Targeting Neuroinflammation in Osteoarthritis with Intra-Articular Adelmidrol
Abstract
:1. Introduction
2. Neuro-Immune Mechanisms Underlying OA
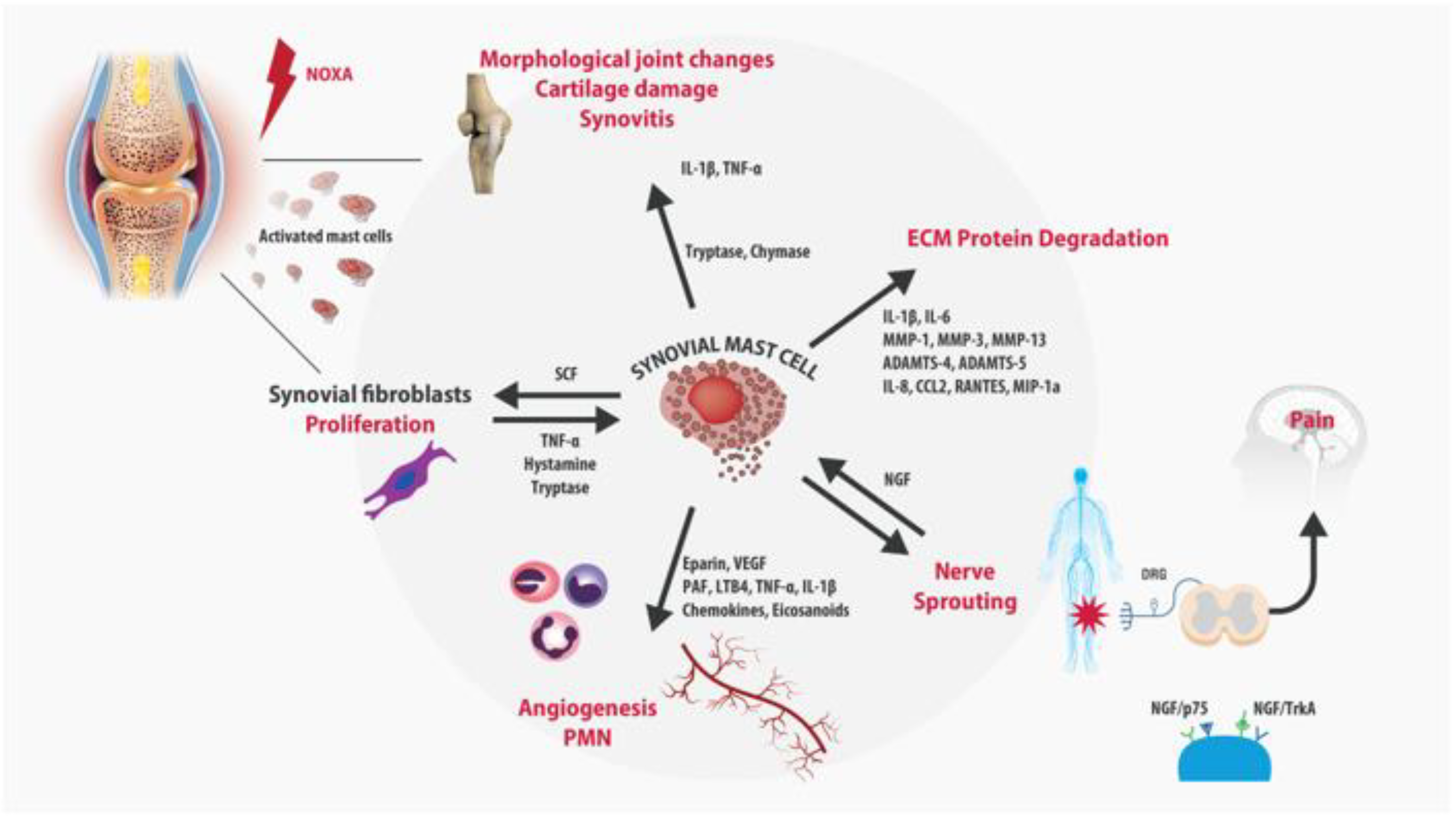
3. Role of Mast Cells in OA
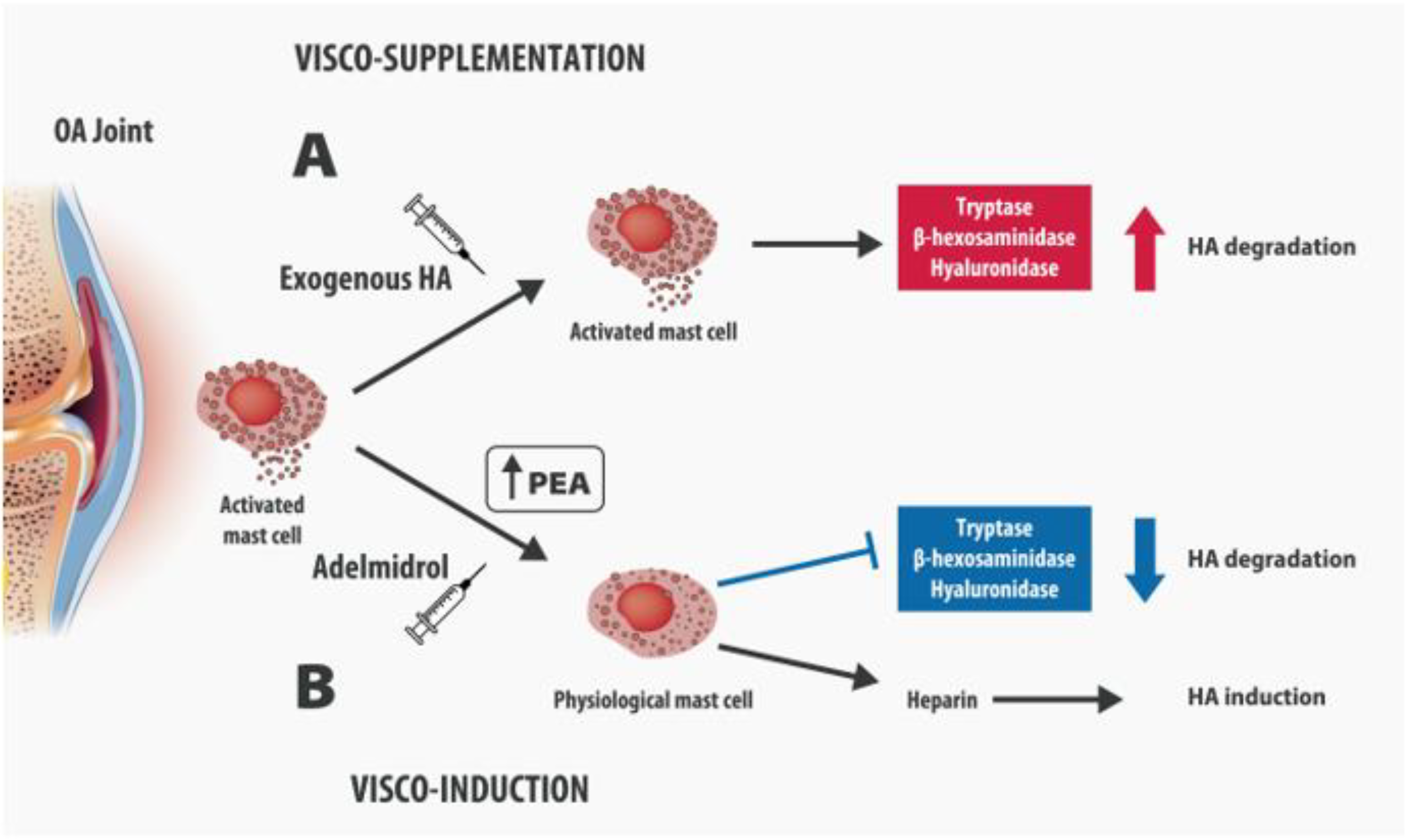
4. Intra-Articular Adelmidrol: A New Therapeutic Option for OA
5. Adelmidrol in OA: Preclinical Evidence
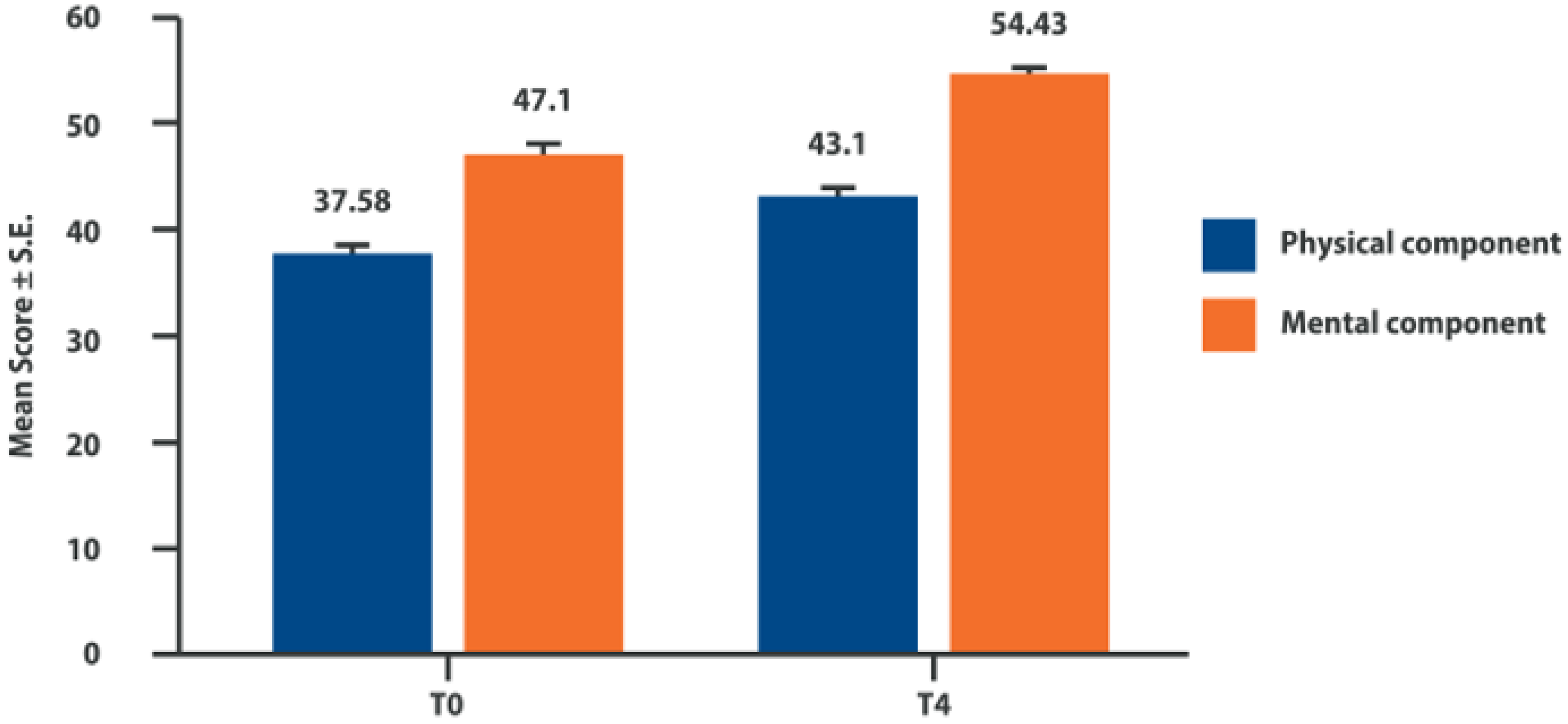
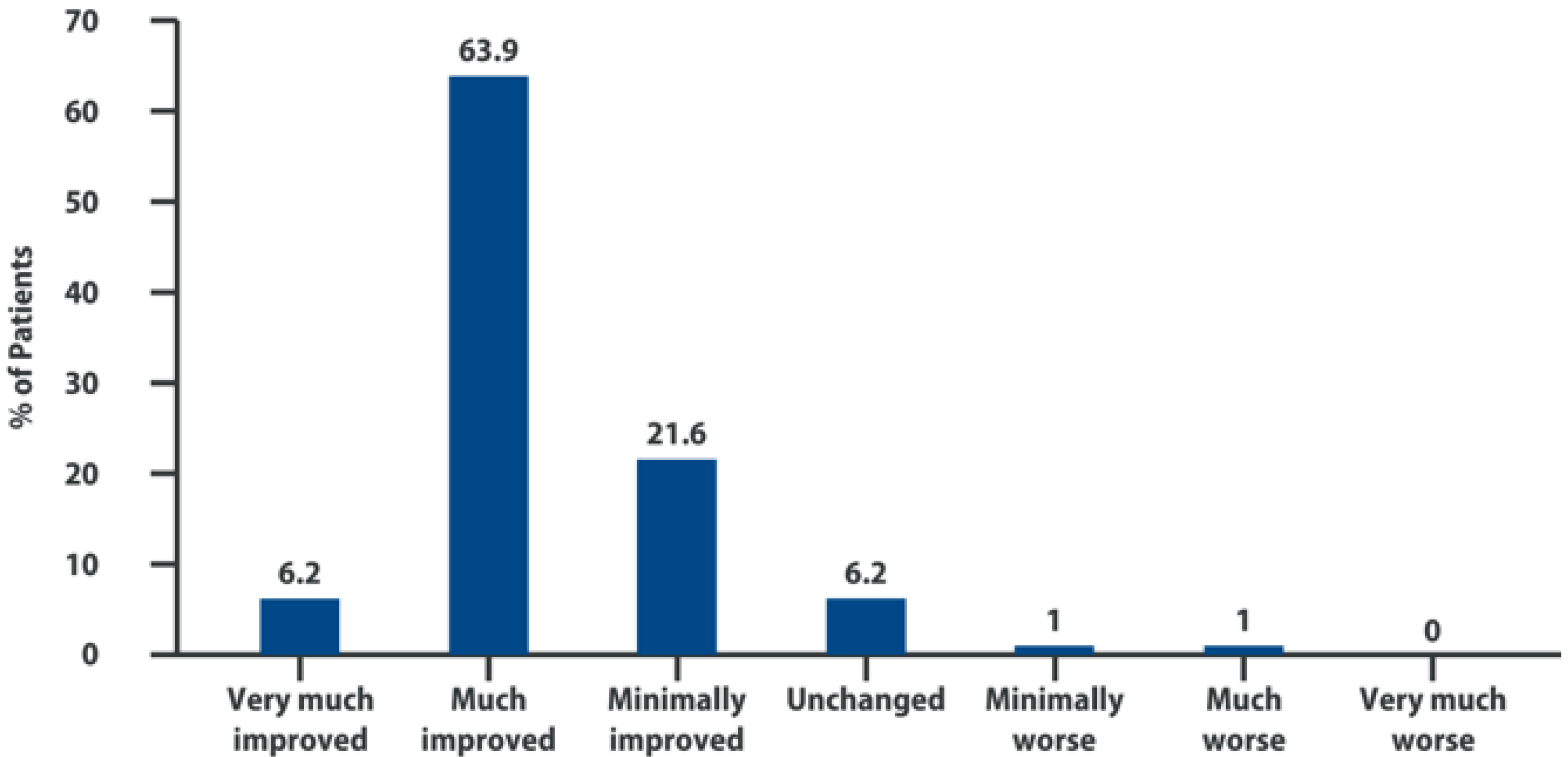
6. Intra-Articular Adelmidrol: Clinical Evidence
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, E.; Lee, Y.C. A Mechanism-Based Approach to the Management of Osteoarthritis Pain. Curr. Osteoporos. Rep. 2015, 13, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, A.; Sagi, V.; Gupta, M.; Gupta, K. Mast Cell Neural Interactions in Health and Disease. Front. Cell. Neurosci. 2019, 13, 110. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.J.; Malfait, A.-M.; Miller, R.E. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr. Cartil. 2020, 28, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.G.; Gallagher, P.J.; Walls, A.F. Mast cell subpopulations in the synovial tissue of patients with osteoarthritis: Selective increase in numbers of tryptase-positive, chymase-negative mast cells. J. Pathol. 1998, 186, 67–74. [Google Scholar] [CrossRef]
- Yu, H.; Huang, T.; Lu, W.W.; Tong, L.; Chen, D. Osteoarthritis Pain. Int. J. Mol. Sci. 2022, 23, 4642. [Google Scholar] [CrossRef] [PubMed]
- Seidel, M.F.; Hügle, T.; Morlion, B.; Koltzenburg, M.; Chapman, V.; MaassenVanDenBrink, A.; Lane, N.E.; Perrot, S.; Zieg-lgänsberger, W. Neurogenic inflammation as a novel treatment target for chronic pain syndromes. Exp. Neurol. 2022, 356, 114108. [Google Scholar] [CrossRef]
- Lambert, C.; Zappia, J.; Sanchez, C.; Florin, A.; Dubuc, J.-E.; Henrotin, Y. The Damage-Associated Molecular Patterns (DAMPs) as Potential Targets to Treat Osteoarthritis: Perspectives from a Review of the Literature. Front. Med. 2020, 7, 607186. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Ebata, T.; Yokota, S.; Takahashi, D.; Endo, T.; Matsumae, G.; Shimizu, T.; Kadoya, K.; Iwasaki, N. Low-Grade Inflammation in the Pathogenesis of Osteoarthritis: Cellular and Molecular Mechanisms and Strategies for Future Therapeutic Intervention. Biomedicines 2022, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [Green Version]
- Martel-Pelletier, J.; McCollum, R.; DiBattista, J.; Faure, M.P.; Chin, J.A.; Fournier, S.; Sarfati, M.; Pelletier, J.P. The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes. Identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum. 1992, 35, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar] [PubMed]
