From Genes to Geography, from Cells to Community, from Biomolecules to Behaviors: The Importance of Social Determinants of Health
Abstract
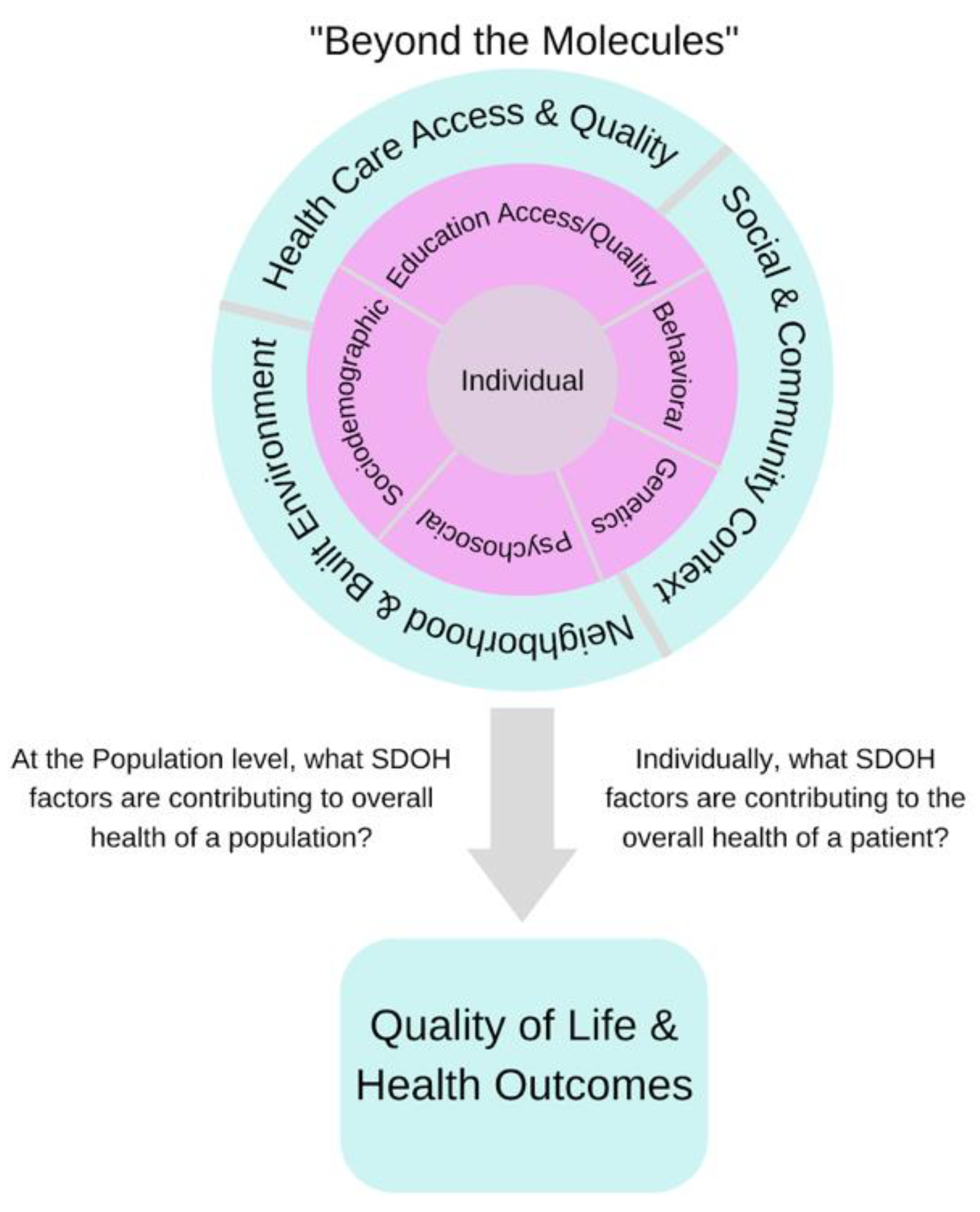
:1. Introduction
2. Social Determinants of Health
3. SDOH Integration into Electronic Health Records
4. The Future of SDOH in Real-World Evidence Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baptista, P.V. Principles in genetic risk assessment. Ther. Clin. Risk Manag. 2005, 1, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Hall, W.D.; Morley, K.I.; Lucke, J.C. The prediction of disease risk in genomic medicine. EMBO Rep. 2004, 5, S22–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraiman, Y.S.; Wojcik, M.H. The influence of social determinants of health on the genetic diagnostic odyssey: Who remains undiagnosed, why, and to what effect? Pediatr. Res. 2020, 89, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Breen, N.; Berrigan, D.; Jackson, J.S.; Wong, D.W.; Wood, F.B.; Denny, J.C.; Zhang, X.; Bourne, P.E. Translational Health Disparities Research in a Data-Rich World. Health Equity 2019, 3, 588–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healthy People 2030, U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. 2018. Available online: https://health.gov/healthypeople/objectives-and-data/social-determinants-health (accessed on 11 March 2022).
