How Streptococcus mutans Affects the Surface Topography and Electrochemical Behavior of Nanostructured Bulk Ti
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Nanostructured Ti
2.2. Bacterial Growth and Adhesion to Nanostructured Ti Surface
2.3. Surface Characterization
3. Results and Discussion
3.1. Microstructure and Surface Topography of Nanostructured Ti
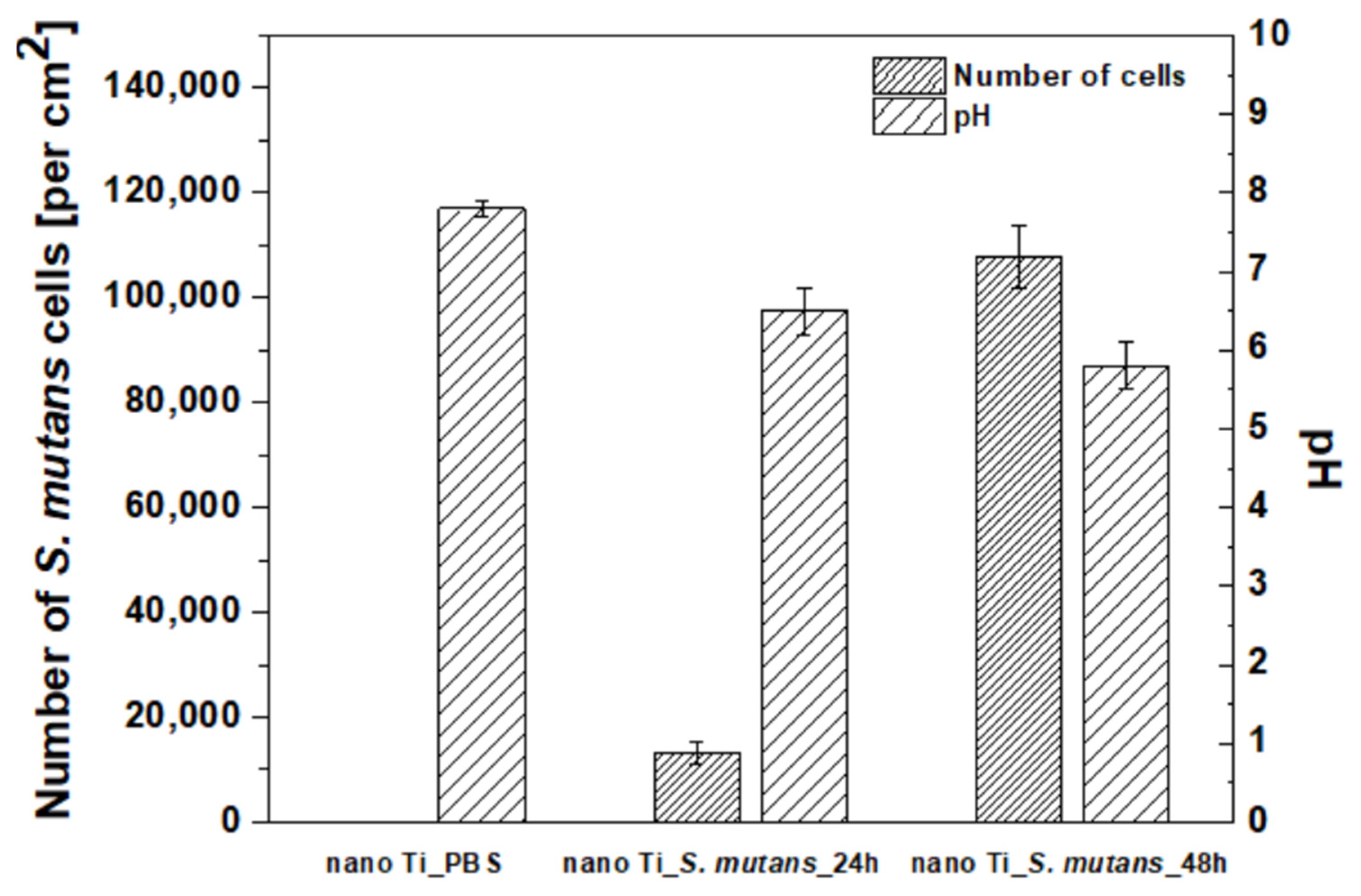
3.2. Bacterial Adhesion to the Nanostructured Ti Surface
3.3. Effect of Bacterial Exposure on the Surface Topography of Nanostructured Ti
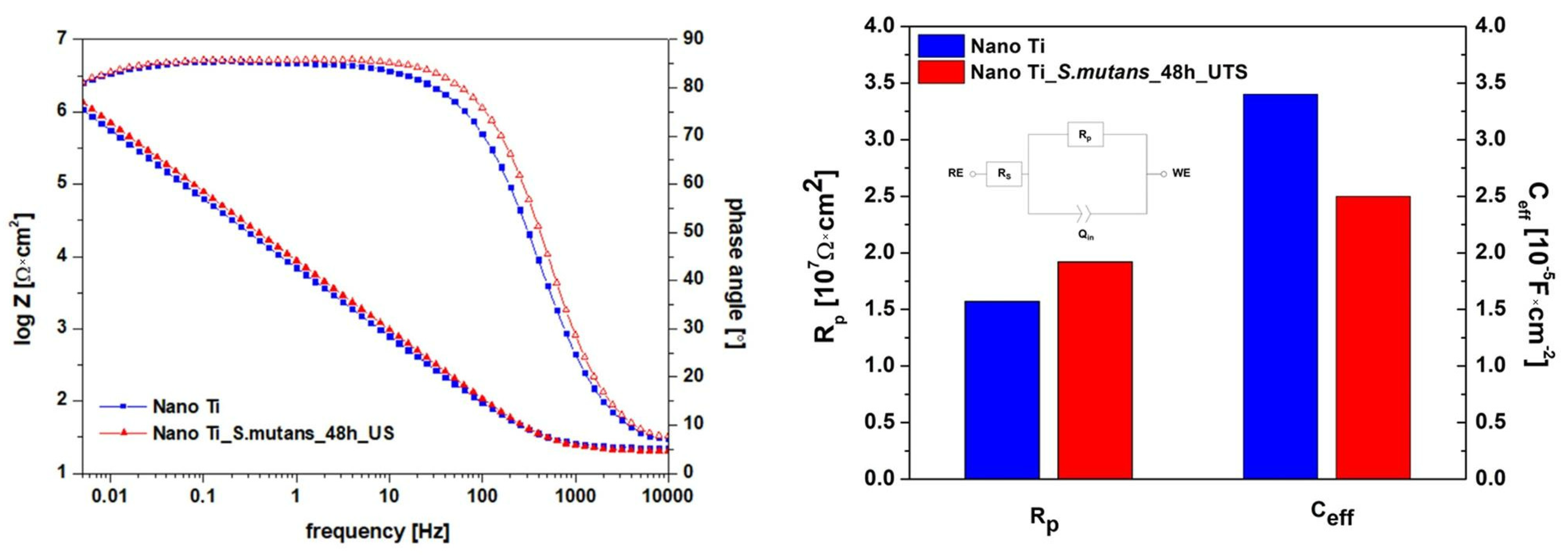
3.4. Effect of Bacterial Exposure on the Electrochemical Response of Nanostructured Ti
4. Future Outlook
5. Conclusions
- Significant coverage of nano Ti surface by S. mutans was observed after 48 h of incubation in a bacteria suspension enriched with glucose. Bacteria adhesion and the beginning of biofilm formation were combined with a slight decline in pH, which could be associated with lactic acid formation owing to glucose metabolization.
- S. mutans incubation resulted in an unexpected, slight reduction in nanoscale surface roughness. Preferential nano Ti dissolution in the closely spaced grain boundaries induced by the pH shift to more negative values could be the reason for the observed phenomenon.
