoxic Trauma in Vitro. J. Neurosci. 2011, 31, 7938–7949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberman, M.C.; Kujawa, S.G. Cochlear Synaptopathy in Acquired Sensorineural Hearing Loss: Manifestations and Mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Lin, H.W.; Furman, A.C.; Kujawa, S.G.; Liberman, M.C. Primary Neural Degeneration in the Guinea Pig Cochlea after Reversible Noise-Induced Threshold Shift. JARO-J. Assoc. Res. Otolaryngol. 2011, 12, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.J.M.; Garcia-Lazaro, J.A.; McAlpine, D.; Schaette, R. Hidden Hearing Loss Impacts the Neural Representation of Speech in Background Noise. Curr. Biol. 2020, 30, 4710–4721.e4. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.I.; Nelson, R.Y.; Concha-Barrientos, M.; Fingerhut, M. The Global Burden of Occupational Noise-Induced Hearing Loss. Am. J. Ind. Med. 2005, 48, 446–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, W.J.; Eichwald, J.; Meinke, D.K.; Chadha, S.; Iskander, J. CDC Grand Rounds: Promoting Hearing Health Across the Lifespan. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Basner, M.; Babisch, W.; Davis, A.; Brink, M.; Clark, C.; Janssen, S.; Stansfeld, S. Auditory and Non-Auditory Effects of Noise on Health. Lancet 2014, 383, 1325–1332. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Koochakzadeh, S.; Nguyen, S.A.; Brennan, E.A.; Meyer, T.A.; Lambert, P.R. Pharmacological Prevention of Noise-Induced Hearing Loss: A Systematic Review. Otol. Neurotol. 2021, 42, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G.; Hammill, T.L.; Murphy, W.J. Noise-Induced Hearing Loss: Translating Risk from Animal Models to Real-World Environments. J. Acoust. Soc. Am. 2019, 146, 3646–3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sha, S.H.; Schacht, J. Emerging Therapeutic Interventions against Noise-Induced Hearing Loss. Expert Opin. Investig. Drugs 2017, 26, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umugire, A.; Lee, S.; Kim, D.; Choi, M.; Kim, H.-S.; Cho, H.-H. Avenanthramide-C Prevents Noise- and Drug-Induced Hearing Loss While Protecting Auditory Hair Cells from Oxidative Stress. Cell Death Discov. 2019, 5, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohinata, Y.; Yamasoba, T.; Schacht, J.; Miller, J.M. Glutathione Limits Noise-Induced Hearing Loss. Hear. Res. 2000, 146, 28–34. [Google Scholar] [CrossRef]
- Xiong, H.; Lai, L.; Ye, Y.; Zheng, Y. Glucose Protects Cochlear Hair Cells Against Oxidative Stress and Attenuates Noise-Induced Hearing Loss in Mice. Neurosci. Bull. 2021, 37, 657–668. [Google Scholar] [CrossRef]
- Shoji, F.; Yamasoba, T.; Magal, E.; Dolan, D.F.; Altschuler, R.A.; Miller, J.M. Glial Cell Line-Derived Neurotrophic Factor Has a Dose Dependent Influence on Noise-Induced Hearing Loss in the Guinea Pig Cochlea. Hear. Res. 2000, 142, 41–55. [Google Scholar] [CrossRef]
- Iwai, K.; Nakagawa, T.; Endo, T.; Matsuoka, Y.; Kita, T.; Kim, T.S.; Tabata, Y.; Ito, J. Cochlear Protection by Local Insulin-like Growth Factor-1 Application Using Biodegradable Hydrogel. Laryngoscope 2006, 116, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Attias, J.; Sapir, S.; Bresloff, I.; Reshef-Haran, I.; Ising, H. Reduction in Noise-Induced Temporary Threshold Shift in Humans Following Oral Magnesium Intake. Clin. Otolaryngol. Allied Sci. 2004, 29, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Rutherford, M.A.; Green, S.H. Protection of Cochlear Synapses from Noise-Induced Excitotoxic Trauma by Blockade of Ca 2+ -Permeable AMPA Receptors. Proc. Natl. Acad. Sci. USA 2020, 117, 201914247. [Google Scholar] [CrossRef]
- Seidman, M.D.; Tang, W.; Shirwany, N.; Bai, U.; Rubin, C.J.; Henig, J.P.; Quirk, W.S. Anti-Intercellular Adhesion Molecule-1 Antibody’s Effect on Noise Damage. Laryngoscope 2009, 119, 707–712. [Google Scholar] [CrossRef]
- Naert, G.; Pasdelou, M.; Le Prell, C.G. Use of the Guinea Pig in Studies on the Development and Prevention of Acquired Sensorineural Hearing Loss, with an Emphasis on Noise. J. Acoust. Soc. Am. 2019, 146, 3743–3769. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Nakagawa, T.; Okano, T.; Hori, R.; Ono, K.; Tabata, Y.; Lee, S.H.; Ito, J. Novel Therapy for Hearing Loss: Delivery of Insulin-like Growth Factor 1 to the Cochlea Using Gelatin Hydrogel. Otol. Neurotol. 2007, 28, 976–981. [Google Scholar] [CrossRef]