- Chevalier, X.; Eymard, F.; Richette, P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat. Rev. Rheumatol. 2013, 9, 400–410. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Li, Y.; Hu, X.; Zhang, Y.; Fan, P. Profiling of inflammatory mediators in the synovial fluid related to pain in knee osteoarthritis. BMC Musculoskelet. Disord. 2020, 21, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Hooge, A.S.K.; Van de Loo, F.A.J.; Bennink, M.B.; Arntz, O.J.; De Hooge, P.; Van den Berg, W.B. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthr. Cartil. 2005, 13, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monibi, F.; Roller, B.L.; Stoker, A.; Garner, B.; Bal, S.; Cook, J.L. Identification of Synovial Fluid Biomarkers for Knee Osteo-arthritis and Correlation with Radiographic Assessment. J. Knee Surg. 2016, 29, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Borzi, R.M.; Mazzetti, I.; Macor, S.; Silvestri, T.; Bassi, A.; Cattini, L.; Facchini, A. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: Constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999, 455, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.E.; Tran, P.B.; Das, R.; Ghoreishi-Haack, N.; Ren, D.; Miller, R.J.; Malfait, A.-M. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc. Natl. Acad. Sci. USA 2012, 109, 20602–20607. [Google Scholar] [CrossRef] [Green Version]
- Beekhuizen, M.; Gierman, L.M.; Van Spil, W.E.; Van Osch, G.J.V.M.; Huizinga, T.W.J.; Saris, D.B.F.; Creemers, L.B.; Zuurmond, A.-M. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthr. Cartil. 2013, 21, 918–922. [Google Scholar] [CrossRef]
- Sandell, L.J.; Xing, X.; Franz, C.; Davies, S.; Chang, L.-W.; Patra, D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthr. Cartil. 2008, 16, 1560–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, S.M.; Chan, C.K.; Teo, S.H.; Singh, S.; Merican, A.; Ng, W.M.; Abbas, A.; Kamarul, T. Elevated plasma and synovial fluid interleukin-8 and interleukin-18 may be associated with the pathogenesis of knee osteoarthritis. Knee 2020, 27, 26–35. [Google Scholar] [CrossRef]
