The Role of Clusterin Transporter in the Pathogenesis of Alzheimer’s Disease at the Blood–Brain Barrier Interface: A Systematic Review
Abstract
1. Introduction
2. Attempts to Use CLU as an AD Biomarker
2.1. Metabolic and Toxic Consequences of CLU-Alpha–Beta Proteins
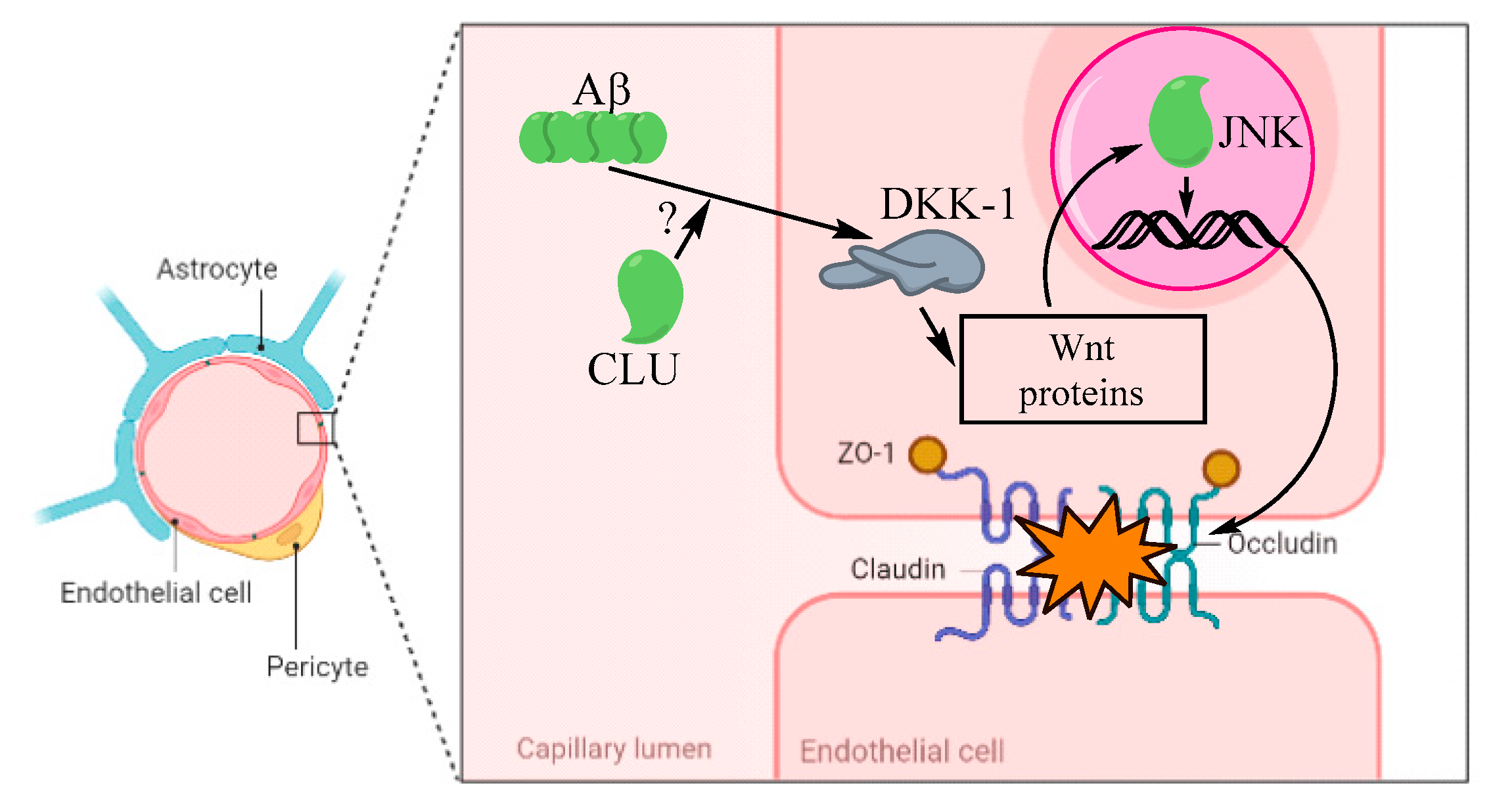
2.2. Aβ Role in Cerebral Amyloid Angiopathy Involving CLU and Wnt at the BBB
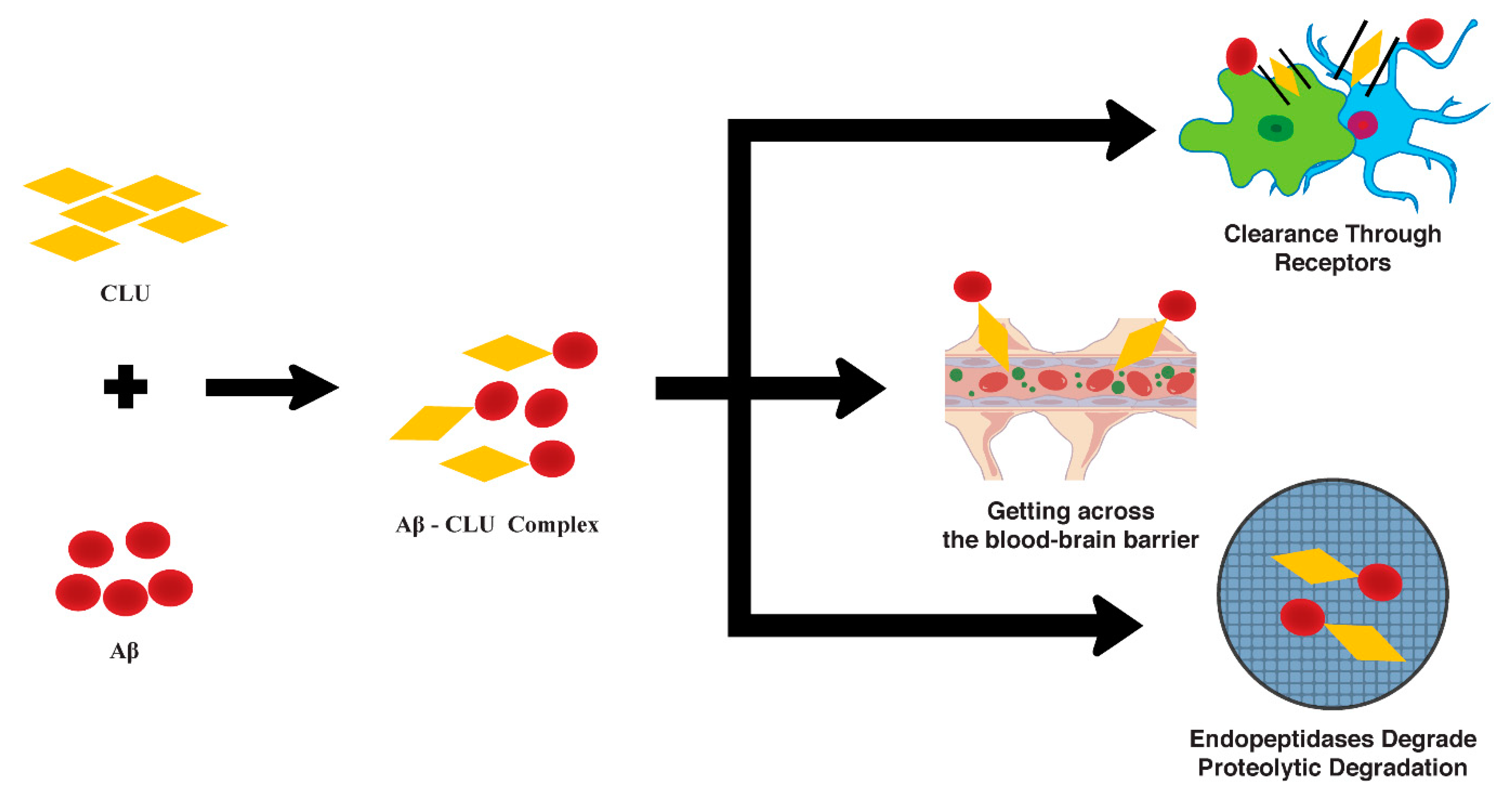
2.3. Aβ Clearance Mechanism (CLU)
3. The Role of CLU in AD-Signaling Channels (Neuro-Inflammatory and Wnt Signaling)
4. The Role of Wnt/β-Catenin Signaling and Neurogenesis in AD
5. The Blood–Brain Barrier (BBB) and Its Dysfunction in the Pathogenesis of AD
6. Wnt-β Signaling and Functionality of Blood–Brain Barrier in AD
7. Cellular Risk Aspects of CLU Protein in AD (Lipid Metabolism, Homeostasis, Neuronal Apoptosis)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ertekin-Taner, N. Genetics of Alzheimer’s disease: A centennial review. Neurol. Clin. 2007, 25, 611–667. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Uddin, M.; Kabir, M.; Khanum, S.; Sarwar, M.; Mathew, B.; Rauf, A.; Ahmed, M.; Ashraf, G.M. Exploring the promise of targeting ubiquitin-proteasome system to combat Alzheimer’s disease. Neurotox. Res. 2020, 38, 8–17. [Google Scholar] [CrossRef]
- Barker, W.W.; Luis, C.A.; Kashuba, A.; Luis, M.; Harwood, D.G.; Loewenstein, D.; Waters, C.; Jimison, P.; Shepherd, E.; Sevush, S. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord. 2002, 16, 203–212. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Santilli, G.; Aronow, B.J.; Sala, A. Essential requirement of apolipoprotein J (clusterin) signaling for IκB expression and regulation of NF-κB activity. J. Biol. Chem. 2003, 278, 38214–38219. [Google Scholar] [CrossRef]
- Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; Tanzi, R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet. 2007, 39, 17–23. [Google Scholar] [CrossRef]
- Satapathy, S.; Wilson, M.R. The dual roles of clusterin in extracellular and intracellular proteostasis. Trends Biochem. Sci. 2021, 46, 652–660. [Google Scholar] [CrossRef]
- Foster, E.M.; Dangla-Valls, A.; Lovestone, S.; Ribe, E.M.; Buckley, N.J. Clusterin in Alzheimer’s disease: Mechanisms, genetics, and lessons from other pathologies. Front. Neurosci. 2019, 13, 164. [Google Scholar] [CrossRef]
- Schuermann, B.; Wiese, B.; Bickel, H.; Weyerer, S.; Riedel-Heller, S.G.; Pentzek, M.; Bachmann, C.; Williams, J.; van den Bussche, H.; Maier, W.; et al. Association of the Alzheimer’s disease clusterin risk allele with plasma clusterin concentration. J. of Alzheimer’s Dis. 2011, 25, 421–424. [Google Scholar] [CrossRef]
- Schrijvers, E.M.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Plasma clusterin and the risk of Alzheimer disease. JAMA 2011, 305, 1322–1326. [Google Scholar] [CrossRef]
- Baig, S.; Palmer, L.E.; Owen, M.J.; Williams, J.; Kehoe, P.G.; Love, S. Clusterin mRNA and protein in Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 28, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lidström, A.M.; Bogdanovic, N.; Hesse, C.; Volkman, I.; Davidsson, P.; Blennow, K. Clusterin (apolipoprotein J) protein levels are increased in hippocampus and in frontal cortex in Alzheimer’s disease. Exp. Neurol. 1998, 154, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Nilselid, A.-M.; Davidsson, P.; Nägga, K.; Andreasen, N.; Fredman, P.; Blennow, K. Clusterin in cerebrospinal fluid: Analysis of carbohydrates and quantification of native and glycosylated forms. Neurochem. Int. 2006, 48, 718–728. [Google Scholar] [CrossRef]
