Immunosenescence, Inflammaging, and Lung Senescence in Asthma in the Elderly
Abstract
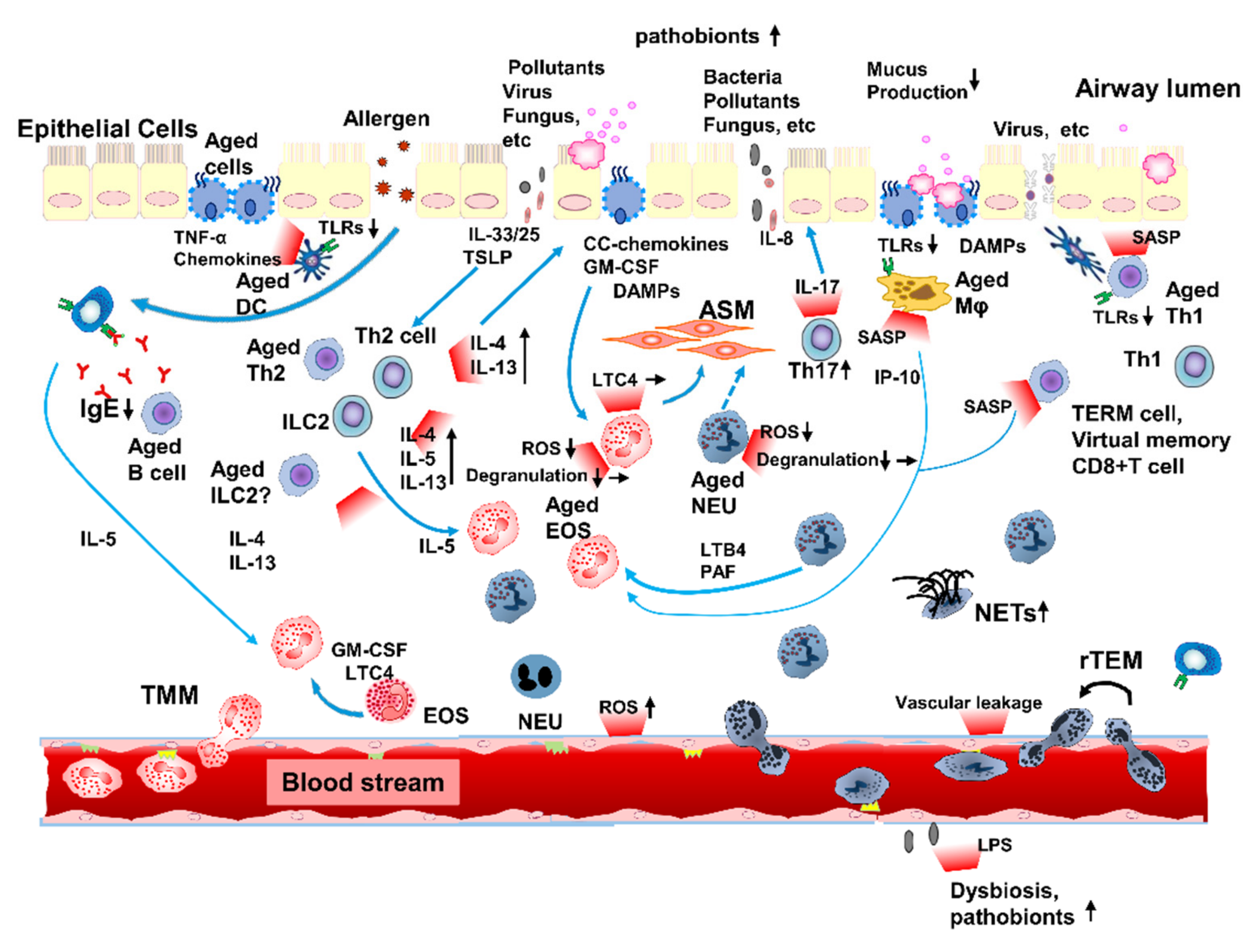
:1. Introduction
2. Alteration in the Immune System through the Lifespan
3. Innate Immune System Senescence Paradoxically Promotes Chronic Inflammation
3.1. Change in PRR with Aging
3.2. Alteration in Neutrophil Function with Advancing Age
3.3. Alteration in Eosinophil Function with Advancing Age
3.4. Alteration in DCs Function with Advancing Age
3.5. Alteration in NK Cell Function with Advancing Age
3.6. Alteration in Monocyte/Macrophage Function with Advancing Age
4. Alteration in Adaptive Immunity Associated with Advancing Age
4.1. Reduced Activeness of Thymus Affects Peripheral T Cell Population
4.2. T Cell Population Paradigm and Function Senescence
4.3. T Cell Senescence
4.4. Contoribution of T Helper 17 (TH17) Cells
4.5. Alteration in B Cell Population and Function with Advancing Age
5. Alteration of Lung Parenchyma with Aging
6. Immunosenescence and Inflammaging with Advancing Age
Initiation of Immunosenescence and Inflammaging
7. Inflammaging Contributes to Immunopathophysiology in Asthma in the Elderly
7.1. Innate Immunosenescence Can Affect Inflammaging in Elderly Patients with Asthma
7.1.1. Contribution of Neutrophils to Inflammation in Elderly Patients with Asthma
7.1.2. Contribution of Eosinophils to Inflammation in Elderly Patients with Asthma
7.1.3. Enhanced Mixed Granulocytic Inflammation in Elderly Patients with Asthma
7.2. Adaptive Immunosenescence Can Affect Inflammaging in Elderly Patients with Asthma
7.2.1. Type-2-High Inflammation in Elderly Patients with Asthma
7.2.2. Type-2-Low (Type-1) Inflammation in Elderly Patients with Asthma
7.2.3. Collaboration of Type-1 and -2 Immune System in Elderly Patients with Asthma
7.2.4. Clinical Impact of Type-1 and -2 Inflammations in Elderly Patients with Asthma
8. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Busse, P.J.; McDonald, V.M.; Wisnivesky, J.P.; Gibson, P.G. Asthma across the ages: Adults. J. Allergy Clin. Immunol. Pract. 2020, 8, 1828–1838. [Google Scholar] [CrossRef]
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Ebmeier, S.; Thayabaran, D.; Braithwaite, I.; Bénamara, C.; Weatherall, M.; Beasley, R. Trends in international asthma mortality: Analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet 2017, 390, 935–945. [Google Scholar] [CrossRef]
- Dunn, R.M.; Busse, P.J.; Wechsler, M.E. Asthma in the elderly and late-onset adult asthma. Allergy 2018, 73, 284–294. [Google Scholar] [CrossRef]
- Gibson, P.G.; McDonald, V.M.; Marks, G.B. Asthma in older adults. Lancet 2010, 376, 803–813. [Google Scholar] [CrossRef]
- Moorman, J.E.; Akinbami, L.J.; Bailey, C.M.; Zahran, H.S.; King, E.M.; Johnson, C.A.; Liu, X. National surveillance ofasthma: United States, 2001–2010. Vital Health Stat. 3 2012, 35, 1–58. [Google Scholar]
- Ichinose, M.; Sugiura, H.; Nagase, H.; Yamaguchi, M.; Inoue, H.; Sagara, H.; Tamaoki, J.; Tohda, Y.; Munakata, M.; Yamauchi, K.; et al. Japanese guidelines for adult asthma 2017. Allergol. Int. 2017, 66, 163–189. [Google Scholar] [CrossRef]
- Baptist, A.P.; Hao, W.; Karamched, K.R.; Kaur, B.; Carpenter, L.; Song, P.X.K. Distinct asthma phenotypes among older adults with asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 244–249.e2. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Song, W.J.; Kim, S.H.; Park, H.K.; Kim, S.H.; Kwon, Y.E.; Kwon, H.S.; Kim, T.B.; Chang, Y.S.; Cho, Y.S.; et al. Classification and implementation of asthma phenotypes in elderly patients. Ann. Allergy Asthma Immunol. 2015, 114, 18–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Moore, W.C.; Hastie, A.T.; Li, X.; Li, H.; Busse, W.W.; Jarjour, N.N.; Wenzel, S.E.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R.; et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J. Allergy Clin. Immunol. 2014, 133, 1557–1563.e5. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Ueki, S.; Tamari, M.; Imoto, Y.; Fujieda, S.; Taniguchi, M. Adult-onset eosinophilic airway diseases. Allergy 2020, 75, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef]
- López-Otín, C.; Kroemer, G. Hallmarks of health. Cell 2021, 84, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Wicher, S.; Khandalavala, K.; Pabelick, C.M.; Britt, R.D., Jr.; Prakash, Y.S. Cellular senescence in the lung across the age spectrum. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L826–L842. [Google Scholar] [CrossRef]
- Schneider, J.L.; Rowe, J.H.; Garcia-de-Alba, C.; Kim, C.F.; Sharpe, A.H.; Haigis, M.C. The aging lung: Physiology, disease, and immunity. Cell 2021, 184, 1990–2019. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of aging: The birth of inflammaging. Clin. Rev. Allergy Immunol. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The integration of inflammaging in age-related diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef]