- Guo, X.; Xu, T.; Zheng, J.; Cui, X.; Li, M.; Wang, K.; Su, M.; Zhang, H.; Zheng, K.; Sun, C.; et al. Accumulation of synovial fluid CD19+CD24hiCD27+ B cells was associated with bone destruction in rheumatoid arthritis. Sci. Rep. 2020, 10, 14386. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.B.; Miller, R.E.; Ishihara, S.; Miller, R.J.; Malfait, A.M. Spinal microglial activation in a murine surgical model of knee osteoarthritis. Osteoarthr. Cartil. 2017, 25, 718–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, E.D.; Sloane, E.M.; Watkins, L.R. Glia in pathological pain: A role for fractalkine. J. Neuroimmunol. 2008, 198, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Luongo, L.; Sajic, M.; Grist, J.; Clark, A.K.; Maione, S.; Malcangio, M. Spinal changes associated with mechanical hypersensi-tivity in a model of Guillain-Barré syndrome. Neurosci. Lett. 2008, 437, 98–102. [Google Scholar] [CrossRef]
- Clark, A.K.; Grist, J.; Al-Kashi, A.; Perretti, M.; Malcangio, M. Spinal cathepsin S and fractalkine contribute to chronic pain in the collagen-induced arthritis model. Arthritis Rheum. 2012, 64, 2038–2047. [Google Scholar] [CrossRef]
- Miller, R.E.; Malfait, A.-M.; Block, J.A. Current status of nerve growth factor antibodies for the treatment of osteoarthritis pain. Clin. Exp. Rheumatol. 2017, 35 (Suppl. S1), 85–87. [Google Scholar]
- Iannone, F.; De Bari, C.; Dell’Accio, F.; Covelli, M.; Patella, V.; Lo Bianco, G.; Lapadula, G. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology 2002, 41, 1413–1418. [Google Scholar] [CrossRef] [Green Version]
- Bryden, L.A.; Nicholson, J.R.; Doods, H.; Pekcec, A. Deficits in spontaneous burrowing behavior in the rat bilateral mono-sodium iodoacetate model of osteoarthritis: An objective measure of pain-related behavior and analgesic efficacy. Osteoarthr. Cartil. 2015, 23, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Nwosu, L.N.; Burston, J.J.; Millns, P.J.; Sagar, D.R.; Mapp, P.I.; Meesawatsom, P.; Li, L.; Bennett, A.J.; Walsh, D.A.; et al. The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthr. Cartil. 2016, 24, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- LaBranche, T.P.; Bendele, A.M.; Omura, B.C.; Gropp, K.E.; Hurst, S.I.; Bagi, C.M.; Cummings, T.R.; Ii, L.E.G.; Shelton, D.L.; Zorbas, M.A. Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann. Rheum. Dis. 2017, 76, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Kroner, J.; Kovtun, A.; Kemmler, J.; Messmann, J.J.; Strauss, G.; Seitz, S.; Schinke, T.; Amling, M.; Kotrba, J.; Froebel, J.; et al. Mast Cells Are Critical Regulators of Bone Fracture-Induced Inflammation and Osteoclast Formation and Activity. J. Bone Miner. Res. 2017, 32, 2431–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragipoglu, D.; Dudeck, A.; Haffner-Luntzer, M.; Voss, M.; Kroner, J.; Ignatius, A.; Fischer, V. The Role of Mast Cells in Bone Metabolism and Bone Disorders. Front. Immunol. