Cyclosporine A Delivery Platform for Veterinary Ophthalmology—A New Concept for Advanced Ophthalmology
Abstract
:1. Introduction
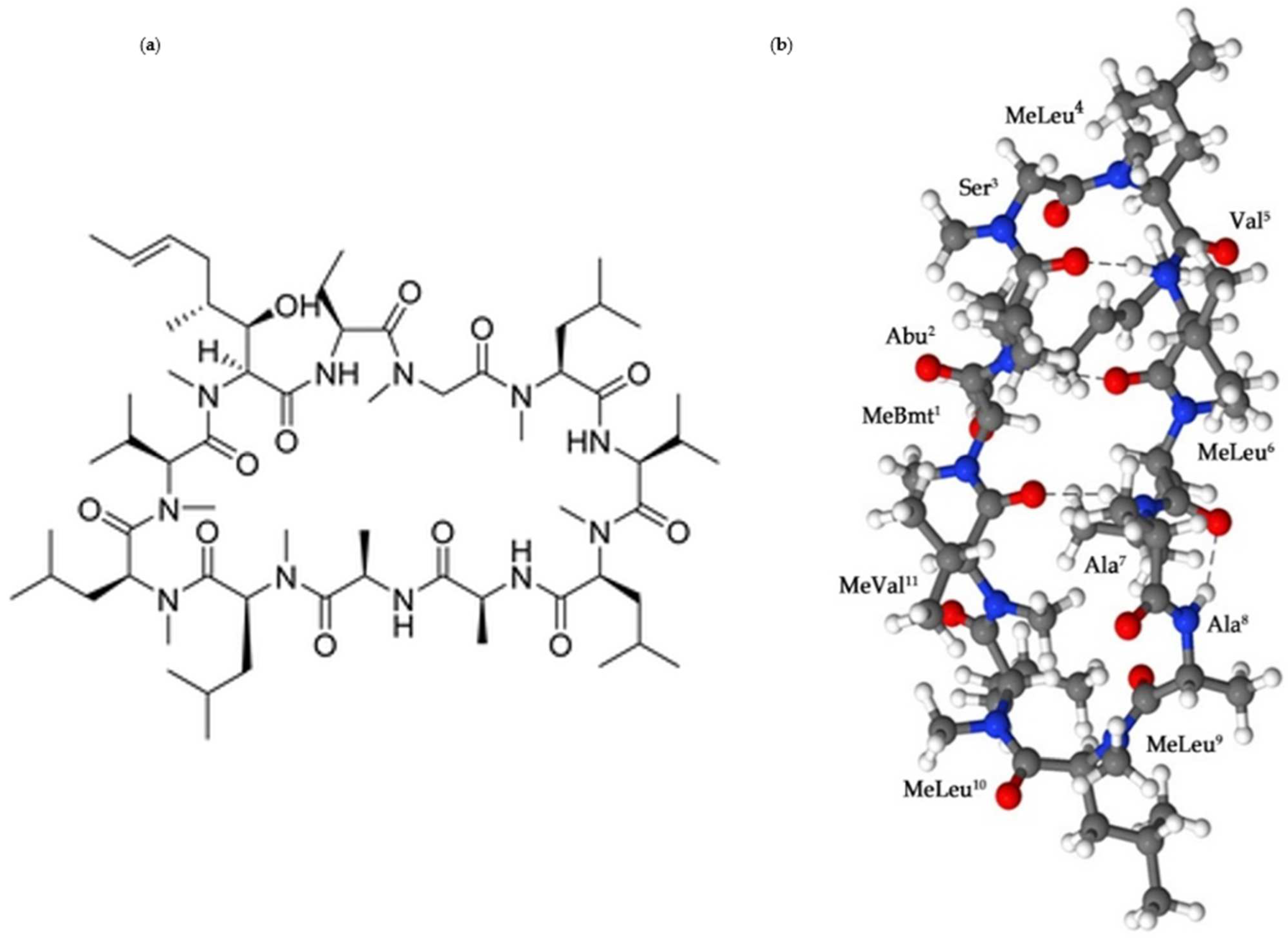
2. Cyclosporine A: The Physicochemical Properties
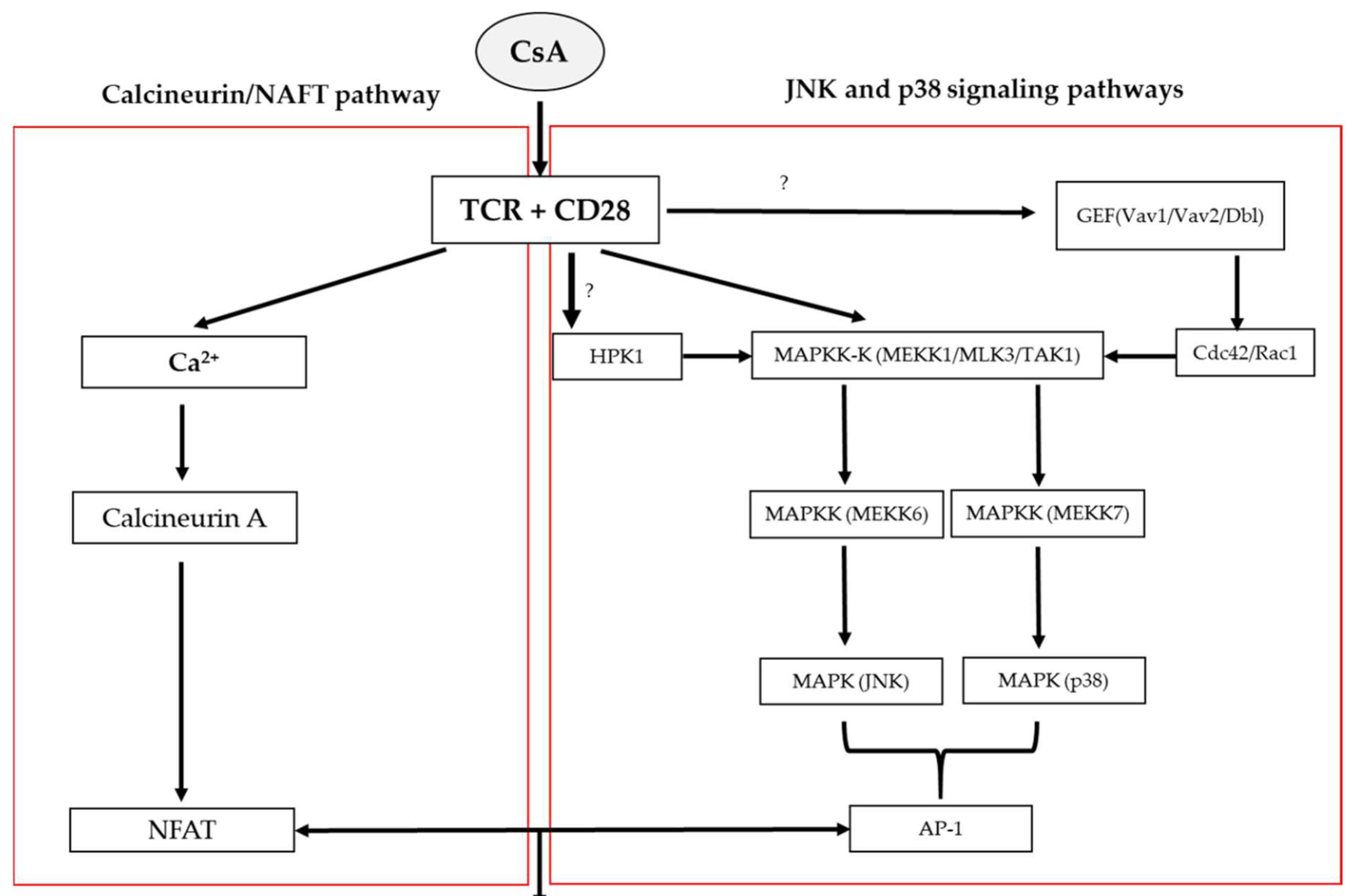
3. The Mechanisms of Action
4. Drug Delivery of Cyclosporine
5. CsA-Implants in Veterinary Ophthalmology
5.1. Keratoconjunctivitis Sicca (KCS)
5.2. Chronic Superficial Keratitis (CSK)
5.3. Immune-Mediated Keratitis (IMMK)
5.4. Equine Recurrent Uveitis (ERU)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Colombo, D.; Ammirati, E. Cyclosporine in transplantation–A history of converging timelines. J. Biol. Regul. Homeost. Agents 2011, 25, 493–504. [Google Scholar] [PubMed]
- Borel, J.F. History of the discovery of cyclosporin and of its early pharmacological development. Wien. Klin. Wochenschr. 2002, 114, 433–437. [Google Scholar] [PubMed]
- Borel, J.F. Comparative study of in vitro and in vivo drug effects on cell-mediated cytotoxicity. Immunology 1976, 31, 631–641. [Google Scholar]
- Faulds, D.; Goa, K.L.; Benfield, P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs 1993, 45, 953–1040. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, A.; Ahmadi, S.R.; Anavri Ardakani, S.; Foroughian, M. Cyclosporine-A-Based Immunosuppressive Therapy-Induced Neurotoxicity: A Case Report. Open Access Emerg. Med. 2020, 12, 93–97. [Google Scholar] [CrossRef]
- Shi, W.; Chen, M.; Xie, L.; Liu, M.; Gao, H.; Wang, T.; Wu, X.; Zhao, J. A novel cyclosporine a drug-delivery system for prevention of human corneal rejection after high-risk keratoplasty: A clinical study. Ophthalmology 2013, 120, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Z.; Liu, T.; Li, S.; Gao, H.; Wei, C.; Shi, W. Cyclosporine a drug-delivery system for high-risk penetrating keratoplasty: Stabilizing the intraocular immune microenvironment. PLoS ONE 2018, 13, e0196571. [Google Scholar] [CrossRef] [Green Version]
- Eperon, S.; Rodriguez-Aller, M.; Balaskas, K.; Gurny, R.; Guex-Crosier, Y. A new drug delivery system inhibits uveitis in an animal model after cataract surgery. Int. J. Pharm. 2013, 443, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Zhang, H.; Tian, F.; Gu, H.Q.; Liu, X.; Sun, J. The study of cyclosporin A modified intraocular lens preventing posterior capsular opacification in rabbit eyes. Zhonghua Yan Ke Za Zhi 2016, 52, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Sun, J.; Wen, K.; Han, G.; Tian, F. Observation of cyclosporin A: Sustained release intraocular lens implantation in rabbit eyes. Curr. Eye Res. 2022, 47, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Han, Y.; Liu, D.; Chen, S.; Qie, J.; Qu, J.; Lin, Q. Centrifugally concentric ring-patterned drug-loaded polymeric coating as an intraocular lens surface modification for efficient prevention of posterior capsular opacification. Acta Biomater. 2022, 138, 327–341. [Google Scholar] [CrossRef]