- Sly, D.J.; Campbell, L.; Uschakov, A.; Saief, S.T.; Lam, M.; O’Leary, S.J. Applying Neurotrophins to the Round Window Rescues Auditory Function and Reduces Inner Hair Cell Synaptopathy after Noise-Induced Hearing Loss. Otol. Neurotol. 2016, 37, 1223–1230. [Google Scholar] [CrossRef]
- Murillo-Cuesta, S.; Rodríguez-de la Rosa, L.; Contreras, J.; Celaya, A.M.; Camarero, G.; Rivera, T.; Varela-Nieto, I. Transforming Growth Factor Β1 Inhibition Protects from Noise-Induced Hearing Loss. Front. Aging Neurosci. 2015, 7, 32. [Google Scholar]
- Brown, J.N.; Miller, J.M.; Altschuler, R.A.; Nuttall, A.L. Osmotic Pump Implant for Chronic Infusion of Drugs into the Inner Ear. Hear. Res. 1993, 70, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Scheper, V.; Paasche, G.; Miller, J.M.; Warnecke, A.; Berkingali, N.; Lenarz, T.; Stöver, T. Effects of Delayed Treatment with Combined GDNF and Continuous Electrical Stimulation on Spiral Ganglioncell Survival in Deafened Guinea Pigs. J. Neurosci. Res. 2009, 87, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Scheper, V.; Seidel-Effenberg, I.; Lenarz, T.; Stöver, T.; Paasche, G. Consecutive Treatment with Brain-Derived Neurotrophic Factor and Electrical Stimulation Has a Protective Effect on Primary Auditory Neurons. Brain Sci. 2020, 10, 559. [Google Scholar] [CrossRef] [PubMed]
- Scheper, V.; Hessler, R.; Hütten, M.; Wilk, M.; Jolly, C.; Lenarz, T.; Paasche, G. Local Inner Ear Application of Dexamethasone in Cochlear Implant Models Is Safe for Auditory Neurons and Increases the Neuroprotective Effect of Chronic Electrical Stimulation. PLoS ONE 2017, 12, e0183820. [Google Scholar] [CrossRef] [Green Version]
- Warnecke, A.; Scheper, V.; Buhr, I.; Wenzel, G.I.; Wissel, K.; Paasche, G.; Berkingali, N.; Jørgensen, J.R.; Lenarz, T.; Stöver, T. Artemin Improves Survival of Spiral Ganglion Neurons in Vivo and in Vitro. Neuroreport 2010, 21, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Prieskorn, D.M.; Miller, J.M. Technical Report: Chronic and Acute Intracochlear Infusion in Rodents. Hear. Res. 2000, 140, 212–215. [Google Scholar] [CrossRef]
- Ramekers, D.; Versnel, H.; Strahl, S.B.; Klis, S.F.L.; Grolman, W. Temporary Neurotrophin Treatment Prevents Deafness- Induced Auditory Nerve Degeneration and Preserves Function. J. Neurosci. 2015, 35, 12331–12345. [Google Scholar] [CrossRef] [Green Version]
- Attias, J.; Hod, R.; Raveh, E.; Mizrachi, A.; Avraham, K.B.; Lenz, D.R.; Nageris, B.I. Hearing Loss Patterns after Cochlear Implantation via the Round Window in an Animal Model. Am. J. Otolaryngol.-Head Neck Med. Surg. 2016, 37, 162–168. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Lang, D.M.; Roell, J.; Van De Water, T.R.; Garnham, C.; Rodrigues, H.; Guardiola, M.; Gupta, C.; Mittal, J. Mechanisms of Programmed Cell Death Signaling in Hair Cells and Support Cells Post-Electrode Insertion Trauma. Acta Otolaryngol. 2015, 135, 328–334. [Google Scholar] [CrossRef]
- Sale, P.J.P.; Uschakov, A.; Saief, T.; Rowe, D.P.; Abbott, C.J.; Luu, C.D.; Hampson, A.J.; O’Leary, S.J.; Sly, D.J. Cannula-Based Drug Delivery to the Guinea Pig Round Window Causes a Lasting Hearing Loss That May Be Temporarily Mitigated by BDNF. Hear. Res. 2017, 356, 104–115. [Google Scholar] [CrossRef]
- Whitlon, D.S.; Young, H.; Barna, M.; Depreux, F.; Richter, C.P. Hearing Differences in Hartley Guinea Pig Stocks from Two Breeders. Hear. Res. 2019, 379, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lexow, G.J.; Schurzig, D.; Gellrich, N.C.; Lenarz, T.; Majdani, O.; Rau, T.S. Visualization, Measurement and Modelling of the Cochlea Using Rotating Midmodiolar Slice Planes. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 1855–1869. [Google Scholar] [CrossRef] [PubMed]
- Statistics Kingdom Shapiro-Wilk Test Calculator. Available online: https://www.statskingdom.com/shapiro-wilk-test-calculator.html (accessed on 5 September 2022).