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannetti, A.; Filice, E.; Caffarelli, C.; Ricci, G.; Pession, A. Mast Cell Activation Disorders. Medicina 2021, 57, 124. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Liu, Q.; Huang, F.; He, H.; Fan, W. Involvement of Mast Cells in the Pathophysiology of Pain. Front. Cell. Neurosci. 2021, 15, 665066. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. Is osteoarthritis a metabolic disease? Jt. Bone Spine 2013, 80, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Farinelli, L.; Aquili, A.; Mattioli-Belmonte, M.; Manzotti, S.; D’Angelo, F.; Ciccullo, C.; Gigante, A. Synovial mast cells from knee and hip osteoarthritis: Histological study and clinical correlations. J. Exp. Orthop. 2022, 9, 13. [Google Scholar] [CrossRef]
- De Lange-Brokaar, B.J.E.; Kloppenburg, M.; Andersen, S.N.; Dorjée, A.L.; Yusuf, E.; Herb-van Toorn, L.; Kroon, H.M.; Zuur-mond, A.-M.; Stojanovic-Susulic, V.; Bloem, J.L.; et al. Characterization of synovial mast cells in knee osteoarthritis: Association with clinical parameters. Osteoarthr. Cartil. 2016, 24, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, J.; Izumi, M.; Habuchi, H.; Habuchi, O.; Takaya, S.; Kasai, Y.; Hayashi, R.; Aso, K.; Ushida, T.; Ikeuchi, M. A novel mice model of acute flares in osteoarthritis elicited by intra-articular injection of cultured mast cells. J. Exp. Orthop. 2021, 8, 75. [Google Scholar] [CrossRef]
- Nigrovic, P.A.; Lee, D.M. Mast cells in inflammatory arthritis. Arthritis Res. Ther. 2005, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magarinos, N.J.; Bryant, K.J.; Fosang, A.J.; Adachi, R.; Stevens, R.L.; McNeil, H.P. Mast cell-restricted, tetramer-forming tryptases induce aggrecanolysis in articular cartilage by activating matrix metalloproteinase-3 and -13 zymogens. J. Immunol. 2013, 191, 1404–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, P.; Harsulkar, A.; Märtson, A.; Suutre, S.; Märtson, A.; Koks, S. Mast cells differentiated in synovial fluid and resident in osteophytes exalt the inflammatory pathology of osteoarthritis. Int. J. Mol. Sci. 2022, 23, 541. [Google Scholar] [CrossRef] [PubMed]
- Rivellese, F.; Rossi, F.W.; Galdiero, M.R.; Pitzalis, C.; De Paulis, A. Mast cells in early rheumatoid arthritis. Int. J. Mol. Sci. 2019, 28, 2040. [Google Scholar] [CrossRef] [Green Version]