- Lyu, N.; Zhao, Y.; Xiang, J.; Fan, X.; Huang, C.; Sun, X.; Xu, J.; Xu, Z.P.; Sun, J. Inhibiting corneal neovascularization by sustainably releasing anti-VEGF and anti-inflammation drugs from silica-thermogel nanohybrids. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112274. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Yu, X.; Hong, J.; Liu, X.; Sun, J.; Sun, X. Development of a novel CsA-PLGA drug delivery system based on a glaucoma drainage device for the prevention of postoperative fibrosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 206–214. [Google Scholar] [CrossRef]
- Velluto, D.; Demurtas, D.; Hubbell, J.A. PEG-b-PPS diblock copolymer aggregates for hydrophobic drug solubilization and release: Cyclosporin A as an example. Mol. Pharm. 2008, 5, 632–642. [Google Scholar] [CrossRef]
- Czogalla, A. Oral cyclosporine A—The current picture of its liposomal and other delivery systems. Cell. Mol. Biol. Lett. 2009, 14, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Bravo González, R.C.; Huwyler, J.; Walter, I.; Mountfield, R.; Bittner, B. Improved oral bioavailability of cyclosporin A in male Wistar rats. Comparison of a Solutol HS 15 containing self-dispersing formulation and a microsuspension. Int. J. Pharm. 2002, 245, 143–151. [Google Scholar] [CrossRef]
- Rose, S.; Jenner, P.; Marsden, C.D. Peripheral pharmacokinetic handling and metabolism of L-dopa in the rat: The effect of route of administration and carbidopa pretreatment. J. Pharm. Pharmacol. 1991, 43, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Barker, S.A.; Craig, D.Q.M. Design and Characterization of Cyclosporine A-Loaded Nanofibers for Enhanced Drug Dissolution. ACS Omega 2020, 5, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.A.; Wang, H.; Napoli, K.L.; Kahan, B.D.; Strobel, H.W. Metabolism of cyclosporine by cytochromes P450 3A9 and 3A4. Eur. J. Drug Metab. Pharmacokinet. 1999, 24, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Price, D.A.; Eng, H.; Farley, K.A.; Goetz, G.H.; Huang, Y.; Jiao, Z.; Kalgutkar, A.S.; Kablaoui, N.M.; Khunte, B.; Liras, S.; et al. Comparative Pharmacokinetic Profile of Cyclosporine (CsA) with a Decapeptide and a Linear Analogue. Org. Biomol. Chem. 2017, 15, 2501–2506. [Google Scholar] [CrossRef]
- Marshall, G.R.; Beusen, D.D.; Nikiforovich, G.V. 5–Peptide Conformation: Stability and Dynamics. In Peptides; Gutte, B., Ed.; Academic Press: San Diego, CA, USA, 1995; pp. 193–245. [Google Scholar] [CrossRef]