- Sihlbom, C.; Davidsson, P.; Sjögren, M.; Wahlund, L.-O.; Nilsson, C.L. Structural and quantitative comparison of cerebrospinal fluid glycoproteins in Alzheimer’s disease patients and healthy individuals. Neurochem. Res. 2008, 33, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Ghiso, J.; Matsubara, E.; Koudinov, A.; Choi-Miura, N.H.; Tomita, M.; Wisniewski, T.; Frangione, B. The cerebrospinal-fluid soluble form of Alzheimer’s amyloid β is complexed to SP-40, 40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem. J. 1993, 293, 27–30. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Martel, C.L.; Matsubara, E.; McComb, J.G.; Zheng, G.; McCluskey, R.T.; Frangione, B.; Ghiso, J. Glycoprotein 330/megalin: Probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc. Natl. Acad. Sci. USA 1996, 93, 4229–4234. [Google Scholar] [CrossRef]
- Schellenberg, G.D.; Montine, T.J. The genetics and neuropathology of Alzheimer’s disease. Acta Neuropathol. 2012, 124, 305–323. [Google Scholar] [CrossRef]
- Deming, Y.; Xia, J.; Cai, Y.; Lord, J.; Holmans, P.; Bertelsen, S.; Holtzman, D.; Morris, J.C.; Bales, K.; Pickering, E.H.; et al. A potential endophenotype for Alzheimer’s disease: Cerebrospinal fluid clusterin. Neurobiol. Aging 2016, 37, 208.e1–208.e9. [Google Scholar] [PubMed]
- Morgan, T.; Laping, N.; Rozovsky, I.; Oda, T.; Hogan, T.; Finch, C.; Pasinetti, G. Clusterin expression by astrocytes is influenced by transforming growth factor β1 and heterotypic cell interactions. J. Neuroimmunol. 1995, 58, 101–110. [Google Scholar] [CrossRef]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Orte, A.; Clarke, R.W.; Bolognesi, B.; Hook, S.; Ganzinger, K.A.; Meehan, S.; Wilson, M.R.; Dobson, C.M.; Klenerman, D. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-β1−40 peptide. Nat. Struct. Mol. Biol. 2012, 19, 79–83. [Google Scholar] [CrossRef]
- Yerbury, J.J.; Wilson, M.R. Extracellular chaperones modulate the effects of Alzheimer’s patient cerebrospinal fluid on Aβ1-42 toxicity and uptake. Cell Stress Chaperones 2010, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Nishida, Y.; Sagare, A.P.; Rege, S.V.; Bell, R.D.; Perlmutter, D.; Sengillo, J.D.; Hillman, S.; Kong, P.; Nelson, A.R. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015, 18, 521–530. [Google Scholar] [CrossRef]
- Miners, J.S.; Clarke, P.; Love, S. Clusterin levels are increased in A lzheimer’s disease and influence the regional distribution of Aβ. Brain Pathol. 2017, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Y.; Wei, X.; Li, Y.; Wu, H.; Zhuang, J.; Zhao, Z. Clusterin in Alzheimer’s disease: A player in the biological behavior of amyloid-beta. Neurosci. Bull. 2014, 30, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Skorb, E.V.; Förster, C.Y.; Dandekar, T. Scaffold Searching of FDA and EMA-Approved Drugs Identifies Lead Candidates for Drug Repurposing in Alzheimer’s Disease. Front. Chem. 2021, 846. [Google Scholar] [CrossRef]
