Stereoselectivity in the Membrane Transport of Phenylethylamine Derivatives by Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3
Abstract
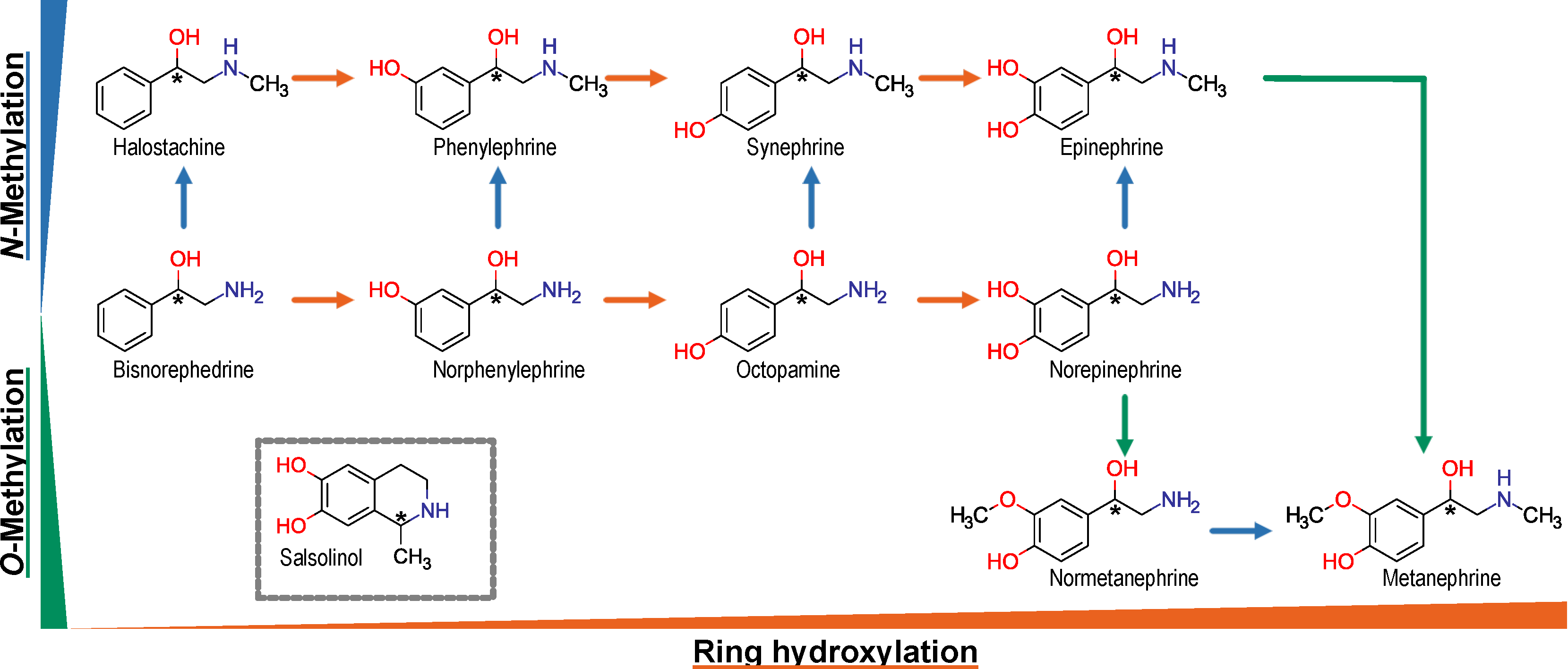
1. Introduction
2. Materials and Methods
2.1. Test Compounds
2.2. In-Vitro Transport Experiments
2.3. Stereoselective Concentration Analyses
2.4. Calculations
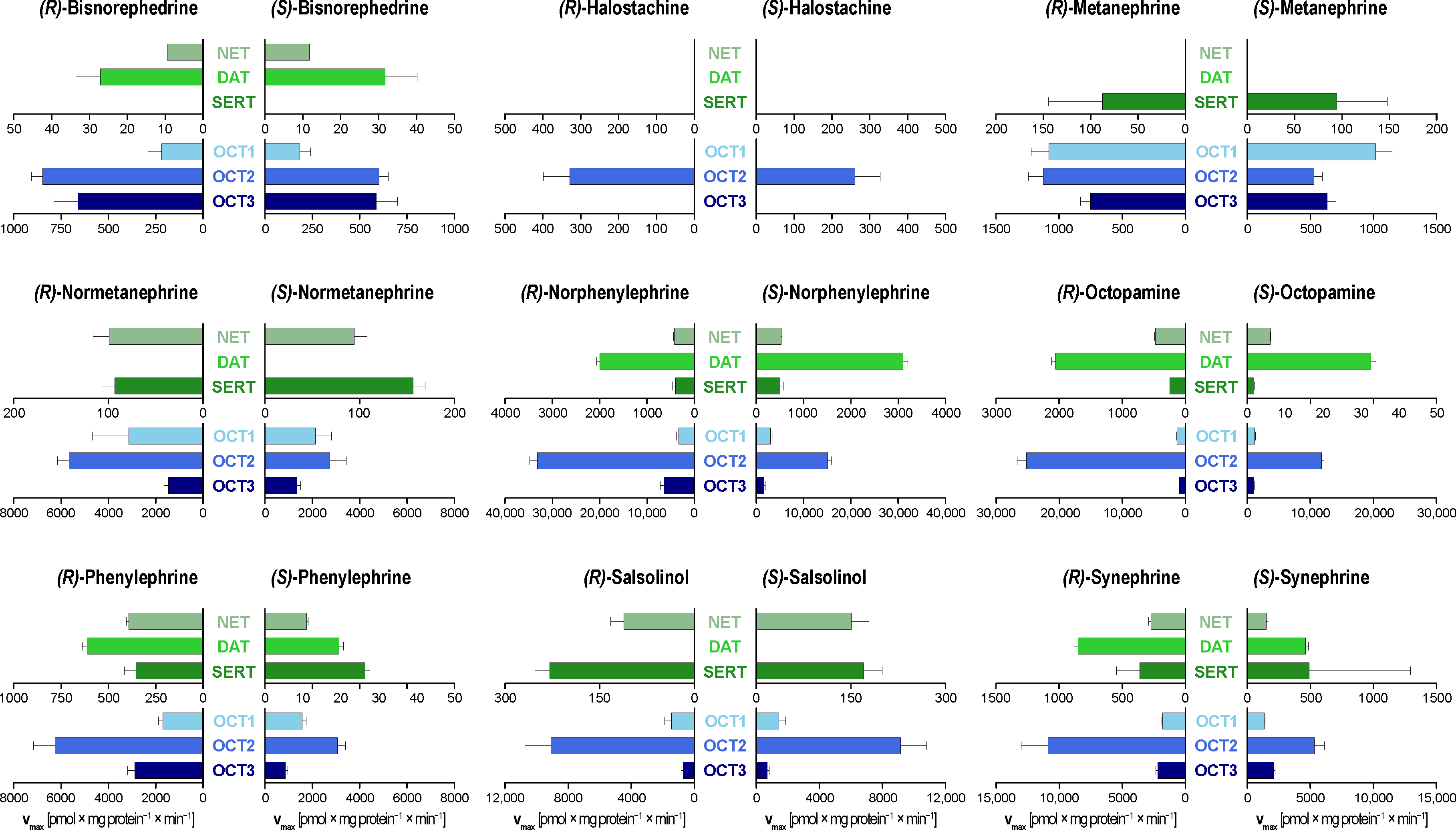
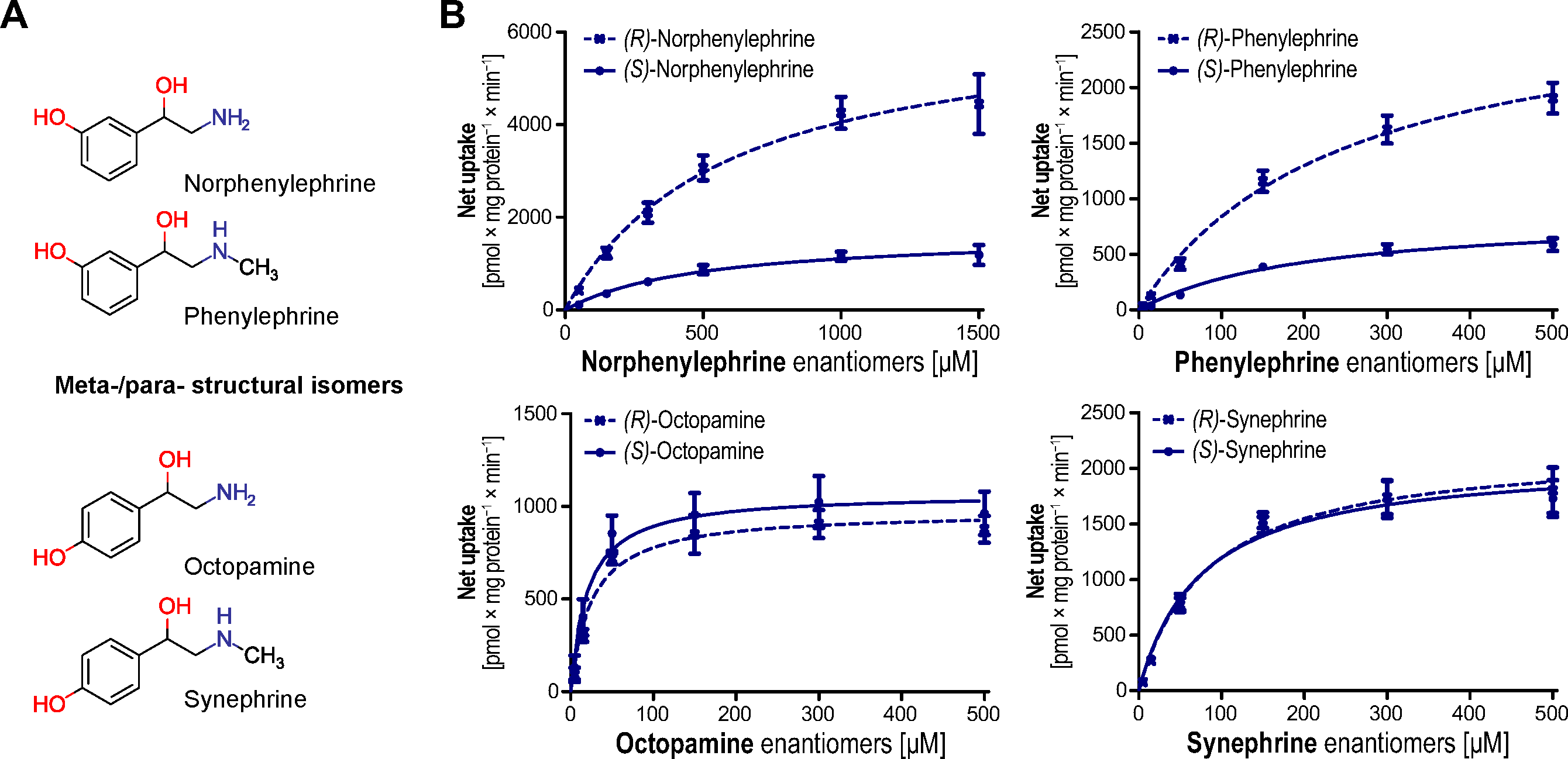
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libersat, F.; Pflueger, H.-J. Monoamines and the Orchestration of Behavior. BioScience 2004, 54, 17–25. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.A. Letter: Amines and theories in psychiatry. Lancet 1974, 2, 52–53. [Google Scholar] [CrossRef]
- Small, K.M.; McGraw, D.W.; Liggett, S.B. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 381–411. [Google Scholar] [CrossRef]
- Hernandez, M.A.; Rathinavelu, A. Basic Pharmacology: Understanding Drug Actions and Reactions; Routledge: London, UK, 2017. [Google Scholar]
- Danielson, T.J.; Boulton, A.A.; Robertson, H.A. m-Octopamine, p-octopamine and phenylethanolamine in rat brain: A sensitive, specific assay and the effects of some drugs. J. Neurochem. 1977, 29, 1131–1135. [Google Scholar] [CrossRef]
- Boulton, A.A.; Wu, P.H. Biosynthesis of cerebral phenolic amines. I. In vivo formation of p-tyramine, octopamine, and synephrine. Can. J. Biochem. 1972, 50, 261–267. [Google Scholar] [CrossRef]
- Boulton, A.A.; Wu, P.H. Biosynthesis of cerebral phenolic amines. II. In vivo regional formation of p-tyramine and octopamine from tyrosine and dopamine. Can. J. Biochem. 1973, 51, 428–435. [Google Scholar] [CrossRef]
- Lamouroux, A.; Vigny, A.; Faucon Biguet, N.; Darmon, M.C.; Franck, R.; Henry, J.P.; Mallet, J. The primary structure of human dopamine-beta-hydroxylase: Insights into the relationship between the soluble and the membrane-bound forms of the enzyme. EMBO J. 1987, 6, 3931–3937. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Dostert, P.; Kohda, K.; Kaiya, T. A novel enzyme enantio-selectively synthesizes (R)-salsolinol, a precursor of a dopaminergic neurotoxin, N-methyl(R)salsolinol. Neurosci. Lett. 1996, 212, 183–186. [Google Scholar] [CrossRef]
- Mack, F.; Bönisch, H. Dissociation constants and lipophilicity of catecholamines and related compounds. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1979, 310, 1–9. [Google Scholar] [CrossRef]