- Haire-Joshu, D.; Hill-Briggs, F. The Next Generation of Diabetes Translation: A Path to Health Equity. Annu. Rev. Public Health 2019, 40, 391–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kind, A.J.; Buckingham, W.R. Making Neighborhood-Disadvantage Metrics Accessible—The Neighborhood Atlas. N. Engl. J. Med. 2018, 378, 2456–2458. [Google Scholar] [CrossRef]
- U.S. Census Bureau. 1994. Census Tracts and Block Numbering Areas. Available online: https://www2.census.gov/geo/pdfs/reference/GARM/Ch10GARM.pdf (accessed on 11 November 2021).
- Kolak, M.; Bhatt, J.; Park, Y.H.; Padrón, N.A.; Molefe, A. Quantification of Neighborhood-Level Social Determinants of Health in the Continental United States. JAMA Netw. Open 2020, 3, e1919928. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.K. Area Deprivation and Widening Inequalities in US Mortality, 1969–1998. Am. J. Public Health 2003, 93, 1137–1143. [Google Scholar] [CrossRef]
- Flanagan, B.E.; Gregory, E.W.; Hallisey, E.J.; Heitgerd, J.L.; Lewis, B. A Social Vulnerability Index for Disaster Management. J. Homel. Secur. Emerg. Manag. 2020, 4–6. [Google Scholar] [CrossRef]
- Nagano, I.; Wu, Z.; Asano, K.; Takahashi, Y. Census Tract Level State Maps of the Modified Retail Food Environment Index (mRFEI). Natl. Cent. Chronic Dis. Prev. Health Promot. Div. 2011, 178, 134–142. [Google Scholar]
- Knighton, A.J.; Savitz, L.; Belnap, T.; Stephenson, B.; VanDerslice, J. Introduction of an Area Deprivation Index Measuring Patient Socio-economic Status in an Integrated Health System: Implications for Population Health. EGEMs (Gener. Evid. Methods Improv. Patient Outcomes) 2016, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Butte, A.J.; Baranzini, S.E. Integrating biomedical research and electronic health records to create knowledge-based biologically meaningful machine-readable embeddings. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Office for Civil Rights (OCR); Malin, B. Guidance Regarding Methods for de-identification of protected health information in accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Health Inf. Priv. 2012, 1–32. Available online: https://privacysecurityacademy.com/wp-content/uploads/2021/03/HHS-OCR-Guidance-on-De-Identification-of-PHI-2012.pdf (accessed on 25 August 2022).