- Cascella, R.; Conti, S.; Tatini, F.; Evangelisti, E.; Scartabelli, T.; Casamenti, F.; Wilson, M.R.; Chiti, F.; Cecchi, C. Extracellular chaperones prevent Aβ42-induced toxicity in rat brains. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 1217–1226. [Google Scholar] [CrossRef][Green Version]
- Sagare, A.; Deane, R.; Bell, R.D.; Johnson, B.; Hamm, K.; Pendu, R.; Marky, A.; Lenting, P.J.; Wu, Z.; Zarcone, T. Clearance of amyloid-β by circulating lipoprotein receptors. Nat. Med. 2007, 13, 1029–1031. [Google Scholar] [CrossRef]
- Cirrito, J.R.; May, P.C.; O’Dell, M.A.; Taylor, J.W.; Parsadanian, M.; Cramer, J.W.; Audia, J.E.; Nissen, J.S.; Bales, K.R.; Paul, S.M.; et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J. Neurosci. 2003, 23, 8844–8853. [Google Scholar] [CrossRef]
- Yu, J.-T.; Tan, L. The role of clusterin in Alzheimer’s disease: Pathways, pathogenesis, and therapy. Mol. Neurobiol. 2012, 45, 314–326. [Google Scholar] [CrossRef]
- Tanzi, R.; Moir, R.; Wagner, S. Clearance of Alzheimer’s Aβ peptide: The many roads to perdition. Neuron 2004, 43, 605–608. [Google Scholar]
- Cirrito, J.R.; Deane, R.; Fagan, A.M.; Spinner, M.L.; Parsadanian, M.; Finn, M.B.; Jiang, H.; Prior, J.L.; Sagare, A.; Bales, K.R. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J. Clin. Investig. 2005, 115, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Situ, M.; Citalan-Madrid, A.F.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Transcriptomic Profile of Blood–Brain Barrier Remodeling in Cerebral Amyloid Angiopathy. Front. Cell. Neurosci. 2022, 16, 931247. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Ando, Y.; Misumi, Y.; Ueda, M. Current management and therapeutic strategies for cerebral amyloid angiopathy. Int. J. Mol. Sci. 2021, 22, 3869. [Google Scholar] [CrossRef]
- Aghaizu, N.D.; Jin, H.; Whiting, P.J. Dysregulated Wnt signalling in the Alzheimer’s brain. Brain Sci. 2020, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- DeMattos, R.B.; O’dell, M.A.; Parsadanian, M.; Taylor, J.W.; Harmony, J.A.; Bales, K.R.; Paul, S.M.; Aronow, B.J.; Holtzman, D.M. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10843–10848. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.R.; Sagare, A.P.; Zlokovic, B.V. Role of clusterin in the brain vascular clearance of amyloid-β. Proc. Natl. Acad. Sci. USA 2017, 114, 8681–8682. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Rose, J.; Tulloch, J.; Henstridge, C.; Smith, C.; Spires-Jones, T.L. Clusterin accumulates in synapses in Alzheimer’s disease and is increased in apolipoprotein E4 carriers. Brain Commun. 2019, 1, fcz003. [Google Scholar] [CrossRef]
- Nordestgaard, L.T.; Tybjærg-Hansen, A.; Rasmussen, K.L.; Nordestgaard, B.G.; Frikke-Schmidt, R. Genetic variation in clusterin and risk of dementia and ischemic vascular disease in the general population: Cohort studies and meta-analyses of 362,338 individuals. BMC Med. 2018, 16, 1–11. [Google Scholar] [CrossRef]