- Kristensen, A.S.; Andersen, J.; Jørgensen, T.N.; Sørensen, L.; Eriksen, J.; Loland, C.J.; Strømgaard, K.; Gether, U. SLC6 neurotransmitter transporters: Structure, function, and regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef] [PubMed]
- Daws, L.C. Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol. Ther. 2009, 121, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wall, S.C.; Rudnick, G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J. Biol. Chem. 1994, 269, 7124–7130. [Google Scholar] [CrossRef]
- Nirenberg, M.J.; Vaughan, R.A.; Uhl, G.R.; Kuhar, M.J.; Pickel, V.M. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 436–447. [Google Scholar] [CrossRef]
- Duan, H.; Wang, J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J. Pharmacol. Exp. Ther. 2010, 335, 743–753. [Google Scholar] [CrossRef]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef]
- Seitz, T.; Stalmann, R.; Dalila, N.; Chen, J.; Pojar, S.; Dos Santos Pereira, J.N.; Krätzner, R.; Brockmöller, J.; Tzvetkov, M.V. Global genetic analyses reveal strong inter-ethnic variability in the loss of activity of the organic cation transporter OCT1. Genome Med. 2015, 7, 56. [Google Scholar] [CrossRef]
- Hilgendorf, C.; Ahlin, G.; Seithel, A.; Artursson, P.; Ungell, A.L.; Karlsson, J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab. Dispos. Biol. Fate Chem. 2007, 35, 1333–1340. [Google Scholar] [CrossRef]
- Wu, X.; Kekuda, R.; Huang, W.; Fei, Y.J.; Leibach, F.H.; Chen, J.; Conway, S.J.; Ganapathy, V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J. Biol. Chem. 1998, 273, 32776–32786. [Google Scholar] [CrossRef]
- Busch, A.E.; Karbach, U.; Miska, D.; Gorboulev, V.; Akhoundova, A.; Volk, C.; Arndt, P.; Ulzheimer, J.C.; Sonders, M.S.; Baumann, C.; et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol. Pharmacol. 1998, 54, 342–352. [Google Scholar] [CrossRef]
- Chen, E.C.; Matsson, P.; Azimi, M.; Zhou, X.; Handin, N.; Yee, S.W.; Artursson, P.; Giacomini, K.M. High Throughput Screening of a Prescription Drug Library for Inhibitors of Organic Cation Transporter 3, OCT3. Pharm. Res. 2022, 39, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Bönisch, H. Extraneuronal transport of catecholamines. Pharmacology 1980, 21, 93–108. [Google Scholar] [PubMed]