- Skaper, S.D. Nerve growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology 2017, 151, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Andrade, J.M.; Mantyh, P.W. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res. Ther. 2012, 14, R101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Coughlin, K.A.; Kaczmarska, M.J.; Castaneda-Corral, G.; Bloom, A.P.; Kuskowski, M.A.; Mantyh, P.W. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful ar-thritic joint. Arthritis Rheum. 2012, 64, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar] [CrossRef]
- Sousa-Valente, J.; Calvo, L.; Vacca, V.; Simeoli, R.; Arévalo, J.C.; Malcangio, M. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthr. Cartil. 2018, 26, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Wei, T.; Hu, N.; Zhou, X. Disrupted homeostasis of synovial hyaluronic acid and its associations with synovial mast cell proteases of rheumatoid arthritis patients and collagen-induced arthritis rats. Immunol. Res. 2021, 69, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Whittlesey, K.; Metcalfe, D.D.; Dastych, J. Human mast cells express the hyaluronic-acid-binding isoform of CD44 and adhere to hyaluronic acid. Clin. Immunol. 2000, 94, 173–178. [Google Scholar] [CrossRef]
- Zhao, D.; Pan, J.-K.; Yang, W.-Y.; Han, Y.-H.; Zeng, L.-F.; Liang, G.-H.; Liu, J. Intra-Articular Injections of Platelet-Rich Plasma, Adipose Mesenchymal Stem Cells, and Bone Marrow Mesenchymal Stem Cells Associated with Better Outcomes Than Hyaluronic Acid and Saline in Knee Osteoarthritis: A Systematic Review and Network Meta-analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 2298–2314.e10. [Google Scholar] [CrossRef] [PubMed]
- Saltychev, M.; Mattie, R.; McCormick, Z.; Laimi, K. The Magnitude and Duration of the Effect of Intra-articular Corticosteroid Injections on Pain Severity in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2020, 99, 617–625. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-articular Tri-amcinolone vs. Saline on Knee Cartilage Volume and Pain in Patients with Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Koenen, M.; Schauer, S.; Wittig-Blaich, S.; Ahmad, M.; Baschant, U.; Tuckermann, J.P. Molecular Actions of Glucocorticoids in Cartilage and Bone During Health, Disease, and Steroid Therapy. Physiol. Rev. 2016, 96, 409–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasavvidis, T.; Totlis, T.; Gilat, R.; Cole, B.J. Platelet-Rich Plasma Combined with Hyaluronic Acid Improves Pain and Function Compared with Hyaluronic Acid Alone in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 1277–1287.e1. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.; Naidoo, P. Viscosupplementation for knee osteoarthritis: A focus on Hylan G-F 20. Orthop. Res. Rev. 2018, 10, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Petrosino, S.; Puigdemont, A.; Della Valle, M.F.; Fusco, M.; Verde, R.; Allarà, M.; Aveta, T.; Orlando, P.; Di Marzo, V. Adelmidrol increases the endogenous concentrations of palmitoylethanolamide in canine keratinocytes and down-regulates an inflammatory reaction in an in vitro model of contact allergic dermatitis. Vet. J. 2016, 207, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Del Re, A.; Palenca, I.; Seguella, L.; Pesce, M.; Corpetti, C.; Steardo, L.; Rurgo, S.; Sarnelli, G.; Esposito, G. Oral Adelmidrol Administration Up-Regulates Palmitoylethanolamide Production in Mice Colon and Duodenum through a PPAR-γ Inde-pendent Action. Metabolites 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; D’Amico, A.; Cinelli, M.P.; Esposito, G.; Di Marzo, V.; Iuvone, T. Adelmidrol, a palmitoylethanolamide analogue, reduces chronic inflammation in a carrageenin-granuloma model in rats. J. Cell. Mol. Med. 2009, 13, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, S.; Brazis, P.; Della Valle, M.F.; Miolo, A.; Puigdemont, A. Inhibitory effect of topical adelmidrol on antigen-induced skin wheal and mast cell behavior in a canine model of allergic dermatitis. BMC Vet. Res. 2012, 8, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punzi, L.; Frigato, M.; Frallonardo, P.; Ramonda, R. Inflammatory osteoarthritis of the hand. Best Pract. Res. Clin. Rheumatol. 2010, 24, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.M.; Beier, F. Novel Insights into Osteoarthritis Joint Pathology from Studies in Mice. Curr. Rheumatol. Rep. 2015, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Luongo, L.; Guida, F.; Boccella, S.; Bellini, G.; Gatta, L.; Rossi, F.; De Novellis, V.; Maione, S. Palmitoylethanolamide reduces formalin-induced neuropathic-like behaviour through spinal glial/microglial phenotypical changes in mice. CNS Neurol. Disord. Drug Targets 2013, 12, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Boccella, S.; Iannotta, M.; De Gregorio, D.D.; Giordano, C.; Belardo, C.; Romano, R.; Palazzo, E.; Scafuro, M.A.; Serra, N.; et al. Palmitoylethanolamide reduces neuropsychiatric behaviors by restoring cortical electrophysiological activity in a mouse model of mild traumatic brain injury. Front. Pharmacol. 2017, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Impellizzeri, D.; Di Paola, R.; Cordaro, M.; Gugliandolo, E.; Casili, G.; Morittu, V.M.; Britti, D.