- Wojtas, A.M.; Kang, S.S.; Olley, B.M.; Gatherer, M.; Shinohara, M.; Lozano, P.A.; Liu, C.-C.; Kurti, A.; Baker, K.E.; Dickson, D.W.; et al. Loss of clusterin shifts amyloid deposition to the cerebrovasculature via disruption of perivascular drainage pathways. Proc. Natl. Acad. Sci. USA 2017, 114, E6962–E6971. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.T.; Bruno, M.A.; Ducatenzeiler, A.; Klein, W.L.; Cuello, A.C. Intracellular Aβ-oligomers and early inflammation in a model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.M.; Pottoo, F.H.; Dahiya, E.S.; Khan, F.A.; Kumar, J.S. Neuron-glia interactions: Molecular basis of alzheimer’s disease and applications of neuroproteomics. Eur. J. Neurosci. 2020, 52, 2931–2943. [Google Scholar] [CrossRef]
- Nuutinen, T.; Huuskonen, J.; Suuronen, T.; Ojala, J.; Miettinen, R.; Salminen, A. Amyloid-β 1–42 induced endocytosis and clusterin/apoJ protein accumulation in cultured human astrocytes. Neurochem. Int. 2007, 50, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Uddin, M.S.; Mathew, B.; Ashraf, G.M. Toxic tau: Structural origins of tau aggregation in Alzheimer’s disease. Neural Regen. Res. 2020, 15, 1417. [Google Scholar]
- Killick, R.; Ribe, E.; Al-Shawi, R.; Malik, B.; Hooper, C.; Fernandes, C.; Dobson, R.; Nolan, P.; Lourdusamy, A.; Furney, S. Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt–PCP–JNK pathway. Mol. Psychiatry 2014, 19, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Falgarone, G.; Chiocchia, G. Clusterin: A multifacet protein at the crossroad of inflammation and autoimmunity. Adv. Cancer Res. 2009, 104, 139–170. [Google Scholar] [PubMed]
- Bell, R.D.; Sagare, A.P.; Friedman, A.E.; Bedi, G.S.; Holtzman, D.M.; Deane, R.; Zlokovic, B.V. Transport pathways for clearance of human Alzheimer’s amyloid β-peptide and apolipoproteins E and J in the mouse central nervous system. J. Cereb. Blood Flow Metab. 2007, 27, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; Lane, S.; Finn, M.B.; Holtzman, D.M.; Zlokovic, B.V. apoE isoform–specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Investig. 2008, 118, 4002–4013. [Google Scholar] [CrossRef]
- Martin-Rehrmann, M.D.; Hoe, H.-S.; Capuani, E.M.; Rebeck, G.W. Association of apolipoprotein J-positive β-amyloid plaques with dystrophic neurites in alzheimer’s disease brain. Neurotox. Res. 2005, 7, 231–241. [Google Scholar] [CrossRef]
- Caricasole, A.; Copani, A.; Caraci, F.; Aronica, E.; Rozemuller, A.J.; Caruso, A.; Storto, M.; Gaviraghi, G.; Terstappen, G.C.; Nicoletti, F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J. Neurosci. 2004, 24, 6021–6027. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, P.; Kövari, E.; French, L.; Viard, I.; Hof, P.; Bouras, C. Possible neuroprotective role of clusterin in Alzheimer’s disease: A quantitative immunocytochemical study. Acta Neuropathol. 1998, 95, 387–394. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Takiuchi, S.; Kamide, K.; Yoshii, M.; Horio, T.; Tanaka, C.; Banno, M.; Miyata, T.; Sasaguri, T.; Kawano, Y. Insertion/deletion polymorphism in clusterin gene influences serum lipid levels and carotid intima-media thickness in hypertensive Japanese females. Biochem. Biophys. Res. Commun. 2005, 331, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Tokuda, T.; Rostagno, A.; Kumar, A.; Zlokovic, B.; Frangione, B.; Ghiso, J. Functional and structural properties of lipid-associated apolipoprotein J (clusterin). Biochem. J. 1999, 344, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Gonos, E.S. Clusterin/apolipoprotein J in human aging and cancer. Int. J. Biochem. Cell Biol. 2002, 34, 1430–1448. [Google Scholar] [CrossRef]