- Gasser, P.J. Organic Cation Transporters in Brain Catecholamine Homeostasis; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Bacq, A.; Balasse, L.; Biala, G.; Guiard, B.; Gardier, A.M.; Schinkel, A.; Louis, F.; Vialou, V.; Martres, M.P.; Chevarin, C.; et al. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol. Psychiatry 2012, 17, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Vialou, V.; Balasse, L.; Callebert, J.; Launay, J.M.; Giros, B.; Gautron, S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J. Neurochem. 2008, 106, 1471–1482. [Google Scholar] [CrossRef]
- Inazu, M.; Takeda, H.; Matsumiya, T. Expression and functional characterization of the extraneuronal monoamine transporter in normal human astrocytes. J. Neurochem. 2003, 84, 43–52. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, L.-S.; Zeng, S. Stereoselectivity of chiral drug transport: A focus on enantiomer–transporter interaction. Drug Metab. Rev. 2014, 46, 283–290. [Google Scholar] [CrossRef]
- Bi, Y.A.; Lin, J.; Mathialagan, S.; Tylaska, L.; Callegari, E.; Rodrigues, A.D.; Varma, M.V.S. Role of Hepatic Organic Anion Transporter 2 in the Pharmacokinetics of R- and S-Warfarin: In Vitro Studies and Mechanistic Evaluation. Mol. Pharm. 2018, 15, 1284–1295. [Google Scholar] [CrossRef]
- Jensen, O.; Rafehi, M.; Tzvetkov, M.V.; Brockmöller, J. Stereoselective cell uptake of adrenergic agonists and antagonists by organic cation transporters. Biochem. Pharmacol. 2020, 171, 113731. [Google Scholar] [CrossRef]
- Gebauer, L.; Arul Murugan, N.; Jensen, O.; Brockmöller, J.; Rafehi, M. Molecular basis for stereoselective transport of fenoterol by the organic cation transporters 1 and 2. Biochem. Pharmacol. 2021, 197, 114871. [Google Scholar] [CrossRef]
- Quinn, D. Does chirality matter? pharmacodynamics of enantiomers of methylphenidate in patients with attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2008, 28 (Suppl. S2), S62–S66. [Google Scholar] [CrossRef]
- Nishimura, M.; Sato, K. Ketamine stereoselectively inhibits rat dopamine transporter. Neurosci. Lett. 1999, 274, 131–134. [Google Scholar] [CrossRef]
- Niello, M.; Cintulová, D.; Raithmayr, P.; Holy, M.; Jäntsch, K.; Colas, C.; Ecker, G.F.; Sitte, H.H.; Mihovilovic, M.D. Effects of Hydroxylated Mephedrone Metabolites on Monoamine Transporter Activity in vitro. Front. Pharmacol. 2021, 12, 654061. [Google Scholar] [CrossRef] [PubMed]