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 2016, 119, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Fusco, R.; Impellizzeri, D.; Cordaro, M.; Britti, D.; Morittu, V.M.; Evangelista, M.; Cuzzocrea, S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodo-acetate-induced osteoarthritis in rats. Arthritis Res. Ther. 2016, 18, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaki, S.; Blaker, C.L.; Little, C.B. OA foundations-experimental models of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 357–380. [Google Scholar] [CrossRef]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Banerji, S.; Wright, A.J.; Noble, M.; Mahoney, D.J.; Campbell, I.D.; Day, A.J.; Jackson, D.G. Structures of the Cd44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat. Struct. Mol. Biol. 2007, 14, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Vulpiani, M.C.; Tucciarone, A.; Vetrano, M.; Trischitta, D.; Santoboni, F.; Nusca, S.M.; Godente, L.; Latini, E.; Ippolito, E. [Il trattamento della gonartrosi mediante infiltrazioni intra-articolari con un’ associazione di acido ialuronico] Knee osteoarthritis treatment by intra-articular injections with a combination of hyaluronic acid and Adelmidrol (Hyadrol®). Giot 2021, 47, 169–179. [Google Scholar] [CrossRef]
- Salaffi, F.; Leardini, G.; Canesi, B.; Mannoni, A.; Fioravanti, A.; Caporali, R.; Lapadula, G.; Punzi, L. Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index in Italian patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2003, 11, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Vulpiani, M.C.; Vetrano, M.; Trischitta, D.; Santoboni, F.; Nusca, S.M.; Latini, E.; Ippolito, E. [Risultati a lungo termine del trattamento della gonartrosi mediante infiltrazioni intra-articolari di Hyadrol ®] Long-term results of knee osteoarthritis treated by intra-articular injections of Hyadrol®. Giot 2022, 48, 100–106. [Google Scholar] [CrossRef]
- Meissner, W.; Huygen, F.; Neugebauer, E.A.M.; Osterbrink, J.; Benhamou, D.; Betteridge, N.; Coluzzi, F.; De Andres, J.; Fawcett, W.; Fletcher, D.; et al. Management of acute pain in the postoperative setting: The importance of quality indicators. Curr. Med. Res. Opin. 2018, 34, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Ambrosio, F.; Finco, G.; Mattia, C.; Mediati, R.; Paoletti, F.; Coluzzi, F.; Piacevoli, Q.; Savoia, G.; Amantea, B.; Aurilio, C.; et al. SIAARTI recommendations for chronic noncancer pain. Minerva Anestesiol. 2006, 72, 859–880. [Google Scholar]