- Martins, I.J.; Berger, T.; Sharman, M.J.; Verdile, G.; Fuller, S.J.; Martins, R.N. Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2009, 111, 1275–1308. [Google Scholar] [CrossRef]
- Deane, R.; Wu, Z.; Sagare, A.; Davis, J.; Du Yan, S.; Hamm, K.; Xu, F.; Parisi, M.; LaRue, B.; Hu, H.W. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 2004, 43, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenco, L.F.; Ranieri, G.R.; de Oliveira Souza, V.C.; Junior, F.B.; Saldiva, P.H.N.; Mauad, T. Edible weeds: Are urban environments fit for foraging? Sci. Total Environ. 2020, 698, 133967. [Google Scholar] [CrossRef] [PubMed]
- Greenough, M.; Ramírez Munoz, A.; Bush, A.; Opazo, C. Metallo-pathways to Alzheimer’s disease: Lessons from genetic disorders of copper trafficking. Metallomics 2016, 8, 831–839. [Google Scholar] [CrossRef]
- Purro, S.A.; Dickins, E.M.; Salinas, P.C. The secreted Wnt antagonist Dickkopf-1 is required for amyloid β-mediated synaptic loss. J. Neurosci. 2012, 32, 3492–3498. [Google Scholar] [CrossRef]
- Liang, W.S.; Dunckley, T.; Beach, T.G.; Grover, A.; Mastroeni, D.; Ramsey, K.; Caselli, R.J.; Kukull, W.A.; McKeel, D.; Morris, J.C. Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: A reference data set. Physiol. Genom. 2008, 33, 240–256. [Google Scholar] [CrossRef]
- Cater, J.H.; Wilson, M.R.; Wyatt, A.R. Alpha-2-macroglobulin, a hypochlorite-regulated chaperone and immune system modulator. Oxid. Med. Cell. Longev. 2019, 2019, 5410657. [Google Scholar] [CrossRef]
- DeMattos, R.B.; Cirrito, J.R.; Parsadanian, M.; May, P.C.; O’Dell, M.A.; Taylor, J.W.; Harmony, J.A.; Aronow, B.J.; Bales, K.R.; Paul, S.M.; et al. ApoE and clusterin cooperatively suppress Aβ levels and deposition: Evidence that ApoE regulates extracellular Aβ metabolism in vivo. Neuron 2004, 41, 193–202. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Essabbani, A.; Margottin-Goguet, F.; Chiocchia, G. Identification of Clusterin Domain Involved in NF-κB Pathway Regulation 2. J. Biol. Chem. 2010, 285, 4273–4277. [Google Scholar] [CrossRef]
- Burek, M.; Förster, C.Y. Cloning and characterization of the murine claudin-5 promoter. Mol. Cell. Endocrinol. 2009, 298, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Salvador, E.; Förster, C.Y. Generation of an immortalized murine brain microvascular endothelial cell line as an in vitro blood brain barrier model. J. Vis. Exp. 2012, e4022. [Google Scholar] [CrossRef]
- Förster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as direct target for glucocorticoid-induced improvement of blood–brain barrier properties in a murine in vitro system. J. Physiol. 2005, 565, 475–486. [Google Scholar] [CrossRef]
- Sagare, A.P.; Bell, R.D.; Zlokovic, B.V. Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a011452. [Google Scholar] [CrossRef]
- Gerhartl, A.; Hahn, K.; Neuhoff, A.; Friedl, H.-P.; Förster, C.Y.; Wunder, C.; Schick, M.; Burek, M.; Neuhaus, W. Hydroxyethylstarch (130/0.4) tightens the blood-brain barrier in vitro. Brain Res. 2020, 1727, 146560. [Google Scholar] [CrossRef]