- Patel, D.; Wairkar, S. Recent advances in cyclosporine drug delivery: Challenges and opportunities. Drug Deliv. Transl. Res. 2019, 9, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Clipstone, N.A.; Crabtree, G.R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 1992, 357, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Koyasu, S. Mechanisms of action of cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Matsuda, S.; Moriguchi, T.; Koyasu, S.; Nishida, E. T lymphocyte activation signals for interleukin-2 production involve activation of MKK6-p38 and MKK7-SAPK/JNK signaling pathways sensitive to cyclosporin A. J. Biol. Chem. 1998, 273, 12378–12382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karin, M.; Delhase, M. JNK or IKK, AP-1 or NF-kappaB, which are the targets for MEK kinase 1 action? Proc. Natl. Acad. Sci. USA 1998, 95, 9067–9069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, T.L.; Deckert, M.; Altman, A. Views on Vav. Immunol. Today 1997, 18, 221–225. [Google Scholar] [CrossRef]
- Matsuda, S.; Shibasaki, F.; Takehana, K.; Mori, H.; Nishida, E.; Koyasu, S. Two distinct action mechanisms of immunophilin-ligand complexes for the blockade of T-cell activation. EMBO Rep. 2000, 1, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, P.; Rupenthal, I.D. Modern approaches to the ocular delivery of cyclosporine A. Drug Discov. Today 2016, 21, 977–988. [Google Scholar] [CrossRef]
- Nussenblatt, R.B.; Palestine, A.G. Cyclosporine: Immunology, pharmacology and therapeutic uses. Surv. Ophthalmol. 1986, 31, 159–169. [Google Scholar] [CrossRef]
- Lallemand, F.; Felt-Baeyens, O.; Besseghir, K.; Behar-Cohen, F.; Gurny, R. Cyclosporine A delivery to the eye: A pharmaceutical challenge. Eur. J. Pharm. Biopharm. 2003, 56, 307–318. [Google Scholar] [CrossRef]
- Mihatsch, M.J.; Kyo, M.; Morozumi, K.; Yamaguchi, Y.; Nickeleit, V.; Ryffel, B. The side-effects of ciclosporine-A and tacrolimus. Clin. Nephrol. 1998, 49, 356–363. [Google Scholar] [PubMed]
- Williams, D.L. A comparative approach to topical cyclosporine therapy. Eye 1997, 11 Pt 4, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Utine, C.A.; Stern, M.; Akpek, E.K. Clinical review: Topical ophthalmic use of cyclosporin A. Ocul. Immunol. Inflamm. 2010, 18, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Hessen, M.; Akpek, E.K. Ocular graft-versus-host disease. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.S.; Shtein, R.M.; Michelotti, M.M.; Cooney, T.M. Topical cyclosporine A 0.05% for recurrent anterior uveitis. Br. J. Ophthalmol. 2016, 100, 345–347. [Google Scholar] [CrossRef]
- Singhal, D.; Sahay, P.; Maharana, P.K.; Raj, N.; Sharma, N.; Titiyal, J.S. Vernal Keratoconjunctivitis. Surv. Ophthalmol. 2019, 64, 289–311. [Google Scholar] [CrossRef]