- Ray, D.; Yung, R. Immune senescence, epigenetics and autoimmunity. Clin. Immunol. 2018, 196, 59–63. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Molony, R.D.; Malawista, A.; Montgomery, R.R. Reduced dynamic range of antiviral innate immune responses in aging. Exp. Gerontol. 2018, 107, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The role of immunosenescence in the development of age-related diseases. Rev. Investig. Clin. 2016, 68, 84–91. [Google Scholar]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Human T cell immunosenescence and inflammation in aging. J. Leukoc Biol. 2017, 102, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Goronzy, J.J.; Weyand, C.M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 2019, 19, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Garcia, D.; Blomberg, B.B. B cell immunosenescence. Annu. Rev. Cell Dev. Biol. 2020, 36, 551–574. [Google Scholar] [CrossRef]
- Hazeldine, J.; Lord, J.M. Innate immunesenescence: Underlying mechanisms and clinical relevance. Biogerontology 2015, 16, 187–201. [Google Scholar] [CrossRef]
- Montgomery, R.R.; Shaw, A.C. Paradoxical changes in innate immunity in aging: Recent progress and new directions. J. Leukoc. Biol. 2015, 98, 937–943. [Google Scholar] [CrossRef] [Green Version]
- Fulop, T.; Larbi, A.; Douziech, N.; Fortin, C.; Guérard, K.P.; Lesur, O.; Khalil, A.; Dupuis, G. Signal transduction and functional changes in neutrophils with aging. Aging Cell 2004, 3, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Arjona, A.; Sapey, E.; Bai, F.; Fikrig, E.; Montgomery, R.R.; Lord, J.M.; Shaw, A.C. Human innate immunosenescence: Causes and consequences for immunity in old age. Trends Immunol. 2009, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.; Tarazona, R.; Gayoso, I.; Lesur, O.; Dupuis, G.; Fulop, T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin. Immunol. 2012, 24, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Salvioli, S.; Garagnani, P.; de Eguileor, M.; Monti, D.; Capri, M. Immunobiography and the heterogeneity of immune responses in the elderly: A focus on inflammaging and trained immunity. Front. Immunol. 2017, 8, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulet, L.-P.; Robitaille, C.; Deschesnes, F.; Villeneuve, H.; Boulay, M.-E. Comparative Clinical, Physiological, and Inflammatory Characteristics of Elderly Subjects with or Without Asthma and Young Subjects with Asthma. Chest 2017, 152, 1203–1213. [Google Scholar] [CrossRef]
- De Vries, M.; Nwozor, K.O.; Muizer, K.; Wisman, M.; Timens, W.; van den Berge, M.; Faiz, A.; Hackett, T.L.; Heijink, I.H.; Brandsma, C.A. The relation between age and airway epithelial barrier function. Respir. Res. 2022, 23, 43. [Google Scholar] [CrossRef]
- Van Duin, D.; Mohanty, S.; Thomas, V.; Ginter, S.; Montgomery, R.R.; Fikrig, E.; Allore, H.G.; Medzhitov, R.; Shaw, A.C. Age-associated defect in human TLR-1/2 function. J. Immunol. 2007, 178, 970–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyugen, J.; Agrawal, S.; Gollapudi, S.; Gupta, S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 2010, 30, 806–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maeyer, R.P.H.; Chambers, E.S. The impact of ageing on monocytes and macrophages. Immunol. Lett. 2021, 230, 1–10. [Google Scholar] [CrossRef]
- Qian, F.; Guo, X.; Wang, X.; Yuan, X.; Chen, S.; Malawista, S.E.; Bockenstedt, L.K.; Allore, H.G.; Montgomery, R.R. Reduced bioenergetics and toll-like receptor 1 function in human polymorphonuclear leukocytes in aging. Aging 2014, 6, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, T.U.; Cubas, R.A.; Ghneim, K.; Cartwright, M.J.; Grevenynghe, J.V.; Richner, J.M.; Olagnier, D.P.; Wilkinson, P.A.; Cameron, M.J.; Park, B.S.; et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 2015, 14, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Crisford, H.; Sapey, E.; Stockley, R.A. Proteinase 3; a potential target in chronic obstructive pulmonary disease and other chronic inflammatory diseases. Respir. Res. 2018, 19, 180. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.L.; Harrison, R.E.; Grinstein, S. Phagocytosis by neutrophils. Microbes Infect. 2003, 5, 1299–1306. [Google Scholar] [CrossRef]
- Korkmaz, B.; Moreau, T.; Gauthier, F. Neutrophil elastase, proteinase 3 and cathepsin G: Physicochemical properties, activity and physiopathological functions. Biochimie 2008, 90, 227–242. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.W.; Liu, G.Y. Expanding roles of neutrophils in aging hosts. Curr. Opin. Immunol. 2014, 29, 43–48. [Google Scholar] [CrossRef]