- Differences in surface nanotopography induced by S. mutans incubation provided virtually no changes in the protectiveness of the passive layer formed on the nanostructured Ti surface in the artificial saliva. This confirms the high application potential of bulk nanostructured Ti in the area of dental implantology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2020, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Sowers, K.T.; Olivares-Navarrete, R. Novel in vitro comparative model of osteogenic and inflammatory cell response to dental implants. Dent. Mater. 2019, 35, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wu, Y.; Diao, X.; Shi, W.; Feng, F.; Qian, F.; Umeda, J.; Kondoh, K.; Xin, H.; Shen, J. Mechanical properties and biocompatibility of titanium with a high oxygen concentration for dental implants. Mater. Sci. Eng. C 2020, 117, 111306. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Fu, S.; Yang, L.; Wang, X.; Wang, Q.; Qin, G.; Chen, D.; Zhang, E. Effect of fluorination/oxidation level of nano-structured titanium on the behaviors of bacteria and osteoblasts. Appl. Surf. Sci. 2020, 502, 144077. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Kolmakov, A.G.; Sergiyenko, K.V.; Kaplan, M.A.; Baikin, A.S.; Gudkov, S.V. Mechanical, physical–chemical and biological properties of the new Ti–30Nb–13Ta–5Zr alloy. J. Mater. Sci. 2020, 55, 14516–14529. [Google Scholar] [CrossRef]
- Duraccio, D.; Mussano, F.; Faga, M.G. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; De Souza, A.; Vazouras, K.; Gholami, H.; Pagni, S.; Weber, H. Survival rates of short dental implants (≤6 mm) compared with implants longer than 6 mm in posterior jaw areas: A meta-analysis. Clin. Oral Implants Res. 2018, 29, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, L.; Gao, Y.; Liu, H.; Dong, H.; Mou, Y. Risk factors and reoperative survival rate of failed narrow-diameter implants in the maxillary anterior region. Clin. Implant Dent. Relat. Res. 2020, 22, 29–41. [Google Scholar] [CrossRef]
- Zweers, J.; van Doornik, A.; Hogendorf, E.A.H.; Quirynen, M.; Van der Weijden, G.A. Clinical and radiographic evaluation of narrow-vs. regular-diameter dental implants: A 3-year follow-up. A retrospective study. Clin. Oral Implants Res. 2015, 26, 149–156. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’yov, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. R Rep. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Ioannidis, A.; Gallucci, G.O.; Jung, R.E.; Borzangy, S.; Hämmerle, C.H.F.; Benic, G.I. Titanium-zirconium narrow-diameter versus titanium regular-diameter implants for anterior and premolar single crowns: 3-year results of a randomized controlled clinical study. J. Clin. Periodontol. 2015, 42, 1060–1070. [Google Scholar] [CrossRef]