- Staessen, J.A.; Wang, J.; Bianchi, G.; Birkenhäger, W.H. Essential hypertension. Lancet 2003, 361, 1629–1641. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Harke, N.; Leers, J.; Kietz, S.; Drenckhahn, D.; Förster, C. Glucocorticoids regulate the human occludin gene through a single imperfect palindromic glucocorticoid response element. Mol. Cell. Endocrinol. 2008, 295, 39–47. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kanekiyo, T. Blood-brain barrier dysfunction and the pathogenesis of Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 1965. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.-O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Neuhaus, W.; Krämer, T.; Neuhoff, A.; Gölz, C.; Thal, S.C.; Förster, C.Y. Multifaceted mechanisms of WY-14643 to stabilize the blood-brain barrier in a model of traumatic brain injury. Front. Mol. Neurosci. 2017, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef]
- Rösing, N.; Salvador, E.; Güntzel, P.; Kempe, C.; Burek, M.; Holzgrabe, U.; Soukhoroukov, V.; Wunder, C.; Förster, C. Neuroprotective effects of isosteviol sodium in murine brain capillary cerebellar endothelial cells (cerebEND) after Hypoxia. Front. Cell. Neurosci. 2020, 14, 573950. [Google Scholar] [CrossRef]
- Deane, R.; Zlokovic, B.V. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.; Schilling, L. Regulation of cerebral blood flow—A brief review. Monit. Cereb. Blood Flow Metab. Intensive Care 1993, 3–10. [Google Scholar]
- Salvador, E.; Burek, M.; Förster, C.Y. Tight junctions and the tumor microenvironment. Curr. Pathobiol. Rep. 2016, 4, 135–145. [Google Scholar] [CrossRef]
- Herz, J.; Strickland, D.K. LRP: A multifunctional scavenger and signaling receptor. J. Clin. Investig. 2001, 108, 779–784. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Du Yan, S.; Yan, S.F.; Stern, D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- de Lau, W.; Peng, W.C.; Gros, P.; Clevers, H. The R-spondin/Lgr5/Rnf43 module: Regulator of Wnt signal strength. Genes Dev. 2014, 28, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Marques, F.; Sousa, J.C.; Sousa, N.; Palha, J.A. Blood–brain-barriers in aging and in Alzheimer’s disease. Mol. Neurodegener. 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Zhou, Y.; Nathans, J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev. Cell 2014, 31, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Smallwood, P.M.; Nathans, J. Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogenesis and blood-brain barrier regulation. Neuron 2017, 95, 1056–1073.e5. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Mancuso, M.R.; Maier, C.; Liang, X.; Yuki, K.; Yang, L.; Kwong, J.W.; Wang, J.; Rao, V.; Vallon, M. Gpr124 is essential for blood–brain barrier integrity in central nervous system disease. Nat. Med. 2017, 23, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Vallon, M.; Yuki, K.; Nguyen, T.D.; Chang, J.; Yuan, J.; Siepe, D.; Miao, Y.; Essler, M.; Noda, M.; Garcia, K.C. A RECK-WNT7 receptor-ligand interaction enables isoform-specific regulation of Wnt bioavailability. Cell Rep. 2018, 25, 339–349.e9. [Google Scholar] [CrossRef]