- Butcher, S.K.; Chahal, H.; Nayak, L.; Sinclair, A.; Henriquez, N.V.; Sapey, E.; O’Mahony, D.; Lord, J.M. Senescence in innate immune responses: Reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J. Leukoc. Biol. 2001, 70, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Fouquet, C.; Allaire, P.; Perrin, N.; Lacombe, G.; Stankova, J.; Rola-Pleszczynski, M.; Gagné, D.; Wagner, J.R.; Khalil, A.; et al. Changes in apoptosis of human polymorphonuclear granulocytes with aging. Mech. Ageing Dev. 1997, 96, 15–34. [Google Scholar] [CrossRef]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes other than the gut: Inflammaging and age-related diseases. Semin. Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef]
- Rylance, J.; Kankwatira, A.; Nelson, D.E.; Toh, E.; Day, R.B.; Lin, H.; Gao, X.; Dong, Q.; Sodergren, E.; Weinstock, G.M.; et al. Household air pollution and the lung microbiome of healthy adults in Malawi: A cross-sectional study. BMC Microbiol. 2016, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Boyton, R.J.; Reynolds, C.J.; Quigley, K.J.; Altmann, D.M. Immune mechanisms and the impact of the disrupted lung microbiome in chronic bacterial lung infection and bronchiectasis. Clin. Exp. Immunol. 2013, 171, 117–123. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mac Aogáin, M.; Fam, K.D.; Chia, K.L.; Binte Mohamed Ali, N.A.B.M.; Yap, M.M.C.; Yap, E.P.H.; Chotirmall, S.H.; Lim, C.L. Airway microbiome composition correlates with lung function and arterial stiffness in an age-dependent manner. PLoS ONE 2019, 14, e0225636. [Google Scholar] [CrossRef] [Green Version]
- Nyenhuis, S.M.; Schwantes, E.A.; Mathur, S.K. Characterization of leukotrienes in a pilot study of older asthma subjects. Immun. Ageing 2010, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Biasi, D.; Carletto, A.; Dell’Agnola, C.; Caramaschi, P.; Montesanti, F.; Zavateri, G.; Zeminian, S.; Bellavite, P.; Bambara, L.M. Neutrophil migration, oxidative metabolism, and adhesion in elderly and young subjects. Inflammation 1996, 20, 673–681. [Google Scholar] [CrossRef]
- Barkaway, A.; Rolas, L.; Joulia, R.; Bodkin, J.; Lenn, T.; Owen-Woods, C.; Reglero-Real, N.; Stein, M.; Vázquez-Martínez, L.; Girbl, T.; et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity 2021, 54, 1494–1510.e7. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Gonzalez, N.A.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; Weber, C.; et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Chen, G.; Manwani, D.; Mortha, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.E.; Scheiermann, C.; et al. Neutrophil ageing is regulated by the microbiome. Nature 2015, 525, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.K.; Schwantes, E.A.; Jarjour, N.N.; Busse, W.W. Age-related changes in eosinophil function in human subjects. Chest 2008, 133, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Soma, T.; Uchida, Y.; Hoshino, Y.; Katayama, K.; Kobayashi, T.; Nakagome, K.; Nagata, M. Relationship between airway inflammation and airflow limitation in elderly asthmatics. Asia Pac. Allergy 2020, 10, e17. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Gupta, S. Impact of aging on dendritic cell functions in humans. Ageing Res. Rev. 2011, 10, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Agrawal, S.; Vahed, H.; Ngyuen, M.; BenMohamed, L.; Gupta, S.; Agrawal, A. Dendritic cells from aged subjects contribute to chronic airway inflammation by activating bronchial epithelial cells under steady state. Mucosal. Immunol. 2014, 7, 1386–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauning, A.; Rae, M.; Zhu, G.; Fulton, E.; Admasu, T.D.; Stolzing, A.; Sharma, A. Aging of the immune system: Focus on natural killer cells phenotype and functions. Cells 2022, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the dysfunctional NK cell: Assessing the clinical relevance of exhaustion, anergy, and senescence. Front. Cell Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Kong, K.F.; Delroux, K.; Wang, X.M.; Qian, F.; Arjona, A.; Malawista, S.E.; Fikrig, E.; Montgomery, R.R. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J. Virol. 2008, 82, 7613–7623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.k.; Smith, C.A.; Sakamoto, K.; Kaminski, N.; Koff, J.L.; Goldstein, D.R. Aging Impairs Alveolar Macrophage Phagocytosis and Increases Influenza-Induced Mortality in Mice. J. Immunol. 2017, 199, 1060–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canan, C.H.; Gokhale, N.S.; Carruthers, B.; Lafuse, W.P.; Schlesinger, L.S.; Torrelles, J.B.; Turner, J. Characterization of lung inflammation and its impact on macrophage function in aging. J. Leukoc. Biol. 2014, 96, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Plataki, M.; Cho, S.J.; Harris, R.M.; Huang, H.R.; Yun, H.S.; Schiffer, K.T.; Stout-Delgado, H.W. Mitochondrial Dysfunction in Aged Macrophages and Lung during Primary Streptococcus pneumoniae Infection is Improved with Pirfenidone. Sci. Rep. 2019, 9, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chougnet, C.A.; Thacker, R.I.; Shehata, H.M.; Hennies, C.M.; Lehn, M.A.; Lages, C.S.; Janssen, E.M. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J. Immunol. 2015, 195, 2624–2632. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, G.; Lee, W.W.; Yuan, M.; Cui, D.; Weyand, C.M.; Goronzy, J.J. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc. Natl. Acad. Sci. USA 2012, 109, E879–E888. [Google Scholar] [CrossRef] [Green Version]