- Zinsli, B.; Sägesser, T.; Mericske, E.; Mericske-Stern, R. Clinical evaluation of small-diameter ITI implants: A prospective study. Int. J. Oral Maxillofac. Implants 2004, 19, 92–99. [Google Scholar]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Neumann, A.; Ng, H.P.; Lapovok, R.; Kasper, C.; Lowe, T.C.; Anumalasetty, V.N.; Estrin, Y. Combined effect of grain refinement and surface modification of pure titanium on the attachment of mesenchymal stem cells and osteoblast-like SaOS-2 cells. Mater. Sci. Eng. C 2017, 71, 483–497. [Google Scholar] [CrossRef]
- Sotniczuk, A.; Garbacz, H. Nanostructured Bulk Titanium with Enhanced Properties–Strategies and Prospects for Dental Applications. Adv. Eng. Mater. 2021, 23, 2000909. [Google Scholar] [CrossRef]
- Topolski, K.; Adamczyk-Cieślak, B.; Garbacz, H. High-strength ultrafine-grained titanium 99.99 manufactured by large strain plastic working. J. Mater. Sci. 2019, 55, 4910–4925. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Mohammadi, M.; Gerlich, A.P. Stability of ultra-fine and nano-grains after severe plastic deformation: A critical review. J. Mater. Sci. 2021, 56, 15513–15537. [Google Scholar] [CrossRef]
- Lowe, T.C.; Valiev, R.Z.; Li, X.; Ewing, B.R. Commercialization of bulk nanostructured metals and alloys. MRS Bull. 2021, 46, 265–272. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Barbosa, S.L.; Ariza, E.A.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.-P.; Rocha, L.A. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater. Sci. Eng. C 2015, 47, 384–393. [Google Scholar] [CrossRef]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater. Sci. Eng. C 2017, 76, 1354–1368. [Google Scholar] [CrossRef]
- Zhang, H.; Man, C.; Wang, L.; Dong, C.; Wang, L.; Kong, D.; Wang, X. Different corrosion behaviors between α and β phases of Ti6Al4V in fluoride-containing solutions: Influence of alloying element Al. Corros. Sci. 2020, 169, 108605. [Google Scholar] [CrossRef]
- Chowdhury, N.D.; Ghosh, K.S. Electrochemical behaviour of dental amalgam in natural, artificial saliva and in 0.90 wt.% NaCl solution. Corros. Sci. 2018, 133, 217–230. [Google Scholar] [CrossRef]
- Hedberg, Y.S.; Gamna, F.; Padoan, G.; Ferraris, S.; Cazzola, M.; Herting, G.; Atapour, M.; Spriano, S.; Wallinder, I.O. Surface modified Ti6Al4V for enhanced bone bonding ability—Effects of silver and corrosivity at simulated physiological conditions from a corrosion and metal release perspective. Corros. Sci. 2020, 168, 108566. [Google Scholar] [CrossRef]