- Tran, K.A.; Zhang, X.; Predescu, D.; Huang, X.; Machado, R.F.; Göthert, J.R.; Malik, A.B.; Valyi-Nagy, T.; Zhao, Y.-Y. Endothelial β-catenin signaling is required for maintaining adult blood–brain barrier integrity and central nervous system homeostasis. Circulation 2016, 133, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Vanhollebeke, B.; Stone, O.A.; Bostaille, N.; Cho, C.; Zhou, Y.; Maquet, E.; Gauquier, A.; Cabochette, P.; Fukuhara, S.; Mochizuki, N. Tip cell-specific requirement for an atypical Gpr124-and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. elife 2015, 4, e06489. [Google Scholar] [CrossRef]
- Main, B.S.; Villapol, S.; Sloley, S.S.; Barton, D.J.; Parsadanian, M.; Agbaegbu, C.; Stefos, K.; McCann, M.S.; Washington, P.M.; Rodriguez, O.C. Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol. Neurodegener. 2018, 13, 1–18. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Tischfield, M.; Williams, J.; Smallwood, P.M.; Rattner, A.; Taketo, M.M.; Nathans, J. Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Investig. 2014, 124, 3825–3846. [Google Scholar] [CrossRef]
- Lim, J.C.; Kania, K.D.; Wijesuriya, H.; Chawla, S.; Sethi, J.K.; Pulaski, L.; Romero, I.A.; Couraud, P.O.; Weksler, B.B.; Hladky, S.B. Activation of β-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J. Neurochem. 2008, 106, 1855–1865. [Google Scholar] [CrossRef]
- Palomer, E.; Buechler, J.; Salinas, P.C. Wnt signaling deregulation in the aging and Alzheimer’s brain.; 2019; Volume 13, p. 227. [Google Scholar]
- Thambisetty, M.; Simmons, A.; Velayudhan, L.; Hye, A.; Campbell, J.; Zhang, Y.; Wahlund, L.-O.; Westman, E.; Kinsey, A.; Güntert, A. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch. Gen. Psychiatry 2010, 67, 739–748. [Google Scholar] [CrossRef]
- Bucossi, S.; Polimanti, R.; Mariani, S.; Ventriglia, M.; Bonvicini, C.; Migliore, S.; Manfellotto, D.; Salustri, C.; Vernieri, F.; Rossini, P.M. Association of K832R and R952K SNPs of Wilson’s disease gene with Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 29, 913–919. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Akasaka, Y.; Ishii, T.; Komiyama, K.; Masuda, S.; Asuwa, N.; Choi-Miura, N.-H.; Tomita, M. Distribution and synthesis of apolipoprotein J in the atherosclerotic aorta. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Polimanti, R.; Bucossi, S.; Ventriglia, M.; Mariani, S.; Manfellotto, D.; Vernieri, F.; Cassetta, E.; Ursini, F.; Rossini, P.M. Linkage disequilibrium and haplotype analysis of the ATP7B gene in Alzheimer’s disease. Rejuvenation Res. 2013, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.A.; Weickert, C.S.; Garner, B. Apolipoproteins in the brain: Implications for neurological and psychiatric disorders. Clin. Lipidol. 2010, 5, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-B.; Jeon, J.-H.; Choi, I.; Kwon, O.-Y.; Yu, K.; You, K.-H. Clusterin, a novel modulator of TGF-β signaling, is involved in Smad2/3 stability. Biochem. Biophys. Res. Commun. 2008, 366, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Materia, S.; Cater, M.A.; Klomp, L.W.; Mercer, J.F.; La Fontaine, S. Clusterin (apolipoprotein J), a molecular chaperone that facilitates degradation of the copper-ATPases ATP7A and ATP7B. J. Biol. Chem. 2011, 286, 10073–10083. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Stomrud, E.; Lindberg, O.; Palmqvist, S.; Zetterberg, H.; Blennow, K.; Hansson, O. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 2018, 91, e867–e877. [Google Scholar] [CrossRef]