- Yager, E.J.; Ahmed, M.; Lanzer, K.; Randall, T.D.; Woodland, D.L.; Blackman, M.A. Ageassociated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 2008, 205, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.; Li, G.; Vallejo, A.N.; Lee, W.W.; Koetz, K.; Bryl, E.; Witkowski, J.; Fulbright, J.; Weyand, C.M.; Goronzy, J.J. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005, 174, 7446–7452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon, B.; Yates, A.J. The natural history of naïve T cells from birth to maturity. Immunol. Rev. 2018, 285, 218–232. [Google Scholar] [CrossRef]
- Busse, P.J.; Mathur, S.K. Age-related changes in immune function: Effect on airway inflammation. J. Allergy Clin. Immunol. 2010, 126, 690–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elyahu, Y.; Hekselman, I.; Eizenberg-Magar, I.; Berner, O.; Strominger, I.; Schiller, M.; Mittal, K.; Nemirovsky, A.; Eremenko, E.; Vital, A.; et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci. Adv. 2019, 5, eaaw8330. [Google Scholar] [CrossRef] [Green Version]
- Ucar, D.; Márquez, E.J.; Chung, C.H.; Marches, R.; Rossi, R.J.; Uyar, A.; Wu, T.C.; George, J.; Stitzel, M.L.; Palucka, A.K.; et al. The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J. Exp. Med. 2017, 214, 3123–3144. [Google Scholar] [CrossRef]
- Thome, J.J.; Grinshpun, B.; Kumar, B.V.; Kubota, M.; Ohmura, Y.; Lerner, H.; Sempowski, G.D.; Shen, Y.; Farber, D.L. Long term maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Sci. Immunol. 2016, 1, eaah6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, C.E.; Cavanagh, M.M.; Jin, J.; Weyand, C.M.; Goronzy, J.J. Functional pathways regulated by microRNA networks in CD8 T cell aging. Aging Cell 2019, 18, e12879. [Google Scholar] [CrossRef]
- Huang, H.; Sikora, M.J.; Islam, S.; Chowdhury, R.R.; Chien, Y.H.; Scriba, T.J.; Davis, M.M.; Steinmetz, L.M. Select sequencing of clonally expanded CD8+ T cells reveals limits to clonal expansion. Proc. Natl. Acad. Sci. USA 2019, 116, 8995–9001. [Google Scholar] [CrossRef] [Green Version]
- Akbar, A.N.; Henson, S.M.; Lanna, A. Senescence of T lymphocytes: Implications for enhancing human immunity. Trends Immunol. 2016, 37, 866–876. [Google Scholar] [CrossRef]
- Akbar, A.N.; Henson, S.M. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011, 11, 289–295. [Google Scholar] [CrossRef]
- Kim, C.; Hu, B.; Jadhav, R.R.; Jin, J.; Zhang, H.; Cavanagh, M.M.; Akondy, R.S.; Ahmed, R.; Weyand, C.M.; Goronzy, J.J. Activation of miR-21-regulated pathways in immune aging selects against signatures characteristic of memory T cells. Cell Rep. 2018, 25, 2148–2162. [Google Scholar] [CrossRef] [Green Version]
- White, J.T.; Cross, E.W.; Burchill, M.A.; Danhorn, T.; McCarter, M.D.; Rosen, H.R.; O’Connor, B.; Kedl, R.M. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat. Commun. 2016, 7, 11291. [Google Scholar] [CrossRef] [Green Version]
- Quinn, K.M.; Fox, A.; Harland, K.L.; Russ, B.E.; Li, J.; Nguyen, T.H.O.; Loh, L.; Olshanksy, M.; Naeem, H.; Tsyganov, K.; et al. Age-related decline in primary CD8+ T cell responses is associated with the development of senescence in virtual memory CD8+ T cells. Cell Rep. 2018, 23, 3512–3524. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; An, J.; Weng, N.P. Telomerase is involved in IL-7-mediated differential survival of naive and memory CD4+ T cells. J. Immunol. 2008, 180, 3775–3781. [Google Scholar] [CrossRef] [Green Version]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage response activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, V.; Rink, L.; Uciechowski, P. The Th17/Treg balance is disturbed during aging. Exp. Gerontol. 2013, 48, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Jagger, A.; Shimojima, Y.; Goronzy, J.J.; Weyand, C.M. Regulatory T cells and the immune aging process: A mini-review. Gerontology 2014, 60, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Dominguez, A.L.; Lustgarten, J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J. Immunol. 2006, 177, 8348–8355. [Google Scholar] [CrossRef] [Green Version]
- Lages, C.S.; Suffia, I.; Velilla, P.A.; Huang, B.; Warshaw, G.; Hildeman, D.A.; Belkaid, Y.; Chougnet, C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J. Immunol. 2008, 181, 1835–1848. [Google Scholar] [CrossRef]