- Gilger, B.C.; Michau, T.M. Equine recurrent uveitis: New methods of management. Vet. Clin. N. Am. Equine Pract. 2004, 20, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.; Gilger, B.C. Equine immune-mediated keratopathies. Vet. Ophthalmol. 2009, 12 (Suppl. 1), 10–16. [Google Scholar] [CrossRef] [PubMed]
- Pearson, P.A.; Jaffe, G.J.; Martin, D.F.; Cordahi, G.J.; Grossniklaus, H.; Schmeisser, E.T.; Ashton, P. Evaluation of a delivery system providing long-term release of cyclosporine. Arch. Ophthalmol. 1996, 114, 311–317. [Google Scholar] [CrossRef]
- Smith, T.J.; Pearson, P.A.; Blandford, D.L.; Brown, J.D.; Goins, K.A.; Hollins, J.L.; Schmeisser, E.T.; Glavinos, P.; Baldwin, L.B.; Ashton, P. Intravitreal sustained-release ganciclovir. Arch. Ophthalmol. 1992, 110, 255–258. [Google Scholar] [CrossRef]
- Enyedi, L.B.; Pearson, P.A.; Ashton, P.; Jaffe, G.J. An intravitreal device providing sustained release of cyclosporine and dexamethasone. Curr. Eye Res. 1996, 15, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Yang, C.S.; Wang, X.C.; Cousins, S.W.; Gallemore, R.P.; Ashton, P. Intravitreal sustained-release cyclosporine in the treatment of experimental uveitis. Ophthalmology 1998, 105, 46–56. [Google Scholar] [CrossRef]
- Izci, C.; Celik, I.; Alkan, F.; Ogurtan, Z.; Ceylan, C.; Sur, E.; Ozkan, Y. Histologic characteristics and local cellular immunity of the gland of the third eyelid after topical ophthalmic administration of 2% cyclosporine for treatment of dogs with keratoconjunctivitis sicca. Am. J. Vet. Res. 2002, 63, 688–694. [Google Scholar] [CrossRef]
- Kaswan, R.L.; Salisbury, M.A.; Ward, D.A. Spontaneous canine keratoconjunctivitis sicca. A useful model for human keratoconjunctivitis sicca: Treatment with cyclosporine eye drops. Arch. Ophthalmol. 1989, 107, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Kaswan, R.L.; Salisbury, M.A. A new perspective on canine keratoconjunctivitis sicca. Treatment with ophthalmic cyclosporine. Vet. Clin. N. Am. Small Anim. Pract. 1990, 20, 583–613. [Google Scholar] [CrossRef]
- Bittencourt, M.K.W.; Barros, M.A.; Martins, J.F.P.; Vasconcellos, J.P.C.; Morais, B.P.; Pompeia, C.; Bittencourt, M.D.; Evangelho, K.D.S.; Kerkis, I.; Wenceslau, C.V. Allogeneic mesenchymal stem cell transplantation in dogs with keratoconjunctivitis sicca. Cell Med. 2016, 8, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Dodi, P.L. Immune-mediated keratoconjunctivitis sicca in dogs: Current perspectives on management. Vet. Med. Res. Rep. 2015, 6, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.L.; Tighe, A.A. Immunohistochemical evaluation of lymphocyte populations in the nictitans glands of normal