- Ewers, M.; Franzmeier, N.; Suárez-Calvet, M.; Morenas-Rodriguez, E.; Caballero, M.A.A.; Kleinberger, G.; Piccio, L.; Cruchaga, C.; Deming, Y.; Dichgans, M.; et al. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci. Transl. Med. 2019, 11, eaav6221. [Google Scholar] [CrossRef]
- Bellaver, B.; Ferrari-Souza, J.P.; da Ros, L.U.; Carter, S.F.; Rodriguez-Vieitez, E.; Nordberg, A.; Pellerin, L.; Rosa-Neto, P.; Leffa, D.T.; Zimmer, E.R. Astrocyte biomarkers in Alzheimer disease: A systematic review and meta-analysis. Neurology 2021, 96, e2944–e2955. [Google Scholar] [CrossRef]
- Moretti, R.M.; Marelli, M.M.; Mai, S.; Cariboni, A.; Scaltriti, M.; Bettuzzi, S.; Limonta, P. Clusterin isoforms differentially affect growth and motility of prostate cells: Possible implications in prostate tumorigenesis. Cancer Res. 2007, 67, 10325–10333. [Google Scholar] [CrossRef]
- Rizzi, F.; Coletta, M.; Bettuzzi, S. Clusterin (CLU): From one gene and two transcripts to many proteins. Adv. Cancer Res. 2009, 104, 9–23. [Google Scholar] [PubMed]
- Xie, Z.; Harris-White, M.; Wals, P.; Frautschy, S.; Finch, C.; Morgan, T.E. Apolipoprotein J (clusterin) activates rodent microglia in vivo and in vitro. J. Neurochem. 2005, 93, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Cunin, P.; Beauvillain, C.; Miot, C.; Augusto, J.-F.; Preisser, L.; Blanchard, S.; Pignon, P.; Scotet, M.; Garo, E.; Fremaux, I. Clusterin facilitates apoptotic cell clearance and prevents apoptotic cell-induced autoimmune responses. Cell Death Dis. 2016, 7, e2215. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, T.; Kiyama, S.; Chi, K.; Miyake, H.; Adomat, H.; Skov, K.; Gleave, M. Overexpression of the cytoprotective protein clusterin decreases radiosensitivity in the human LNCaP prostate tumour model. BJU Int. 2003, 92, 463–469. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fareed, M.M.; Qasmi, M.; Aziz, S.; Völker, E.; Förster, C.Y.; Shityakov, S. The Role of Clusterin Transporter in the Pathogenesis of Alzheimer’s Disease at the Blood–Brain Barrier Interface: A Systematic Review. Biomolecules 2022, 12, 1452. https://doi.org/10.3390/biom12101452
Fareed MM, Qasmi M, Aziz S, Völker E, Förster CY, Shityakov S. The Role of Clusterin Transporter in the Pathogenesis of Alzheimer’s Disease at the Blood–Brain Barrier Interface: A Systematic Review. Biomolecules. 2022; 12(10):1452. https://doi.org/10.3390/biom12101452
Chicago/Turabian StyleFareed, Muhammad Mazhar, Maryam Qasmi, Shaan Aziz, Elisabeth Völker, Carola Yvette Förster, and Sergey Shityakov. 2022. "The Role of Clusterin Transporter in the Pathogenesis of Alzheimer’s Disease at the Blood–Brain Barrier Interface: A Systematic Review" Biomolecules 12, no. 10: 1452. https://doi.org/10.3390/biom12101452
APA StyleFareed, M. M., Qasmi, M., Aziz, S., Völker, E., Förster, C. Y., & Shityakov, S. (2022). The Role of Clusterin Transporter in the Pathogenesis of Alzheimer’s Disease at the Blood–Brain Barrier Interface: A Systematic Review. Biomolecules, 12(10), 1452. https://doi.org/10.3390/biom12101452