- Carvalho, G.J.; Lalwani, A.K. The Effect of Cochleostomy and Intracochlear Infusion on Auditory Brain Stem Response Threshold in the Guinea Pig. Am. J. Otol. 1999, 20, 87–90. [Google Scholar]
- Fernandez, K.A.; Watabe, T.; Tong, M.; Meng, X.; Tani, K.; Kujawa, S.G.; Edge, A.S.B. Trk Agonist Drugs Rescue Noise-Induced Hidden Hearing Loss. JCI Insight 2021, 6, e142572. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.; Chambers, S.; Hampson, A.; Eastwood, H.; Campbell, L.; O’Leary, S. Delayed Low Frequency Hearing Loss Caused by Cochlear Implantation Interventions via the Round Window but Not Cochleostomy. Hear. Res. 2016, 333, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.J.; Ni, G.; Verschuur, C.A. Modelling the Effect of Round Window Stiffness on Residual Hearing after Cochlear Implantation. Hear. Res. 2016, 341, 155–167. [Google Scholar] [CrossRef]
- Tanaka, C.; Nguyen-Huynh, A.; Loera, K.; Stark, G.; Reiss, L. Factors Associated with Hearing Loss in a Normal-Hearing Guinea Pig Model of Hybrid Cochlear Implants. Hear. Res. 2014, 316, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, R.; Coco, A. Chronic Depolarization Enhances the Trophic Effects of BDNF in Rescuing Auditory Neurons Following a Sensorineural Hearing Loss. J. Comp. Neurol. 2005, 486, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.-H.; Oghalai, J.S. Perilymph Osmolality Modulates Cochlear Function. Laryngoscope 2008, 118, 1621–1629. [Google Scholar] [CrossRef] [Green Version]
- Ghiz, A.F.; Salt, A.N.; DeMott, J.E.; Henson, M.M.; Henson, O.W.; Gewalt, S.L. Quantitative Anatomy of the Round Window and Cochlear Aqueduct in Guinea Pigs. Hear. Res. 2001, 162, 105–112. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.; Oh, S.; Jung, G.; Jeong, K.J.; Tan Tran, V.; Hwang, D.; Kim, S., II; Song, J.J.; Suh, M.W.; et al. Contralateral Spreading of Substances Following Intratympanic Nanoparticle-Conjugated Gentamicin Injection in a Rat Model. Sci. Rep. 2020, 10, 18636. [Google Scholar] [CrossRef] [PubMed]
- Canlon, B.; Fransson, A. Morphological and Functional Preservation of the Outer Hair Cells from Noise Trauma by Sound Conditioning. Hear. Res. 1995, 84, 112–124. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Mancuso, C.; Eramo, S.L.M.; Ralli, M.; Piacentini, R.; Barone, E.; Paludetti, G.; Troiani, D. In Vivo Protective Effect of Ferulic Acid against Noise-Induced Hearing Loss in the Guinea-Pig. Neuroscience 2010, 169, 1575–1588. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, S.; Cazevieille, C. N-Acetylcysteine Treatment Reduces Noise-Induced Hearing Loss in Guinea Pig. J. Community Prev. Med. 2020, 3, 1. [Google Scholar] [CrossRef]
- Fernandez, K.A.; Guo, D.; Micucci, S.; De Gruttola, V.; Liberman, M.C.; Kujawa, S.G. Noise-Induced Cochlear Synaptopathy with and Without Sensory Cell Loss. Neuroscience 2020, 427, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, P.W.C.; Bourien, J.; Diuba, A.; Puel, J.L.; Kujawa, S.G. Noise-Induced Hearing Loss in Gerbil: Round Window Assays of Synapse Loss. Front. Cell. Neurosci. 2021, 15, 286. [Google Scholar] [CrossRef]