- Lepola, U.; Wade, A.; Andersen, H.F. Do equivalent doses of escitalopram and citalopram have similar efficacy? A pooled analysis of two positive placebo-controlled studies in major depressive disorder. Int. Clin. Psychopharmacol. 2004, 19, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Auclair, A.L.; Martel, J.C.; Assié, M.B.; Bardin, L.; Heusler, P.; Cussac, D.; Marien, M.; Newman-Tancredi, A.; O’Connor, J.A.; Depoortère, R. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: Profile in vitro and in models of depression and anxiety. Neuropharmacology 2013, 70, 338–347. [Google Scholar] [CrossRef]
- Verrico, C.D.; Miller, G.M.; Madras, B.K. MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: Implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology 2007, 189, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, L.; Jensen, O.; Neif, M.; Brockmöller, J.; Dücker, C. Overlap and Specificity in the Substrate Spectra of Human Monoamine Transporters and Organic Cation Transporters 1, 2 and 3. Int. J. Mol. Sci. 2021, 22, 12816. [Google Scholar] [CrossRef]
- Jensen, O.; Rafehi, M.; Gebauer, L.; Brockmöller, J. Cellular Uptake of Psychostimulants—Are High- and Low-Affinity Organic Cation Transporters Drug Traffickers? Front. Pharmacol. 2021, 11, 609811. [Google Scholar] [CrossRef]
- Dos Santos Pereira, J.N.; Tadjerpisheh, S.; Abu Abed, M.; Saadatmand, A.R.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Brockmöller, J.; Tzvetkov, M.V. The poorly membrane permeable antipsychotic drugs amisulpride and sulpiride are substrates of the organic cation transporters from the SLC22 family. AAPS J. 2014, 16, 1247–1258. [Google Scholar] [CrossRef]
- Saadatmand, A.R.; Tadjerpisheh, S.; Brockmöller, J.; Tzvetkov, M.V. The prototypic pharmacogenetic drug debrisoquine is a substrate of the genetically polymorphic organic cation transporter OCT1. Biochem. Pharmacol. 2012, 83, 1427–1434. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Melegari, M. Enantioselective LC analysis of synephrine in natural products on a protein-based chiral stationary phase. J. Pharm. Biomed. Anal. 2005, 37, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Wieland, K.; Zuurmond, H.M.; Krasel, C.; Ijzerman, A.P.; Lohse, M.J. Involvement of Asn-293 in stereospecific agonist recognition and in activation of the beta 2-adrenergic receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 9276–9281. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, M.L.R.; Herman, L.W. Ulotaront: A TAAR1 Agonist for the Treatment of Schizophrenia. ACS Med. Chem. Lett. 2022, 13, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.J.; Miller, D.D.; Patil, P.N. Epinephrine enantiomers: Affinity, efficacy and potency relationships in rat smooth muscle tissues. J. Pharmacol. Exp. Ther. 1989, 249, 242–248. [Google Scholar]
- Brown, E.M.; Fedak, S.A.; Woodard, C.J.; Aurbach, G.D. Beta-Adrenergic receptor interactions. Direct comparison of receptor interaction and biological activity. J. Biol. Chem. 1976, 251, 1239–1246. [Google Scholar] [CrossRef]
- Brown, C.M.; McGrath, J.C.; Midgley, J.M.; Muir, A.G.; O’Brien, J.W.; Thonoor, C.M.; Williams, C.M.; Wilson, V.G. Activities of octopamine and synephrine stereoisomers on alpha-adrenoceptors. Br. J. Pharmacol. 1988, 93, 417–429. [Google Scholar] [CrossRef]
- Eisenhofer, G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol. Ther. 2001, 91, 35–62. [Google Scholar] [CrossRef]
- Iversen, L.L. The uptake of catechol amines at high perfusion concentrations in the rat isolated heart: A novel catechol amine uptake process. Br. J. Pharmacol. Chemother. 1965, 25, 18–33. [Google Scholar] [CrossRef]
- Hendrickx, R.; Johansson, J.G.; Lohmann, C.; Jenvert, R.-M.; Blomgren, A.; Börjesson, L.; Gustavsson, L. Identification of Novel Substrates and Structure–Activity Relationship of Cellular Uptake Mediated by Human Organic Cation Transporters 1 and 2. J. Med. Chem. 2013, 56, 7232–7242. [Google Scholar] [CrossRef]
- Berry, M.D.; Hart, S.; Pryor, A.R.; Hunter, S.; Gardiner, D. Pharmacological characterization of a high-affinity p-tyramine transporter in rat brain synaptosomes. Sci. Rep. 2016, 6, 38006. [Google Scholar] [CrossRef]
- Taubert, D.; Grimberg, G.; Stenzel, W.; Schömig, E. Identification of the endogenous key substrates of the human organic cation transporter OCT2 and their implication in function of dopaminergic neurons. PLoS ONE 2007, 2, e385. [Google Scholar] [CrossRef] [PubMed]