dogs and dogs with keratoconjunctivitis sicca. Open Vet. J. 2018, 8, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Csaky, K.G.; Gilger, B.C.; Dunn, J.P.; Lee, S.S.; Tremblay, M.; de Monasterio, F.; Tansey, G.; Yuan, P.; Bungay, P.M.; et al. Preclinical evaluation of a novel episcleral cyclosporine implant for ocular graft-versus-host disease. Investig. Ophthalmol. Vis. Sci. 2005, 46, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Acton, A.E.; Beale, A.B.; Gilger, B.C.; Stoskopf, M.K. Sustained release cyclosporine therapy for bilateral keratoconjunctivitis sicca in a red wolf (Canis rufus). J. Zoo Wildl. Med. 2006, 37, 562–564. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, H.; Wang, N.S.; Bungay, P.M.; Gilger, B.C.; Yuan, P.; Kim, J.; Csaky, K.G.; Robinson, M.R. A pharmacokinetic and safety evaluation of an episcleral cyclosporine implant for potential use in high-risk keratoplasty rejection. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2023–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barachetti, L.; Rampazzo, A.; Mortellaro, C.M.; Scevola, S.; Gilger, B.C. Use of episcleral cyclosporine implants in dogs with keratoconjunctivitis sicca: Pilot study. Vet. Ophthalmol. 2015, 18, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Li, Y.; Jin, R.; Shrestha, T.; Choi, J.S.; Lee, W.J.; Moon, M.J.; Ju, H.T.; Choi, W.; Yoon, K.C. The Efficiency of Cyclosporine A-Eluting Contact Lenses for the Treatment of Dry Eye. Curr. Eye Res. 2019, 44, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Soluri, A.; Hui, A.; Jones, L. Delivery of ketotifen fumarate by commercial contact lens materials. Optom. Vis. Sci. 2012, 89, 1140–1149. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact lenses: Promising devices for ocular drug delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Shaikh, A.A.; Lakdawala, D.H.; Desai, A.R.; Pandya, M.M.; Singhania, S.S.; Vaidya, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Design and optimization of a novel implantation technology in contact lenses for the treatment of dry eye syndrome: In vitro and in vivo evaluation. Acta Biomater. 2017, 53, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Chandasana, H.; Prasad, Y.D.; Chhonker, Y.S.; Chaitanya, T.K.; Mishra, N.N.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Corneal targeted nanoparticles for sustained natamycin delivery and their PK/PD indices: An approach to reduce dose and dosing frequency. Int. J. Pharm. 2014, 477, 317–325. [Google Scholar] [CrossRef]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef]
- Shirley, M. Bimatoprost implant: First approval. Drugs Aging 2020, 37, 457–462. [Google Scholar] [CrossRef]