- Kaur, T.; Clayman, A.C.; Nash, A.J.; Schrader, A.D.; Warchol, M.E.; Ohlemiller, K.K. Lack of Fractalkine Receptor on Macrophages Impairs Spontaneous Recovery of Ribbon Synapses after Moderate Noise Trauma in C57bl/6 Mice. Front. Neurosci. 2019, 13, 620. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, D.; Minami, S.B.; Kanzaki, S.; Ogawa, K.; Miller, J.M. Bcl-2 Genes Regulate Noise-Induced Hearing Loss. J. Neurosci. Res. 2008, 86, 920–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasoba, T.; Schacht, J.; Shoji, F.; Miller, J.M. Attenuation of Cochlear Damage from Noise Trauma by an Iron Chelator, a Free Radical Scavenger and Glial Cell Line-Derived Neurotrophic Factor in Vivo. Brain Res. 1999, 815, 317–325. [Google Scholar] [CrossRef]
- Ji, L.; Lee, H.J.; Wan, G.; Wang, G.P.; Zhang, L.; Sajjakulnukit, P.; Schacht, J.; Lyssiotis, C.A.; Corfas, G. Auditory Metabolomics, an Approach to Identify Acute Molecular Effects of Noise Trauma. Sci. Rep. 2019, 9, 9273. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Liu, K.; Wang, H.; Zhang, Y.; Hong, Z.; Wang, M.; Wang, X.; Jiang, X.; Yang, S. Noise Induced Reversible Changes of Cochlear Ribbon Synapses Contribute to Temporary Hearing Loss in Mice. Acta Otolaryngol. 2015, 135, 1093–1102. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, N.; Yan, K.; Liu, X.; Zhang, Y.; Hong, Z.; Wang, M.; Yin, Q.; Wu, F.; Lei, Y.; et al. Inner Hair Cell Ribbon Synapse Plasticity Might Be Molecular Basis of Temporary Hearing Threshold Shifts in Mice. Int. J. Clin. Exp. Pathol. 2015, 8, 8680–8691. [Google Scholar] [PubMed]
- Lee, S.Y.; Han, J.J.; Lee, S.Y.; Jung, G.; Min, H.J.; Song, J.J.; Koo, J.W. Outcomes of Peptide Vaccine GV1001 Treatment in a Murine Model of Acute Noise-Induced Hearing Loss. Antioxidants 2020, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.B.; Nowak, N.; Mull, K.; Goldring, A.; Lehar, M.; Fuchs, P.A. Acoustic Trauma Increases Ribbon Number and Size in Outer Hair Cells of the Mouse Cochlea. JARO-J. Assoc. Res. Otolaryngol. 2021, 22, 19–31. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Freitas, M.A.; Neves, M.P.; Silva, M.V. Evaluation of the Noise Exposure of Symphonic Orchestra Musicians. Noise Health 2014, 16, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Schink, T.; Kreutz, G.; Busch, V.; Pigeot, I.; Ahrens, W. Incidence and Relative Risk of Hearing Disorders in Professional Musicians. Occup. Environ. Med. 2014, 71, 472–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickman, T.T.; Hashimoto, K.; Liberman, L.D.; Liberman, M.C. Synaptic Migration and Reorganization after Noise Exposure Suggests Regeneration in a Mature Mammalian Cochlea. Sci. Rep. 2020, 10, 19945. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.A.; Wolfovitz, A.; Yilmazer, R.; Garnham, C.; Yilmazer, A.B.; Bas, E.; Ashman, P.; Roell, J.; Bohorquez, J.; Mittal, R.; et al. Otoprotection to Implanted Cochlea Exposed to Noise Trauma With Dexamethasone Eluting Electrode. Front. Cell. Neurosci. 2019, 13, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Arteaga, D.N.; Aksit, A.; Chiang, H.; Olson, E.S.; Kysar, J.W.; Lalwani, A.K. Anatomical and Functional Consequences of Microneedle Perforation of Round Window Membrane. Otol. Neurotol. 2020, 41, e280–e287. [Google Scholar] [CrossRef]