- Leabman, M.K.; Huang, C.C.; Kawamoto, M.; Johns, S.J.; Stryke, D.; Ferrin, T.E.; DeYoung, J.; Taylor, T.; Clark, A.G.; Herskowitz, I.; et al. Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function. Pharmacogenetics 2002, 12, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Song, I.S. Genetic variants of organic cation transporter 1 (OCT1) and OCT2 significantly reduce lamivudine uptake. Biopharm. Drug Dispos. 2012, 33, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.P.; Accordi, F.; Chimenti, C.; Civinini, A.; Crivellato, E. Catecholaminergic System of Invertebrates: Comparative and Evolutionary Aspects in Comparison With the Octopaminergic System. Int. Rev. Cell Mol. Biol. 2016, 322, 363–394. [Google Scholar] [PubMed]
- Lane, R.M.; Baker, G.B. Chirality and drugs used in psychiatry: Nice to know or need to know? Cell. Mol. Neurobiol. 1999, 19, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Bergqvist, P.B.F.; Brennum, L.T.; Gupta, S.; Hogg, S.; Larsen, A.; Wiborg, O. Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology 2003, 167, 353–362. [Google Scholar] [CrossRef]
- Koh, A.H.W.; Chess-Williams, R.; Lohning, A.E. Racemic synephrine found in Citrus aurantium-listing pre-workout supplements suggests a non-plant-based origin. Drug Test. Anal. 2021, 13, 1569–1575. [Google Scholar] [CrossRef]
- Ranieri, R.L.; McLaughlin, J.L. Cactus alkaloids. XXVIII. .beta.-Phenethylamine and tetrahydroisoquinoline alkaloids from the Mexican cactus Dolichothele longimamma. J. Org. Chem. 1976, 41, 319–323. [Google Scholar] [CrossRef]
- Jordan, R.; Midgley, J.M.; Thonoor, C.M.; Williams, C.M. Beta-adrenergic activities of octopamine and synephrine stereoisomers on guinea-pig atria and trachea. J. Pharm. Pharmacol. 1987, 39, 752–754. [Google Scholar] [CrossRef]
- da Silva-Pereira, J.F.; Bubna, G.A.; Gonçalves Gde, A.; Bracht, F.; Peralta, R.M.; Bracht, A. Fast hepatic biotransformation of p-synephrine and p-octopamine and implications for their oral intake. Food Funct. 2016, 7, 1483–1491. [Google Scholar] [CrossRef]
- Melzig, M.F.; Putscher, I.; Henklein, P.; Haber, H. In vitro pharmacological activity of the tetrahydroisoquinoline salsolinol present in products from Theobroma cacao L. like cocoa and chocolate. J. Ethnopharmacol. 2000, 73, 153–159. [Google Scholar] [CrossRef]