- Vanslette, A.; Blizzard, C.D.; Haberman, P.; Tomaszewski, J.; Rosales, C.; Metzinger, J.L.; Goldstein, M.H.; Driscoll, A. Pharmacokinetics of OTX-CSI, a Sustained Release Cyclosporine Intracanalicular Insert in Beagles. Investig. Ophthalmol. Vis. Sci. 2019, 60, 285. [Google Scholar]
- Slatter, D.H.; Lavach, J.D.; Severin, G.A.; Young, S. Uberreiter’s syndrome (chronic superficial keratitis) in dogs in the Rocky Mountain area—A study of 463 cases. J. Small Anim. Pract. 1977, 18, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Andrew, S.E. Immune-mediated canine and feline keratitis. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Bedford, P.G.; Longstaffe, J.A. Corneal pannus (chronic superficial keratitis) in the German shepherd dog. J. Small Anim. Pract. 1979, 20, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, J.D.; Lavach, J.D.; Gould, D.H.; Severin, G.A.; Paulsen, M.E.; Jones, R.L. Immunohistochemical staining patterns of canine eyes affected with chronic superficial keratitis. Am. J. Vet. Res. 1986, 47, 1952–1955. [Google Scholar] [PubMed]
- Williams, D.L. Histological and immunohistochemical evaluation of canine chronic superficial keratitis. Res. Vet. Sci. 1999, 67, 191–195. [Google Scholar] [CrossRef]
- Williams, D.L.; Cribb, A.; Scott, J.E. Proteoglycan-collagen interactions in chronic superficial keratitis in the dog. Biochem. Soc. Trans. 1991, 19, 353S. [Google Scholar] [CrossRef]
- Drahovska, Z.; Balicki, I.; Trbolova, A.; Mihalova, M.; Holickova, M. A retrospective study of the occurrence of chronic superficial keratitis in 308 German Shepherd dogs: 1999–2010. Pol. J. Vet. Sci. 2014, 17, 543–546. [Google Scholar] [CrossRef] [Green Version]
- Chavkin, M.J.; Roberts, S.M.; Salman, M.D.; Severin, G.A.; Scholten, N.J. Risk factors for development of chronic superficial keratitis in dogs. J. Am. Vet. Med. Assoc. 1994, 204, 1630–1634. [Google Scholar]
- Denk, N.; Fritsche, J.; Reese, S. The effect of UV-blocking contact lenses as a therapy for canine chronic superficial keratitis. Vet. Ophthalmol. 2011, 14, 186–194. [Google Scholar] [CrossRef]
- Williams, D.L.; Hoey, A.J.; Smitherman, P. Comparison of topical cyclosporin and dexamethasone for the treatment of chronic superficial keratitis in dogs. Vet. Rec. 1995, 137, 635–639. [Google Scholar]
- Gilger, B.C.; Michau, T.M.; Salmon, J.H. Immune-mediated keratitis in horses: 19 cases (1998–2004). Vet. Ophthalmol. 2005, 8, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Gilger, B.C.; Stoppini, R.; Wilkie, D.A.; Clode, A.B.; Pinto, N.H.; Hempstead, J.; Gerding, J.; Salmon, J.H. Treatment of immune-mediated keratitis in horses with episcleral silicone matrix cyclosporine delivery devices. Vet. Ophthalmol. 2014, 17 (Suppl. 1), 23–30. [Google Scholar] [CrossRef] [PubMed]