- Pergolizzi, J.V., Jr.; Varrassi, G.; Magnusson, P.; Breve, F.; Raffa, R.B.; Christo, P.J.; Chopra, M.; Paladini, A.; LeQuang, J.A.; Mitchell, K.; et al. Pharmacologic agents directed at the treatment of pain associated with maladaptive neuronal plasticity. Expert Opin. Pharmacother. 2022, 23, 105–116. [Google Scholar] [CrossRef]
- Morlion, B.; Coluzzi, F.; Aldington, D.; Kocot-Kepska, M.; Pergolizzi, J.; Mangas, A.C.; Ahlbeck, K.; Kalso, E. Pain chronification: What should a non-pain medicine specialist know? Curr. Med. Res. Opin. 2018, 34, 1169–1178. [Google Scholar] [CrossRef]
- Coluzzi, F.; Fornasari, D.; Pergolizzi, J.; Romualdi, P. From acute to chronic pain: Tapentadol in the progressive stages of this disease entity. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1672–1683. [Google Scholar]
| T0 | T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|---|
| Basal (before 1st infiltration) | 1st w | 2nd ws | 3rd ws | 1st w after 4th infiltration | 4th w after 4th infiltration | |
| N patients | 102 | 102 | 101 | 98 | 97 | 95 |
| Pain | 22.5 ± 0.76 | 16.8 ± 0.87 | 12.2 ± 0.81 | 9.4 ± 0.82 | 7.0 ± 0.76 | 5.2 ± 0.63 |
| Stiffness | 7.8 ± 0.49 | 6.3 ± 0.48 | 4.7 ± 0.43 | 3.6 ± 0.39 | 2.5 ± 0.33 | 1.9 ± 0.29 |
| Articular function | 67.3 ± 3.24 | 58.4 ± 3.10 | 46.3 ± 2.84 | 36.0 ± 2.83 | 28.1 ± 2.66 | 21.4 ± 2.13 |
| Total WOMAC score | 97.6 ± 4.11 | 81.5 ± 4.08 | 63.3 ± 3.78 | 49.1 ± 3.77 | 37.6 ± 3.53 | 28.5 ± 2.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guida, F.; Rocco, M.; Luongo, L.; Persiani, P.; Vulpiani, M.C.; Nusca, S.M.; Maione, S.; Coluzzi, F. Targeting Neuroinflammation in Osteoarthritis with Intra-Articular Adelmidrol. Biomolecules 2022, 12, 1453. https://doi.org/10.3390/biom12101453
Guida F, Rocco M, Luongo L, Persiani P, Vulpiani MC, Nusca SM, Maione S, Coluzzi F. Targeting Neuroinflammation in Osteoarthritis with Intra-Articular Adelmidrol. Biomolecules. 2022; 12(10):1453. https://doi.org/10.3390/biom12101453
Chicago/Turabian StyleGuida, Francesca, Monica Rocco, Livio Luongo, Pietro Persiani, Maria Chiara Vulpiani, Sveva Maria Nusca, Sabatino Maione, and Flaminia Coluzzi. 2022. "Targeting Neuroinflammation in Osteoarthritis with Intra-Articular Adelmidrol" Biomolecules 12, no. 10: 1453. https://doi.org/10.3390/biom12101453
APA StyleGuida, F., Rocco, M., Luongo, L., Persiani, P., Vulpiani, M. C., Nusca, S. M., Maione, S., & Coluzzi, F. (2022). Targeting Neuroinflammation in Osteoarthritis with Intra-Articular Adelmidrol. Biomolecules, 12(10), 1453. https://doi.org/10.3390/biom12101453