- Busse, P.J.; Birmingham, J.M.; Calatroni, A.; Manzi, J.; Goryachokovsky, A.; Fontela, G.; Federman, A.D.; Wisnivesky, J.P. Effect of aging on sputum inflammation and asthma control. J. Allergy Clin. Immunol. 2017, 139, 1808–1818.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molet, S.; Hamid, Q.; Davoine, F.; Nutku, E.; Taha, R.; Pagé, N.; Olivenstein, R.; Elias, J.; Chakir, J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001, 108, 430–438. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B.; Garcia, D.; Keilich, S.R.; Haynes, L. Age-related factors that affect B cell responses to vaccination in mice and humans. Immunol. Rev. 2020, 296, 142–154. [Google Scholar] [CrossRef]
- Claes, N.; Fraussen, J.; Vanheusden, M.; Hellings, N.; Stinissen, P.; Van Wijmeersch, B.; Hupperts, R.; Somers, V. Age-associated B cells with proinflammatory characteristics are expanded in a proportion of multiple sclerosis patients. J. Immunol. 2016, 197, 4576–4583. [Google Scholar] [CrossRef] [Green Version]
- Illingworth, J.; Butler, N.S.; Roetynck, S.; Mwacharo, J.; Pierce, S.K.; Bejon, P.; Crompton, P.D.; Marsh, K.; Ndungu, F.M. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J. Immunol. 2013, 190, 1038–1047. [Google Scholar] [CrossRef] [Green Version]
- Murasko, D.M.; Bernstein, E.D.; Gardner, E.M.; Gross, P.; Munk, G.; Dran, S.; Abrutyn, E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp. Gerontol 2002, 37, 427–439. [Google Scholar] [CrossRef]
- Pritz, T.; Lair, J.; Ban, M.; Keller, M.; Weinberger, B.; Krismer, M.; Grubeck-Loebenstein, B. Plasma cell numbers decrease in bone marrow of old patients. Eur. J. Immunol. 2015, 45, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.N. Affinity enhancement of antibodies: How low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol. Res. 2014, 2, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.; Kelsoe, G. Ig V-H hypermutation is absent in the germinal-centers of aged mice. J. Immunol. 1995, 155, 3377–3384. [Google Scholar]
- Frasca, D.; Diaz, A.; Romero, M.; Blomberg, B.B. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp. Gerontol. 2017, 87, 113–120. [Google Scholar] [CrossRef]
- Brandenberger, C.; Mühlfeld, C. Mechanisms of lung aging. Cell Tissue Res. 2017, 367, 469–480. [Google Scholar] [CrossRef]
- Janssens, J.P.; Pache, J.C.; Nicod, L.P. Physiological changes in respiratory function associated with ageing. Eur. Respir. J. 1999, 13, 197–205. [Google Scholar] [CrossRef]
- McClaran, S.R.; Babcock, M.A.; Pegelow, D.F.; Reddan, W.G.; Dempsey, J.A. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. J. Appl. Physiol. 1995, 78, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R. Structural and physiological age-associated changes in aging lungs. Semin Respir. Crit. Care Med. 2010, 31, 521–527. [Google Scholar] [CrossRef]
- Quirk, J.D.; Sukstanskii, A.L.; Woods, J.C.; Lutey, B.A.; Conradi, M.S.; Gierada, D.S.; Yusen, R.D.; Castro, M.; Yablonskiy, D.A. Experimental evidence of age-related adaptive changes in human acinar airways. J. Appl. Physiol. 2016, 120, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, H.; Mühlfeld, C.; Brandenberger, C. Age-related structural and functional changes in the mouse lung. Front. Physiol. 2019, 10, 1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, J.; Woldhuis, R.R.; Boudewijn, I.M.; van den Berg, A.; Kluiver, J.; Kok, K.; Terpstra, M.M.; Guryev, V.; de Vries, M.; Vermeulen, C.J.; et al. Age-related gene and miRNA expression changes in airways of healthy individuals. Sci. Rep. 2019, 9, 3765. [Google Scholar] [CrossRef] [Green Version]
- Angelidis, I.; Simon, L.M.; Fernandez, I.E.; Strunz, M.; Mayr, C.H.; Greiffo, F.R.; Tsitsiridis, G.; Ansari, M.; Graf, E.; Strom, T.M.; et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 2019, 10, 963. [Google Scholar] [CrossRef] [Green Version]
- Chilosi, M.; Carloni, A.; Rossi, A.; Poletti, V. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl. Res. 2013, 162, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Svartengren, M.; Falk, R.; Philipson, K. Long-term clearance from small airways decreases with age. Eur. Respir. J. 2005, 26, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, K.L.; Bonasera, S.J.; Wilderdyke, M.; Hanisch, B.W.; Pavlik, J.A.; DeVasure, J.; Robinson, J.E.; Sisson, J.H.; Wyatt, T.A. Aging causes a slowing in ciliary beat frequency, mediated by PKCε. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L584–L589. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martínez, M.; Rodríguez-Flores, L.E.; Ancer-Arellano, A.; Cerda-Flores, R.M.; de-la-Garza-González, C.; Ancer-Rodríguez, J.; Jaramillo-Rangel, G. Analysis of cell turnover in the bronchiolar epithelium through the normal aging process. Lung 2016, 194, 581–587. [Google Scholar] [CrossRef]