- Sandmeyer, L.S.; Bauer, B.S.; Feng, C.X.; Grahn, B.H. Equine recurrent uveitis in western Canadian prairie provinces: A retrospective study (2002–2015). Can. Vet. J. 2017, 58, 717–722. [Google Scholar] [PubMed]
- Gerding, J.C.; Gilger, B.C. Prognosis and impact of equine recurrent uveitis. Equine Vet. J. 2016, 48, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Kalsow, C.M.; Dwyer, A.E.; Smith, A.W.; Nifong, T.P. Pinealitis accompanying equine recurrent uveitis. Br. J. Ophthalmol. 1993, 77, 46–48. [Google Scholar] [CrossRef]
- Deeg, C.A.; Ehrenhofer, M.; Thurau, S.R.; Reese, S.; Wildner, G.; Kaspers, B. Immunopathology of recurrent uveitis in spontaneously diseased horses. Exp. Eye Res. 2002, 75, 127–133. [Google Scholar] [CrossRef]
- Malalana, F.; Stylianides, A.; McGowan, C. Equine recurrent uveitis: Human and equine perspectives. Vet. J. 2015, 206, 22–29. [Google Scholar] [CrossRef]
- Allbaugh, R.A. Equine recurrent uveitis: A review of clinical assessment and management. Equine Vet. Educ. 2017, 29, 279–288. [Google Scholar] [CrossRef]
- Lorenz, L.; Amann, B.; Hirmer, S.; Degroote, R.L.; Hauck, S.M.; Deeg, C.A. NEU1 is more abundant in uveitic retina with concomitant desialylation of retinal cells. Glycobiology 2021, 31, 873–883. [Google Scholar] [CrossRef]
- McMullen, R.J.; Fischer, B.M. Medical and surgical management of equine recurrent uveitis. Vet. Clin. N. Am. Equine Pract. 2017, 33, 465–481. [Google Scholar] [CrossRef]
- Gilger, B.C.; Malok, E.; Stewart, T.; Horohov, D.; Ashton, P.; Smith, T.; Jaffe, G.J.; Allen, J.B. Effect of an intravitreal cyclosporine implant on experimental uveitis in horses. Vet. Immunol. Immunopathol. 2000, 76, 239–255. [Google Scholar] [CrossRef]
- Gilger, B.C.; Malok, E.; Stewart, T.; Ashton, P.; Smith, T.; Jaffe, G.J.; Allen, J.B. Long-term effect on the equine eye of an intravitreal device used for sustained release of cyclosporine A. Vet. Ophthalmol. 2000, 3, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Gilger, B.C.; Wilkie, D.A.; Davidson, M.G.; Allen, J.B. Use of an intravitreal sustained-release cyclosporine delivery device for treatment of equine recurrent uveitis. Am. J. Vet. Res. 2001, 62, 1892–1896. [Google Scholar] [CrossRef] [PubMed]
- Shane, T.S.; Martin, D.F.; Endopthalmitis-Gancioclovir Implant Study Group. Endophthalmitis after ganciclovir implant in patients with AIDS and cytomegalovirus retinitis. Am. J. Ophthalmol. 2003, 136, 649–654. [Google Scholar] [CrossRef]