- Trolic, I.M.; Serdarevic, N.L.; Todoric, Z.; Budimir, A.; Spalj, S.; Curkovic, H.O. Corrosion of orthodontic archwires in artificial saliva in the presence of Lactobacillus reuteri. Surf. Coat. Technol. 2019, 370, 44–52. [Google Scholar] [CrossRef]
- Eduok, U.; Szpunar, J. In vitro corrosion studies of stainless-steel dental substrates during Porphyromonas gingivalis biofilm growth in artificial saliva solutions: Providing insights into the role of resident oral bacterium. RSC Adv. 2020, 10, 31280–31294. [Google Scholar] [CrossRef]
- Xavier, J.G.; Geremias, T.C.; Montero, J.F.D.; Vahey, B.R.; Benfatti, C.A.M.; Souza, J.C.M.; Magini, R.S.; Pimenta, A.L. Lactam inhibiting Streptococcus mutans growth on titanium. Mater. Sci. Eng. C 2016, 68, 837–841. [Google Scholar] [CrossRef]
- Yang, Y.; Masoumeh, M.; Zhou, E.; Liu, D.; Song, Y.; Xu, D.; Wang, F.; Smith, J.A. Streptococcus mutans biofilms induce metabolite-mediated corrosion of 316 L stainless steel in a simulated oral environment. Corros. Sci. 2021, 182, 109286. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Ponthiaux, P.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.P.; Rocha, L.A. Corrosion behaviour of titanium in the presence of Streptococcus mutans. J. Dent. 2013, 41, 528–534. [Google Scholar] [CrossRef]
- Mabilleau, G.; Bourdon, S.; Joly-Guillou, M.L.; Filmon, R.; Baslé, M.F.; Chappard, D. Influence of fluoride, hydrogen peroxide and lactic acid on the corrosion resistance of commercially pure titanium. Acta Biomater. 2006, 2, 121–129. [Google Scholar] [CrossRef]
- Miyamoto, H. Corrosion of Ultrafine Grained Materials by Severe Plastic Deformation, an Overview. Mater. Trans. 2016, 575, 559–572. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Huo, W.; Zhang, W.; Zhao, Y.; Zhang, Y. Electrochemical corrosion characteristics and biocompatibility of nanostructured titanium for implants. Appl. Surf. Sci. 2018, 434, 63–72. [Google Scholar] [CrossRef]
- Kubacka, D.; Yamamoto, A.; Wiecinski, P.; Garbacz, H. Biological behavior of titanium processed by severe plastic deformation. Appl. Surf. Sci. 2019, 472, 54–63. [Google Scholar] [CrossRef]
- Sotniczuk, A.; Kuczyńska-Zemła, D.; Królikowski, A.; Garbacz, H. Enhancement of the corrosion resistance and mechanical properties of nanocrystalline titanium by low-temperature annealing. Corros. Sci. 2019, 147, 342–349. [Google Scholar] [CrossRef]