- Gyo, K. Healing of experimentally produced round window membrane rupture. Acta Otolaryngol. 1989, 107, 85–89. [Google Scholar] [CrossRef]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The Role of Oxidative Stress in Noise-Induced Hearing Loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, J.H.; Kim, Y.; Yi, J.; Chung, J.W. Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Okudaira, N.; Ishii, N.; Yotsuyanagi, H.; Nagai, T.; Tokudome, S.; Fujioka, T.; Takahashi, S. Comparison of the Antioxidant Activity of Albumin from Various Animal Species. Zoolog. Sci. 2008, 25, 172–177. [Google Scholar] [CrossRef]
- Xiong, M.; He, Q.; Lai, H.; Wang, J. Oxidative Stress in Spiral Ganglion Cells of Pigmented and Albino Guinea Pigs Exposed to Impulse Noise. Acta Otolaryngol. 2011, 131, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Shuster, B.; Casserly, R.; Lipford, E.; Olszewski, R.; Milon, B.; Viechweg, S.; Davidson, K.; Enoch, J.; McMurray, M.; Rutherford, M.A.; et al. Estradiol Protects against Noise-Induced Hearing Loss and Modulates Auditory Physiology in Female Mice. Int. J. Mol. Sci. 2021, 22, 12208. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Park, J.; Basinou, V.; Weger, B.D.; Tserga, E.; Sarlus, H.; Magnusson, A.K.; Kadri, N.; Gachon, F.; Canlon, B. Circadian Regulation of Cochlear Sensitivity to Noise by Circulating Glucocorticoids. Curr. Biol. 2019, 29, 2477–2487.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.W.; Ahn, J.H.; Kim, J.Y.; Lee, H.J.; Kang, H.H.; Lee, Y.K.; Kim, J.U.; Koo, S.W. The Effect of Isoflurane, Halothane and Pentobarbital on Noise-Induced Hearing Loss in Mice. Anesth. Analg. 2007, 104, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.R.; Chole, R.A. Hypothermia Protects the Cochlea from Noise Damage. Hear. Res. 1984, 16, 225–230. [Google Scholar] [CrossRef]












| Day | Implanted Ear | Not Implanted Ear |
|---|---|---|
| −7 | Click, frequency specific | Click |
| 0 pre noise | Click, frequency specific | Click, frequency specific |
| 0 post noise | Click, frequency specific | Click, frequency specific |
| 1 | Click, frequency specific | Click |
| 7 | Click, frequency specific | Click, frequency specific |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malfeld, K.; Baumhoff, P.; Volk, H.A.; Lenarz, T.; Scheper, V. Local Long-Term Inner Ear Drug Delivery in Normal Hearing Guinea Pig—An Animal Model to Develop Preventive Treatment for Noise-Induced Hearing Loss. Biomolecules 2022, 12, 1427. https://doi.org/10.3390/biom12101427
Malfeld K, Baumhoff P, Volk HA, Lenarz T, Scheper V. Local Long-Term Inner Ear Drug Delivery in Normal Hearing Guinea Pig—An Animal Model to Develop Preventive Treatment for Noise-Induced Hearing Loss. Biomolecules. 2022; 12(10):1427. https://doi.org/10.3390/biom12101427
Chicago/Turabian StyleMalfeld, Kathrin, Peter Baumhoff, Holger A. Volk, Thomas Lenarz, and Verena Scheper. 2022. "Local Long-Term Inner Ear Drug Delivery in Normal Hearing Guinea Pig—An Animal Model to Develop Preventive Treatment for Noise-Induced Hearing Loss" Biomolecules 12, no. 10: 1427. https://doi.org/10.3390/biom12101427
APA StyleMalfeld, K., Baumhoff, P., Volk, H. A., Lenarz, T., & Scheper, V. (2022). Local Long-Term Inner Ear Drug Delivery in Normal Hearing Guinea Pig—An Animal Model to Develop Preventive Treatment for Noise-Induced Hearing Loss. Biomolecules, 12(10), 1427. https://doi.org/10.3390/biom12101427