- Watson, J.K.; Sanders, P.; Dunmore, R.; Rosignoli, G.; Julé, Y.; Rawlins, E.L.; Mustelin, T.; May, R.; Clarke, D.; Finch, D.K. Distal lung epithelial progenitor cell function declines with age. Sci. Rep. 2020, 10, 10490. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, F.; Wang, R.A.; Wang, J.; Zhao, J.; Yang, M.; Gong, W.; Cui, R.; Dong, L. Central role of cellular senescence in TSLP-induced airway remodeling in asthma. PLoS ONE 2013, 8, e77795. [Google Scholar] [CrossRef] [Green Version]
- Alder, J.K.; Barkauskas, C.E.; Limjunyawong, N.; Stanley, S.E.; Kembou, F.; Tuder, R.M.; Hogan, B.L.; Mitzner, W.; Armanios, M. Telomere dysfunction causes alveolar stem cell failure. Proc. Natl. Acad. Sci. USA 2015, 112, 5099–5104. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhang, K.; Chen, H.; Zhao, X.; Wang, J.; Li, L.; Cong, Y.; Ju, Z.; Xu, D.; Williams, B.R.; et al. Telomerase deficiency causes alveolar stem cell senescence-associated low-grade inflammation in lungs. J. Biol. Chem. 2015, 290, 30813–30829. [Google Scholar] [CrossRef] [Green Version]
- Kotton, D.N.; Morrisey, E.E. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014, 20, 822–832. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Rabold, R.; Schofield, B.; Mitzner, W.; Tankersley, C.G. Age-dependent changes of airway and lung parenchyma in C57BL/6J mice. J. Appl. Physiol. 2007, 102, 200–206. [Google Scholar] [CrossRef]
- Yang, S.R.; Park, J.R.; Kang, K.S. Reactive oxygen species in mesenchymal stem cell aging: Implication to lung diseases. Oxid. Med. Cell Longev. 2015, 2015, 486263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. an evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Busse, P.J.; Zhang, T.F.; Srivastava, K.; Schofield, B.; Li, X.M. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin. Exp. Allergy 2007, 37, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.A.; Jeong, J.J.; Yoo, S.Y.; Kim, D.H. Gut microbiota lipopolysaccharide accelerates inflammaging in mice. BMC Microbiol. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.J.; Nariya, S.; Harris, J.M.; Lynch, S.V.; Choy, D.F.; Arron, J.R.; Boushey, H. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J. Allergy Clin. Immunol. 2015, 136, 874–884. [Google Scholar] [CrossRef] [Green Version]
- Calmes, D.; Huynen, P.; Paulus, V.; Henket, M.; Guissard, F.; Moermans, C.; Louis, R.; Schleich, F. Chronic infection with Chlamydia pneumoniae in asthma: A type-2 low infection related phenotype. Respir. Res. 2021, 22, 72. [Google Scholar] [CrossRef]
- Crisford, H.; Sapey, E.; Rogers, G.B.; Taylor, S.; Nagakumar, P.; Lokwani, R.; Simpson, J.L. Neutrophils in asthma: The good, the bad and the bacteria. Thorax 2021, 76, 835–844. [Google Scholar] [CrossRef]
- Sverrild, A.; Kiilerich, P.; Brejnrod, A.; Pedersen, R.; Porsbjerg, C.; Bergqvist, A.; Erjefält, J.S.; Kristiansen, K.; Backer, V.J. Eosinophilic airway inflammation in asthmatic patients is associated with an altered airway microbiome. J. Allergy Clin. Immunol. 2017, 140, 407–417.e11. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.I.; Vijverberg, S.J.H.; Neerincx, A.H.; Kraneveld, A.D.; Maitland-van der Zee, A.H. The crosstalk between microbiome and asthma: Exploring associations and challenges. Clin. Exp. Allergy 2019, 49, 1067–1086. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Qiu, R.; Yang, Z.; Li, J.; Chung, K.F.; Zhong, N.; Zhang, Q. Sputum microbiota in severe asthma patients: Relationship to eosinophilic inflammation. Respir. Med. 2017, 131, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Zamudio, R.I.; Robinson, L.; Roux, P.F.; Bischof, O. Snap Shot: Cellular senescence pathways. Cell 2017, 170, 816. [Google Scholar] [CrossRef]
- Azim, A.; Green, B.; Lau, L.; Rupani, H.; Jayasekera, N.; Bruce, K.; Howarth, P. Peripheral airways type 2 inflammation, neutrophilia and microbial dysbiosis in severe asthma. Allergy 2021, 76, 2070–2078. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouzaki, H.; Iijima, K.; Kobayashi, T.; O’Grady, S.M.; Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 2011, 186, 4375–4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef] [Green Version]
- Idzko, M.; Hammad, H.; Van Nimwegen, M.; Kool, M.; Willart, M.A.; Muskens, F.; Hoogsteden, H.C.; Luttmann, W.; Ferrari, D.; Di Virgilio, F.; et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 2007, 13, 913–919. [Google Scholar] [CrossRef]
- Kobayashi, T.; Soma, T.; Noguchi, T.; Nakagome, K.; Nakamoto, H.; Kita, H.; Nagata, M. ATP drives eosinophil effector responses through P2 purinergic receptors. Allergol. Int. 2015, 64, S30–S36. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Nakagome, K.; Noguchi, T.; Kobayashi, K.; Ueda, Y.; Soma, T.; Ikebuchi, K.; Nakamoto, H.; Nagata, M. Elevated uric acid and adenosine triphosphate concentrations in bronchoalveolar lavage fluid of eosinophilic pneumonia. Allergol. Int. 2017, 66S, S27–S34. [Google Scholar] [CrossRef]