- Shortreed, S.M.; Cook, A.J.; Coley, R.Y.; Bobb, J.F.; Nelson, J.C. Challenges and opportunities for using big health care data to advance medical science and public health. Am. J. Epidemiol. 2019, 188, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Rudrapatna, V.A.; Butte, A.J. Opportunities and challenges in using real-world data for health care. J. Clin. Investig. 2020, 130, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Cantor, M.N.; Thorpe, L. Integrating data on social determinants of health into electronic health records. Health Aff. 2018, 37, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M. Social determinants of health inequalities. Lancet 2005, 365, 1099–1104. [Google Scholar] [CrossRef]
- Thornton, P.L.; Kumanyika, S.K.; Gregg, E.W.; Araneta, M.R.; Baskin, M.L.; Chin, M.H.; Mangione, C.M. New research directions on disparities in obesity and type 2 diabetes. Ann. N. Y. Acad. Sci. 2020, 1461, 5–24. [Google Scholar] [CrossRef]
- IOM (Institute of Medicine). Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 2; National Academies Press: Washington, DC, USA, 2015; pp. 1–351. [Google Scholar] [CrossRef]
- Hamilton, C.M.; Strader, L.C.; Pratt, J.G.; Maiese, D.; Hendershot, T.; Kwok, R.K.; Hammond, J.A.; Huggins, W.; Jackman, D.; Pan, H.; et al. The PhenX Toolkit: Get the Most From Your Measures. Am. J. Epidemiol. 2011, 174, 253–260. [Google Scholar] [CrossRef]
- Brämer, G.R. International Statistical Classification of Diseases and Related Health Problems: Tenth Revision, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- MIT Critical Data. Secondary Analysis of Electronic Health Records. In Secondary Analysis of Electronic Health Records; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1–427. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. mHealth: New Horizons for Health through Mobile Technologies: Second Global Survey on Ehealth; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Akbari, P.; Sosina, O.A.; Bovijn, J.; Landheer, K.; Nielsen, J.B.; Kim, M.; Aykul, S.; De, T.; Haas, M.E.; Hindy, G.; et al. Multiancestry exome sequencing reveals INHBE mutations associated with favorable fat distribution and protection from diabetes. Nat. Commun. 2022, 13, 4844. [Google Scholar] [CrossRef]
- Hughes, M.M.; Wang, A.; Grossman, M.K.; Pun, E.; Whiteman, A.; Deng, L.; Hallisey, E.; Sharpe, J.D.; Ussery, E.N.; Stokley, S.; et al. County-Level COVID-19 Vaccination Coverage and Social Vulnerability—United States, 14 December 2020–1 March 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Azap, R.A.; Paredes, A.Z.; Diaz, A.; Hyer, J.M.; Pawlik, T.M. The association of neighborhood social vulnerability with surgical textbook outcomes among patients undergoing hepatopancreatic surgery. Surgery 2020, 168, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Ghirimoldi, F.M.; Schmidt, S.; Simon, R.C.; Wang, C.-P.; Wang, Z.; Brimhall, B.B.; Damien, P.; Moffett, E.E.; Manuel, L.S.; Sarwar, Z.U.; et al. Association of Socioeconomic Area Deprivation Index with Hospital Readmissions After Colon and Rectal Surgery. J. Gastrointest. Surg. 2020, 25, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.Q.; Althouse, A.D.; Sabik, L.; Arnold, R.; Chu, E.; Smith, T.J.; Smith, K.; White, D.; Schenker, Y. The Association Between Area Deprivation Index and Patient-Reported Outcomes in Patients with Advanced Cancer. Health Equity 2021, 5, 8–16. [Google Scholar] [CrossRef]
| ICD-10 CM Code | SDoH Categories |
|---|---|
| Z55 | Problems related to education and literacy |
| Z56 | Problems related to employment and unemployment |
| Z57 | Occupational exposure to risk factors |
| Z58 | Problems related to physical environment |
| Z59 | Problems related to housing and economic circumstances |
| Z60 | Problems related to social environment |
| Z62 | Problems related to upbringing |
| Z63 | Other problems related to primary support group, including family circumstances |
| Z64 | Problems related to certain psychosocial circumstances |
| Z65 | Problems related to other psychosocial circumstances |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidson, J.; Vashisht, R.; Butte, A.J. From Genes to Geography, from Cells to Community, from Biomolecules to Behaviors: The Importance of Social Determinants of Health. Biomolecules 2022, 12, 1449. https://doi.org/10.3390/biom12101449
Davidson J, Vashisht R, Butte AJ. From Genes to Geography, from Cells to Community, from Biomolecules to Behaviors: The Importance of Social Determinants of Health. Biomolecules. 2022; 12(10):1449. https://doi.org/10.3390/biom12101449
Chicago/Turabian StyleDavidson, Jaysón, Rohit Vashisht, and Atul J. Butte. 2022. "From Genes to Geography, from Cells to Community, from Biomolecules to Behaviors: The Importance of Social Determinants of Health" Biomolecules 12, no. 10: 1449. https://doi.org/10.3390/biom12101449
APA StyleDavidson, J., Vashisht, R., & Butte, A. J. (2022). From Genes to Geography, from Cells to Community, from Biomolecules to Behaviors: The Importance of Social Determinants of Health. Biomolecules, 12(10), 1449. https://doi.org/10.3390/biom12101449