- Wang, C.T.; Fox, A.G.; Langdon, T.G. Microstructural evolution in ultrafine-grained titanium processed by high-pressure torsion under different pressures. J. Mater. Sci. 2014, 49, 6558–6564. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Attarilar, S. Grain Refinement Affected Machinability in Commercial Pure Titanium. Metall. Mater. Trans. A 2021, 52, 1282–1292. [Google Scholar] [CrossRef]
- Semenova, I.P.; Polyakova, V.V.; Dyakonov, G.S.; Polyakov, A.V. Ultrafine-Grained Titanium-Based Alloys: Structure and Service Properties for Engineering Applications. Adv. Eng. Mater. 2020, 22, 1900651. [Google Scholar] [CrossRef]
- Wojtas, D.; Wierzbanowski, K.; Chulist, R.; Pachla, W.; Bieda-Niemiec, M.; Jarzębska, A.; Maj, Ł.; Kawałko, J.; Marciszko-Wiąckowska, M.; Wroński, M. Microstructure-strength relationship of ultrafine-grained titanium manufactured by unconventional severe plastic deformation process. J. Alloys Compd. 2020, 837, 155576. [Google Scholar] [CrossRef]
- Ostrovska, L.; Vistejnova, L.; Dzugan, J.; Slama, P.; Kubina, T.; Ukraintsev, E.; Kubies, D.; Kralickova, M.; Hubalek Kalbacova, M. Biological evaluation of ultra-fine titanium with improved mechanical strength for dental implant engineering. J. Mater. Sci. 2016, 51, 3097–3110. [Google Scholar] [CrossRef]
- Sandeep Kranthi Kiran, A.; Sireesha, M.; Ramalingam, R.; Kizhakeyil, A.; Verma, N.K.; Lakshminarayanan, R.; Sampath Kumar, T.S.; Doble, M.; Ramakrishna, S. Modulation of biological properties by grain refinement and surface modification on titanium surfaces for implant-related infections. J. Mater. Sci. 2019, 54, 13265–13282. [Google Scholar] [CrossRef]
- Truong, V.K.; Rundell, S.; Lapovok, R.; Estrin, Y.; Wang, J.Y.; Berndt, C.C.; Barnes, D.G.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. Effect of ultrafine-grained titanium surfaces on adhesion of bacteria. Appl. Microbiol. Biotechnol. 2009, 83, 925–937. [Google Scholar] [CrossRef]
- Elias, C.N.; Fernandes, D.J.; de Souza, F.M.; Monteiro, E.D.S.; Biasi, R.S. De Mechanical and clinical properties of titanium and titanium-based alloys (Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. J. Mater. Res. Technol. 2019, 8, 1060–1069. [Google Scholar] [CrossRef]
- Garbacz, H.; Topolski, K.; Motyka, M. Hydrostatic extrusion. In Nanocrystalline Titanium; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–53. [Google Scholar]
- Giordano, C.; Saino, E.; Rimondini, L.; Pedeferri, M.P.; Visai, L.; Cigada, A.; Chiesa, R. Electrochemically induced anatase inhibits bacterial colonization on Titanium Grade 2 and Ti6Al4V alloy for dental and orthopedic devices. Colloids Surf. B Biointerfaces 2011, 88, 648–655. [Google Scholar] [CrossRef]