- Davalos, A.R.; Kawahara, M.; Malhotra, G.K.; Schaum, N.; Huang, J.; Ved, U.; Beausejour, C.M.; Coppe, J.P.; Rodier, F.; Campisi, J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013, 201, 613–629. [Google Scholar] [CrossRef]
- Youm, Y.H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013, 18, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Summer, R.; Shaghaghi, H.; Schriner, D.; Roque, W.; Sales, D.; Cuevas-Mora, K.; Desai, V.; Bhushan, A.; Ramirez, M.I.; Romero, F. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L1049–L1060. [Google Scholar] [CrossRef]
- Calado, R.T.; Young, N.S. Telomere diseases. N. Engl. J. Med. 2009, 361, 2353–2365. [Google Scholar] [CrossRef]
- Moon, J.S.; Goeminne, L.J.E.; Kim, J.T.; Tian, J.W.; Kim, S.-H.; Nga, H.T.; Kang, S.G.; Kang, B.E.; Byun, J.-S.; Lee, Y.S.; et al. Growth differentiation factor 15 protects against the aging-mediated systemic inflammatory response in humans and mice. Aging Cell 2020, 19, e13195. [Google Scholar] [CrossRef]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Landi, F.; Bernabei, R.; Marzetti, E. Fueling inflamm-aging through mitochondrial dysfunction: Mechanisms and molecular targets. Int. J. Mol. Sci. 2017, 18, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vital, M.; Harkema, J.R.; Rizzo, M.; Tiedje, J.; Brandenberger, C. Alterations of the Murine gut microbiome with age and allergic airway disease. J. Immunol. Res. 2015, 2015, 892568. [Google Scholar] [CrossRef] [Green Version]
- Schleich, F.N.; Graff, S.; Guissard, F.; Henket, M.; Paulus, V.; Louis, R. Asthma in elderly is characterized by increased sputum neutrophils, lower airway caliber variability and air trapping. Respir. Res. 2021, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Good, J.; Rollins, D.; Verma, M.; Chu, H.; Pham, T.H.; Martin, R.J. Airway and serum biochemical correlates of refractory neutrophilic asthma. J. Allergy Clin. Immunol. 2017, 140, 1004–1014.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, C.R.; Gibson, P.G.; Douwes, J.; Van Dalen, C.J.; Simpson, J.L. Relationship between airway neutrophilia and ageing in asthmatics and non-asthmatics. Respirology 2013, 18, 857–865. [Google Scholar] [CrossRef]
- Birmingham, J.M.; Patil, S.; Li, X.M.; Busse, P.J. The effect of oral tolerance on the allergic airway response in younger and aged mice. J. Asthma 2013, 50, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Busse, P.J.; Lurslurchachai, L.; Sampson, H.A.; Halm, E.A.; Wisnivesky, J. Perennial allergen-specific immunoglobulin E levels among inner-city elderly asthmatics. J. Asthma 2010, 47, 781–785. [Google Scholar] [CrossRef]
- Teague, W.G.; Phillips, B.R.; Fahy, J.V.; Wenzel, S.E.; Fitzpatrick, A.M.; Moore, W.C.; Hastie, A.T.; Bleecker, E.R.; Meyers, D.A.; Peters, S.P.; et al. Baseline features of the severe asthma research program (SARP III) cohort: Differences with age. J. Allergy Clin. Immunol. Pract. 2018, 6, 545–554.e4. [Google Scholar] [CrossRef]
- Simpson, J.L.; Scott, R.; Boyle, M.J.; Gibson, P.G. Inflammatory subtypes in asthma: Assessment and identification using induced sputum. Res.pirology 2006, 11, 54–61. [Google Scholar] [CrossRef]
- Tanaka, A.; Sato, H.; Akimoto, K.; Matsunaga, T.; Sagara, H. Spontaneous sputum discriminates inflammatory phenotypes in patients with asthma. Ann. Allergy Asthma Immunol. 2021, 126, 54–60. [Google Scholar] [CrossRef]
- Kikuchi, S.; Nagata, M.; Kikuchi, I.; Hagiwara, K.; Kanazawa, M. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. Int. Arch. Allergy Immunol. 2005, 137 (Suppl. 1), 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hinks, T.S.C.; Brown, T.; Lau, L.C.K.; Rupani, H.; Barber, C.; Elliott, S.; Ward, J.A.; Ono, J.; Ohta, S.; Izuhara, K.; et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J. Allergy Clin. Immunol. 2016, 138, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, I.; Kikuchi, S.; Kobayashi, T.; Hagiwara, K.; Sakamoto, Y.; Kanazawa, M.; Nagata, M. Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. Am. J. Respir. Cell Mol. Biol. 2006, 34, 760–765. [Google Scholar] [CrossRef]
- Nishihara, F.; Nakagome, K.; Kobayashi, T.; Noguchi, T.; Araki, R.; Uchida, Y.; Soma, T.; Nagata, M. Trans-basement membrane migration of eosinophils induced by LPS-stimulated neutrophils from human peripheral blood in vitro. ERJ. Open Res. 2015, 1, 00003–02015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenberger, C.; Li, N.; Jackson-Humbles, D.N.; Rockwell, C.E.; Wagner, J.G.; Harkema, J.R. Enhanced allergic airway disease in old mice is associated with a Th17 response. Clin. Exp. Allergy 2014, 44, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Vale-Pereira, S.; Todo-Bom, A.; Geraldes, L.; Schmidt-Weber, C.; Akdis, C.A.; Mota-Pinto, A. FoxP3, GATA-3 and T-bet expression in elderly asthma. Clin. Exp. Allergy 2011, 41, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Kerr, S.; Dunican, E.M.; Woodruff, P.G.; Fajt, M.L.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J. Allergy Clin. Immunol. 2019, 143, 104–113.e14. [Google Scholar] [CrossRef]