- Sakurai, E.; Nozaki, M.; Okabe, K.; Kunou, N.; Kimura, H.; Ogura, Y. Scleral plug of biodegradable polymers containing tacrolimus (FK506) for experimental uveitis. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4845–4852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, H.; Ogura, Y.; Hashizoe, M.; Kunou, N.; Honda, Y.; Ikada, Y. Biodegradable scleral implant for intravitreal controlled release of fluconazole. Curr. Eye Res. 1997, 16, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Kimura, H.; Okabe, J.; Kato, A.; Kunou, N.; Ogura, Y. Intraocular tissue distribution of betamethasone after intrascleral administration using a non-biodegradable sustained drug delivery device. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2702–2707. [Google Scholar] [CrossRef] [Green Version]
- Okabe, J.; Kimura, H.; Kunou, N.; Okabe, K.; Kato, A.; Ogura, Y. Biodegradable intrascleral implant for sustained intraocular delivery of betamethasone phosphate. Investig. Ophthalmol. Vis. Sci. 2003, 44, 740–744. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Kimura, H.; Okabe, K.; Okabe, J.; Kunou, N.; Ogura, Y. Feasibility of drug delivery to the posterior pole of the rabbit eye with an episcleral implant. Investig. Ophthalmol. Vis. Sci. 2004, 45, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Gilger, B.C.; Salmon, J.H.; Wilkie, D.A.; Cruysberg, L.P.J.; Kim, J.; Hayat, M.; Kim, H.; Kim, S.; Yuan, P.; Lee, S.S.; et al. A novel bioerodible deep scleral lamellar cyclosporine implant for uveitis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2596–2605. [Google Scholar] [CrossRef]
- Cislo-Pakuluk, A.; Smieszek, A.; Kucharczyk, N.; Bedford, P.G.C.; Marycz, K. Intra-Vitreal Administration of Microvesicles Derived from Human Adipose-Derived Multipotent Stromal Cells Improves Retinal Functionality in Dogs with Retinal Degeneration. J. Clin. Med. 2019, 8, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cislo-Pakuluk, A.; Marycz, K. A Promising Tool in Retina Regeneration: Current Perspectives and Challenges When Using Mesenchymal Progenitor Stem Cells in Veterinary and Human Ophthalmological Applications. Stem Cell Rev. Rep. 2017, 13, 598–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padjasek, M.; Qasem, B.; Cisło-Pakuluk, A.; Marycz, K. Cyclosporine A Delivery Platform for Veterinary Ophthalmology—A New Concept for Advanced Ophthalmology. Biomolecules 2022, 12, 1525. https://doi.org/10.3390/biom12101525
Padjasek M, Qasem B, Cisło-Pakuluk A, Marycz K. Cyclosporine A Delivery Platform for Veterinary Ophthalmology—A New Concept for Advanced Ophthalmology. Biomolecules. 2022; 12(10):1525. https://doi.org/10.3390/biom12101525
Chicago/Turabian StylePadjasek, Martyna, Badr Qasem, Anna Cisło-Pakuluk, and Krzysztof Marycz. 2022. "Cyclosporine A Delivery Platform for Veterinary Ophthalmology—A New Concept for Advanced Ophthalmology" Biomolecules 12, no. 10: 1525. https://doi.org/10.3390/biom12101525