- Wejrzanowski, T.; Kurzydlowski, K.J. Stereology of grains in nano-crystals. In Solid State Phenomena; Trans Tech Publications Ltd.: Bäch, Switzerland, 2003; pp. 221–228. [Google Scholar]
- Wejrzanowski, T.; Lewandowska, M.; Kurzydłowski, K.J. Stereology of nano-materials. Image Anal. Stereol. 2010, 29, 1–12. [Google Scholar] [CrossRef]
- Sotniczuk, A.; Kuczyńska-Zemła, D.; Kwaśniak, P.; Thomas, M.; Garbacz, H. Corrosion behavior of Ti-29Nb-13Ta-4.6Zr and commercially pure Ti under simulated inflammatory conditions—Comparative effect of grain refinement and non-toxic β phase stabilizers. Electrochim. Acta 2019, 312, 369–379. [Google Scholar] [CrossRef]
- Ferraris, S.; Vitale, A.; Bertone, E.; Guastella, S.; Cassinelli, C.; Pan, J.; Spriano, S. Multifunctional commercially pure titanium for the improvement of bone integration: Multiscale topography, wettability, corrosion resistance and biological functionalization. Mater. Sci. Eng. C 2016, 60, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Perero, S.; Costa, P.; di Confiengo, G.G.; Cochis, A.; Rimondini, L.; Renaux, F.; Vernè, E.; Ferraris, M.; Spriano, S. Antibacterial inorganic coatings on metallic surfaces for temporary fixation devices. Appl. Surf. Sci. 2020, 508, 144707. [Google Scholar] [CrossRef]
- Rajab, F.H.; Tayf Tariq, A.S.; Al-Jumaily, A.K.; AlShaer, A.W.; Li, L.; Whitehead, K.A. The influence of picosecond laser generated periodic structures on bacterial behaviour. Appl. Surf. Sci. 2021, 540, 148292. [Google Scholar] [CrossRef]
- Bazaka, O.; Bazaka, K.; Truong, V.K.; Levchenko, I.; Jacob, M.V.; Estrin, Y.; Lapovok, R.; Chichkov, B.; Fadeeva, E.; Kingshott, P. Effect of titanium surface topography on plasma deposition of antibacterial polymer coatings. Appl. Surf. Sci. 2020, 521, 146375. [Google Scholar] [CrossRef]
- Koike, M.; Fujii, H. The corrosion resistance of pure titanium in organic acids. Biomaterials 2001, 22, 2931–2936. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-phase-element behavior caused by resistivity distributions in films II. Applications. J. Electrochem. Soc. 2010, 157, C458–C463. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J. Is niobium more corrosion-resistant than commercially pure titanium in fluoride-containing artificial saliva? Electrochim. Acta 2017, 233, 151–166. [Google Scholar] [CrossRef]
- Gurao, N.P.; Manivasagam, G.; Govindaraj, P.; Asokamani, R.; Suwas, S. Effect of texture and grain size on bio-corrosion response of ultrafine-grained titanium. In Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science; Springer: Berlin/Heidelberg, Germany, 2013; pp. 5602–5610. [Google Scholar]