- Lee, J.; Ramchandani, V.A.; Hamazaki, K.; Engleman, E.A.; McBride, W.J.; Li, T.-K.; Kim, H.-Y. A critical evaluation of influence of ethanol and diet on salsolinol enantiomers in humans and rats. Alcohol. Clin. Exp. Res. 2010, 34, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Kurnik-Łucka, M.; Panula, P.; Bugajski, A.; Gil, K. Salsolinol: An Unintelligible and Double-Faced Molecule—Lessons Learned from In Vivo and In Vitro Experiments. Neurotox. Res. 2018, 33, 485–514. [Google Scholar] [CrossRef]
- Takahashi, T.; Maruyama, W.; Deng, Y.; Dostert, P.; Nakahara, D.; Niwa, T.; Ohta, S.; Naoi, M. Cytotoxicity of endogenous isoquinolines to human dopaminergic neuroblastoma SH-SY5Y cells. J. Neural Transm. 1997, 104, 59–66. [Google Scholar] [CrossRef] [PubMed]


| Transporter | Substrate | Km ± SEM [µM] | Vmax ± SEM [pmol × mg Protein−1 × min−1] | Clint ± SEM [mL × g Protein−1 × min−1] | Stereoselectivity | ||
|---|---|---|---|---|---|---|---|
| Km | Vmax | ClInt | |||||
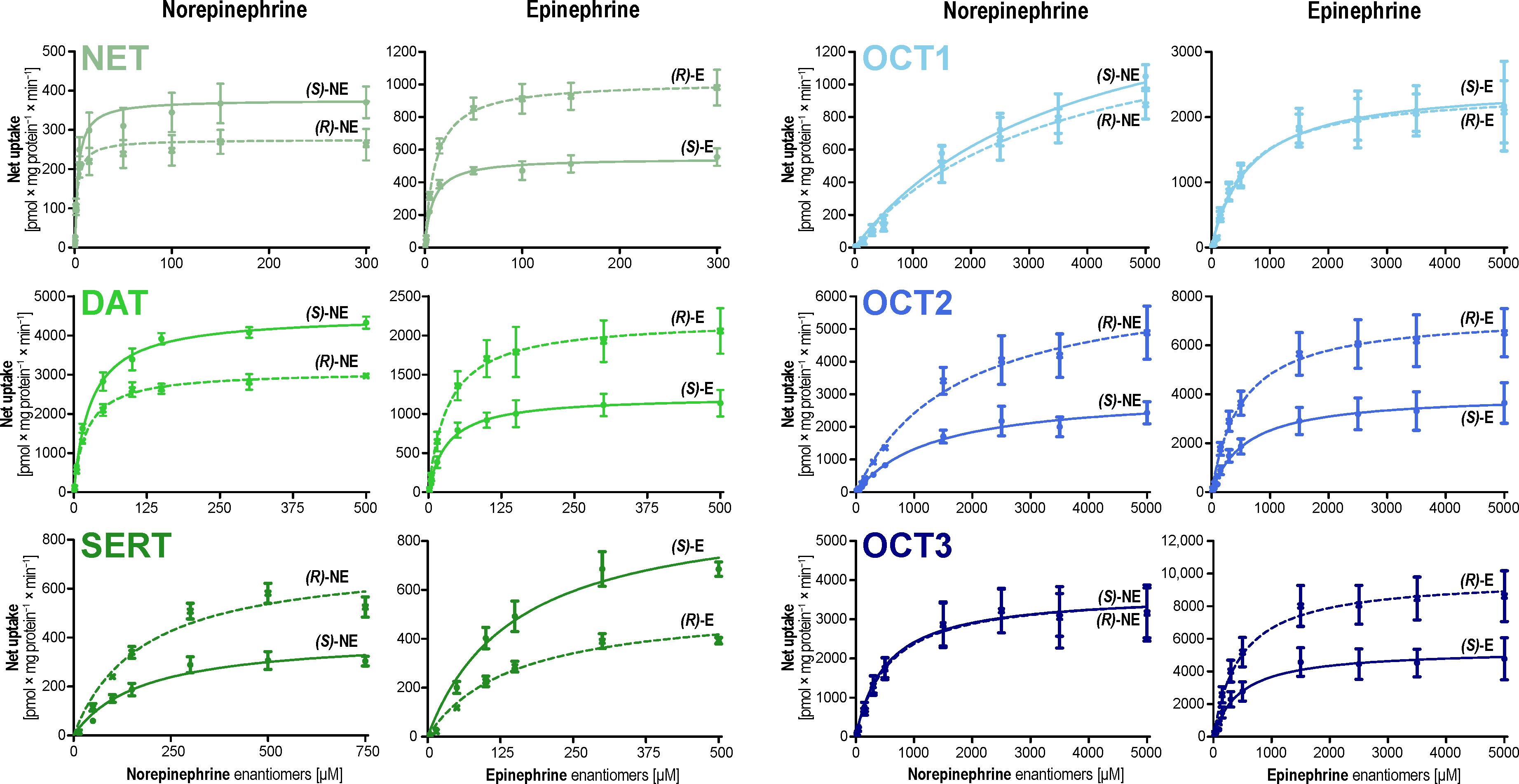
| NET | (R)-Norepinephrine | 2.31 ± 0.84 | 262 ± 15 | 113.3 ± 47.6 | 1.32-fold for (S) | 1.38-fold for (S) ** | 1.05-fold for (S) |
| (S)-Norepinephrine | 3.05 ± 1.01 | 362 ± 20 | 118.7 ± 46.0 | ||||
| (R)-Epinephrine | 11.2 ± 2.2 | 1027 ± 37 | 91.7 ± 21.3 | 1.31-fold for (R) | 1.84-fold for (R) *** | 1.41-fold for (R) | |
| (S)-Epinephrine | 8.52 ± 1.91 | 557 ± 22 | 65.4 ± 17.3 | ||||
| DAT | (R)-Norepinephrine | 22.2 ± 2.6 | 3085 ± 81 | 138.8 ± 19.7 | 1.31-fold for (S) | 1.47-fold for (S) *** | 1.12-fold for (S) |
| (S)-Norepinephrine | 29.1 ± 3.2 | 4524 ± 122 | 155.5 ± 21.4 | ||||
| (R)-Epinephrine | 34.0 ± 11.6 | 2201 ± 173 | 64.7 ± 26.7 | 1.05-fold for (R) | 1.80-fold for (R) ** | 1.71-fold for (R) | |
| (S)-Epinephrine | 32.3 ± 10.3 | 1226 ± 90 | 37.9 ± 14.8 | ||||
| SERT | (R)-Norepinephrine | 176.5 ± 41.7 | 727 ± 60 | 4.12 ± 1.31 | 1.01-fold for (R) | 1.79-fold for (R) ** | 1.78-fold for (R) |
| (S)-Norepinephrine | 175.4 ± 52.23 | 405 ± 42 | 2.31 ± 0.93 | ||||
| (R)-Epinephrine | 147.1 ± 27.6 | 542 ± 41 | 3.68 ± 0.97 | 1.02-fold for (S) | 1.76-fold for (S) ** | 1.73-fold for (S) | |
| (S)-Epinephrine | 149.6 ± 35.6 | 951 ± 91 | 6.35 ± 2.12 | ||||
| OCT1 | (R)-Norepinephrine | 3394 ± 1417 | 1524 ± 330 | 0.45 ± 0.28 | 1.09-fold for (S) | 1.16-fold for (S) | 1.06-fold for (S) |
| (S)-Norepinephrine | 3710 ± 1011 | 1766 ± 258 | 0.48 ± 0.20 | ||||
| (R)-Epinephrine | 596.1 ± 154.6 | 2419 ± 189 | 4.06 ± 1.37 | 1.13-fold for (S) | 1.04-fold for (S) | 1.08-fold for (R) | |