- Peters, M.C.; Ringel, L.; Dyjack, N.; Herrin, R.; Woodruff, P.G.; Rios, C.; O’Connor, B.; Fahy, J.V.; Seibold, M.A. A transcriptomic method to determine airway immune dysfunction in T2-high and T2-low asthma. Am. J. Respir. Crit Care Med. 2019, 199, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.L.; Wardzynska, A.; Studzinska, M.; Pawelczyk, M.; Lesnikowski, Z.J.; Paradowska, E. Cytomegalovirus DNA is highly prevalent in the blood of patients with asthma and is associated with age and asthma traits. Allergy 2017, 72, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Kinyanjui, M.W.; Shan, J.; Nakada, E.M.; Qureshi, S.T.; Fixman, E.D. Dose-dependent effects of IL-17 on IL-13-induced airway inflammatory responses and airway hyperresponsiveness. J. Immunol. 2013, 190, 3859–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, M.; Melton, A.C.; Chen, C.; Engler, M.B.; Huang, K.E.; Ren, X.; Wang, Y.; Bernstein, X.; Li, J.T.; Atabai, K.; et al. IL-17A produced by ab T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 2012, 18, 547–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Thai, P.; Zhao, Y.H.; Ho, Y.S.; DeSouza, M.M.; Wu, R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem 2003, 278, 17036–17043. [Google Scholar] [CrossRef] [Green Version]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Sorbello, V.; Folino, A.; Gallo, F.; Massaglia, G.M.; Favatà, G.; Conticello, S.; Vallese, D.; Gani, F.; Malerba, M.; et al. Identification of IL-17F/frequent exacerbator endotype in asthma. J. Allergy Clin. Immunol. 2017, 140, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Takaku, Y.; Soma, T.; Uchida, Y.; Kobayashi, T.; Nakagome, K.; Nagata, M. CXC chemokine superfamily induced by interferon-γ in asthma: A cross-sectional observational study. Asthma Res. Pract. 2016, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takaku, Y.; Nakagome, K.; Kobayashi, T.; Hagiwara, K.; Kanazawa, M.; Nagata, M. IFN-γ-inducible protein of 10 kDa upregulates the effector functions of eosinophils through β2 integrin and CXCR3. Respir. Res. 2011, 12, 138. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Takaku, Y.; Yokote, A.; Miyazawa, H.; Soma, T.; Hagiwara, K.; Kanazawa, M.; Nagata, M. Interferon-beta augments eosinophil adhesion-inducing activity of endothelial cells. Eur. Respir. J. 2008, 32, 1540–1547. [Google Scholar] [CrossRef]
- Hastie, A.T.; Mauger, D.T.; Denlinger, L.C.; Coverstone, A.; Castro, M.; Erzurum, S.; Jarjour, N.; Levy, B.D.; Meyers, D.A.; Moore, W.C.; et al. Mixed sputum granulocyte longitudinal impact on lung function in the severe asthma research program. Am. J. Respir. Crit. Care Med. 2021, 203, 882–892. [Google Scholar] [CrossRef]
- Abdo, M.; Pedersen, F.; Kirsten, A.M.; Veith, V.; Biller, H.; Trinkmann, F.; von Mutius, E.; Kopp, M.; Hansen, G.; Rabe, K.F.; et al. Longitudinal Impact of sputum inflammatory Phenotypes on Small Airway Dysfunction and disease Outcomes in Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 1545–1553.e2. [Google Scholar] [CrossRef]
- Schleich, F.N.; Manise, M.; Sele, J.; Henket, M.; Seidel, L.; Louis, R. Distribution of sputum cellular phenotype in a large asthma cohort: Predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm. Med. 2013, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Hastie, A.T.; Mauger, D.T.; Denlinger, L.C.; Coverstone, A.; Castro, M.; Erzurum, S.; Jarjour, N.; Levy, B.D.; Meyers, D.A.; Moore, W.C.; et al. NHLBI SARP 3 investigators. Baseline sputum eosinophil + neutrophil subgroups’ clinical characteristics and longitudinal trajectories for NHLBI Severe Asthma Research Program (SARP 3) cohort. J. Allergy Clin. Immunol. 2020, 146, 222–226. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soma, T.; Nagata, M. Immunosenescence, Inflammaging, and Lung Senescence in Asthma in the Elderly. Biomolecules 2022, 12, 1456. https://doi.org/10.3390/biom12101456
Soma T, Nagata M. Immunosenescence, Inflammaging, and Lung Senescence in Asthma in the Elderly. Biomolecules. 2022; 12(10):1456. https://doi.org/10.3390/biom12101456
Chicago/Turabian StyleSoma, Tomoyuki, and Makoto Nagata. 2022. "Immunosenescence, Inflammaging, and Lung Senescence in Asthma in the Elderly" Biomolecules 12, no. 10: 1456. https://doi.org/10.3390/biom12101456
APA StyleSoma, T., & Nagata, M. (2022). Immunosenescence, Inflammaging, and Lung Senescence in Asthma in the Elderly. Biomolecules, 12(10), 1456. https://doi.org/10.3390/biom12101456