- Cvijović-Alagić, I.; Cvijović, Z.; Bajat, J.; Rakin, M. Composition and processing effects on the electrochemical characteristics of biomedical titanium alloys. Corros. Sci. 2014, 83, 245–254. [Google Scholar] [CrossRef]
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2020, 268, 120595. [Google Scholar] [CrossRef]
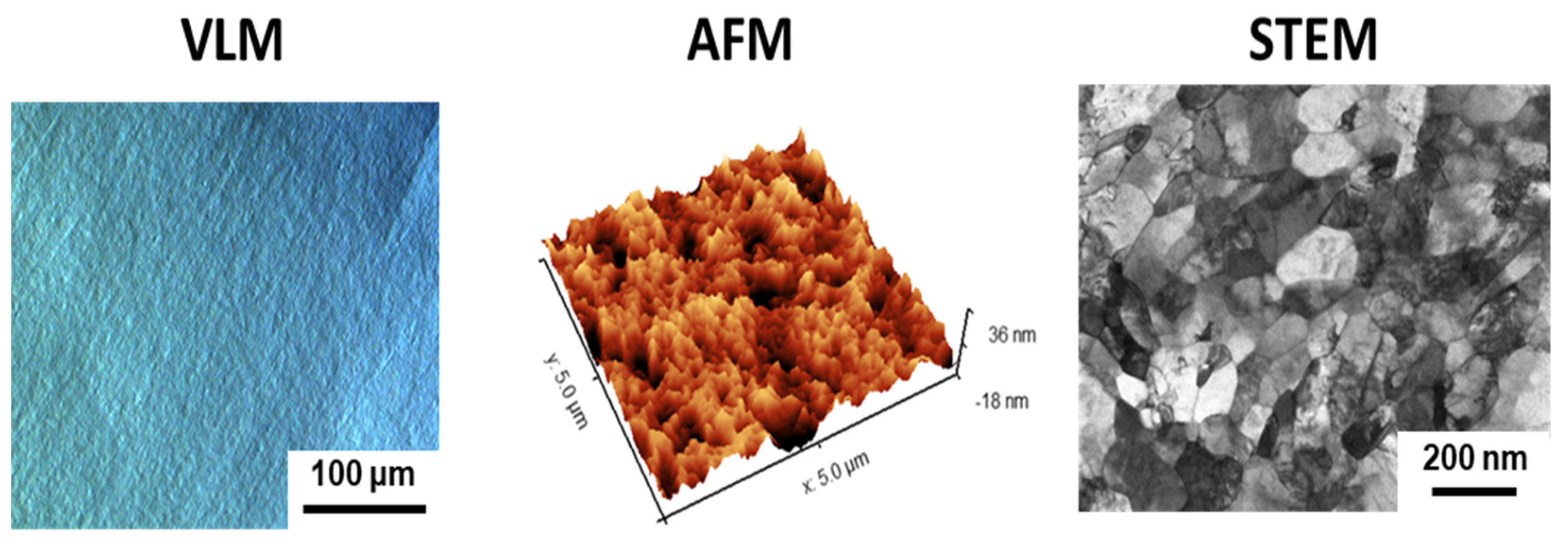
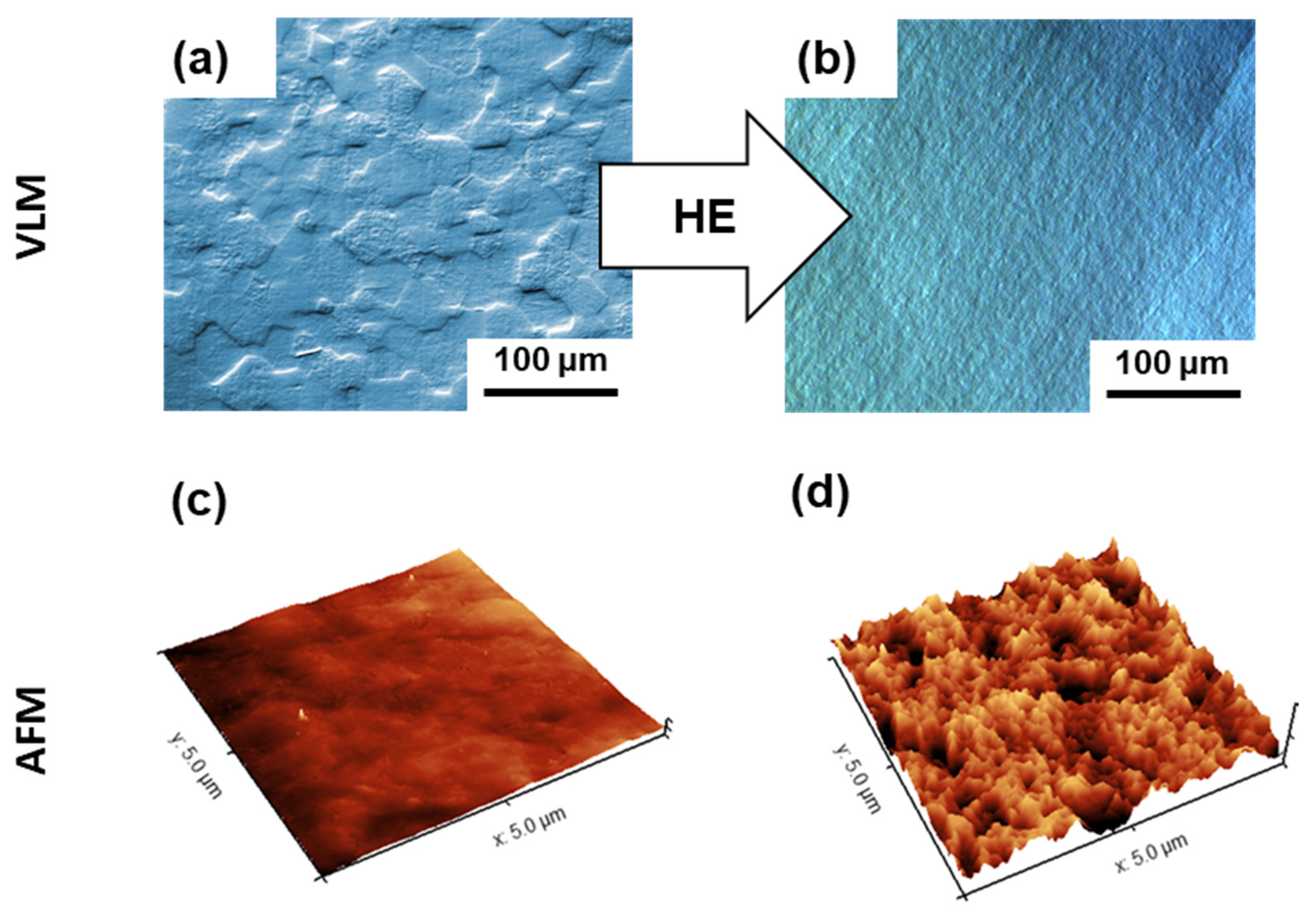



| Sample | Surface Preparation | Exposure | Surface Preparation after Exposure | Number of Samples |
|---|---|---|---|---|
| Nano Ti | Polishing | - | - | 3 |
| nano Ti_PBS (control) | Polishing | 48 h of exposure in PBS | Sputtering with gold layer–SEM | 1 |
| nano Ti_S. mutans_24 h | Polishing | 24 h of exposure in S. mutans suspension | Sputtering with gold layer–SEM | 6 |
| nano Ti_S. mutans_48 h | Polishing | 48 h of exposure in S. mutans suspension | Sputtering with gold layer–SEM | 3 |
| nano Ti_S. mutans_48 h_US | Polishing | 48 h of exposure in S. mutans suspension | Ultrasonication (bacteria removal)–AFM and corrosion | 3 |
| Nano Ti_PBS (Control) | Nano Ti_ S. mutans_24 h | Nano Ti_ S. mutans_48 h | |
|---|---|---|---|
| Number of S. mutans cells [per cm2] | 0 | 13 176 ± 2 260 | 107 764 ± 5 808 |
| pH | 7.8 ± 0.1 | 6.5 ± 0.3 | 5.8 ± 0.3 |
| Nano Ti | Nano Ti_S. mutans_48 h_US | |
|---|---|---|
| Ra [nm] | 3.78 ± 0.10 | 2.89 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotniczuk, A.; Jastrzębska, A.; Chlanda, A.; Kwiatek, A.; Garbacz, H. How Streptococcus mutans Affects the Surface Topography and Electrochemical Behavior of Nanostructured Bulk Ti. Biomolecules 2022, 12, 1515. https://doi.org/10.3390/biom12101515
Sotniczuk A, Jastrzębska A, Chlanda A, Kwiatek A, Garbacz H. How Streptococcus mutans Affects the Surface Topography and Electrochemical Behavior of Nanostructured Bulk Ti. Biomolecules. 2022; 12(10):1515. https://doi.org/10.3390/biom12101515
Chicago/Turabian StyleSotniczuk, Agata, Agnieszka Jastrzębska, Adrian Chlanda, Agnieszka Kwiatek, and Halina Garbacz. 2022. "How Streptococcus mutans Affects the Surface Topography and Electrochemical Behavior of Nanostructured Bulk Ti" Biomolecules 12, no. 10: 1515. https://doi.org/10.3390/biom12101515
APA StyleSotniczuk, A., Jastrzębska, A., Chlanda, A., Kwiatek, A., & Garbacz, H. (2022). How Streptococcus mutans Affects the Surface Topography and Electrochemical Behavior of Nanostructured Bulk Ti. Biomolecules, 12(10), 1515. https://doi.org/10.3390/biom12101515