| (S)-Epinephrine | 670.7 ± 230.3 | 2514 ± 266 | 3.75 ± 1.68 | ||||
| OCT2 | (R)-Norepinephrine | 1731 ± 417.5 | 6603 ± 664 | 3.81 ± 1.30 | 1.32-fold for (R) | 2.17-fold for (R) ** | 1.64-fold for (R) |
| (S)-Norepinephrine | 1309 ± 290.9 | 3042 ± 258 | 2.32 ± 0.71 | ||||
| (R)-Epinephrine | 513.1 ± 110.4 | 7277 ± 483 | 14.2 ± 4.0 | 1.14-fold for (S) | 1.82-fold for (R) ** | 2.08-fold for (R) | |
| (S)-Epinephrine | 584.6 ± 167.4 | 3993 ± 360 | 6.83 ± 2.57 | ||||
| OCT3 | (R)-Norepinephrine | 557.1 ± 200.0 | 3683 ± 360 | 6.61 ± 3.02 | 1.07-fold for (R) | 1.01-fold for (R) | 1.07-fold for (S) |
| (S)-Norepinephrine | 519.2 ± 158.5 | 3662 ± 299 | 7.05 ± 2.73 | ||||
| (R)-Epinephrine | 466.5 ± 116.0 | 9742 ± 698 | 20.9 ± 6.7 | 1.03-fold for (R) | 1.83-fold for (R) ** | 1.78-fold for (S) | |
| (S)-Epinephrine | 454.9 ± 149.0 | 5336 ± 502 | 11.7 ± 4.9 | ||||
| Substance | Enantiomeric Activity Ratio (R/S) in Receptor Binding | References | ||
|---|---|---|---|---|
| Alpha-1 | Alpha-2 | Beta | ||
| Epinephrine | 49 | 125 | [46,47] | |
| Norepinephrine | 33 | [48] | ||
| Norphenylephrine | 8 | 3 | [48] | |
| Octopamine | 5 | 3 | [48] | |
| Phenylephrine | 420 | 45 | [48] | |
| Synephrine | 75 | [48] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebauer, L.; Rafehi, M.; Brockmöller, J. Stereoselectivity in the Membrane Transport of Phenylethylamine Derivatives by Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3. Biomolecules 2022, 12, 1507. https://doi.org/10.3390/biom12101507
Gebauer L, Rafehi M, Brockmöller J. Stereoselectivity in the Membrane Transport of Phenylethylamine Derivatives by Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3. Biomolecules. 2022; 12(10):1507. https://doi.org/10.3390/biom12101507
Chicago/Turabian StyleGebauer, Lukas, Muhammad Rafehi, and Jürgen Brockmöller. 2022. "Stereoselectivity in the Membrane Transport of Phenylethylamine Derivatives by Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3" Biomolecules 12, no. 10: 1507. https://doi.org/10.3390/biom12101507
APA StyleGebauer, L., Rafehi, M., & Brockmöller, J. (2022). Stereoselectivity in the Membrane Transport of Phenylethylamine Derivatives by Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3. Biomolecules, 12(10), 1507. https://doi.org/10.3390/biom12101507







