Modelling Hyperglycaemia in an Epithelial Membrane Model: Biophysical Characterisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
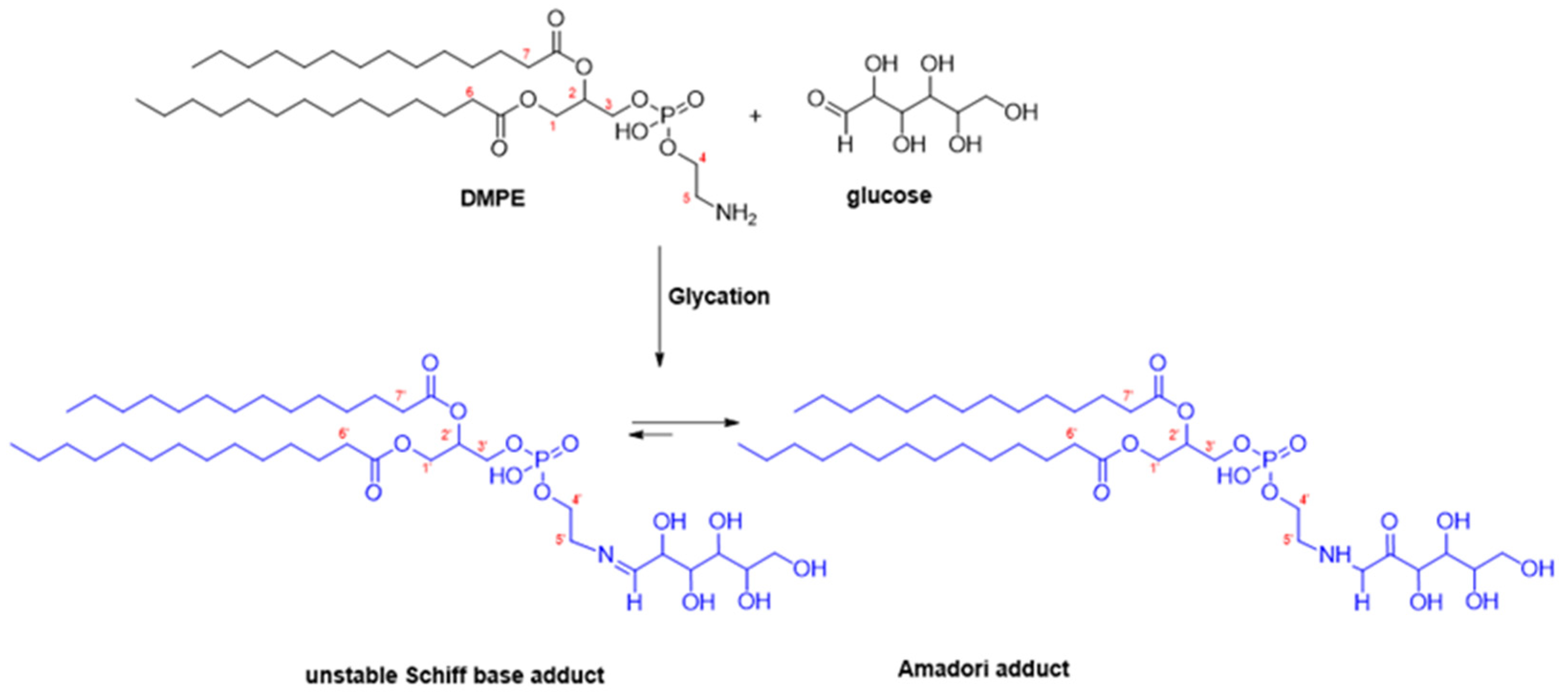
2.2. Synthesis of DMPE-Glucose Adducts
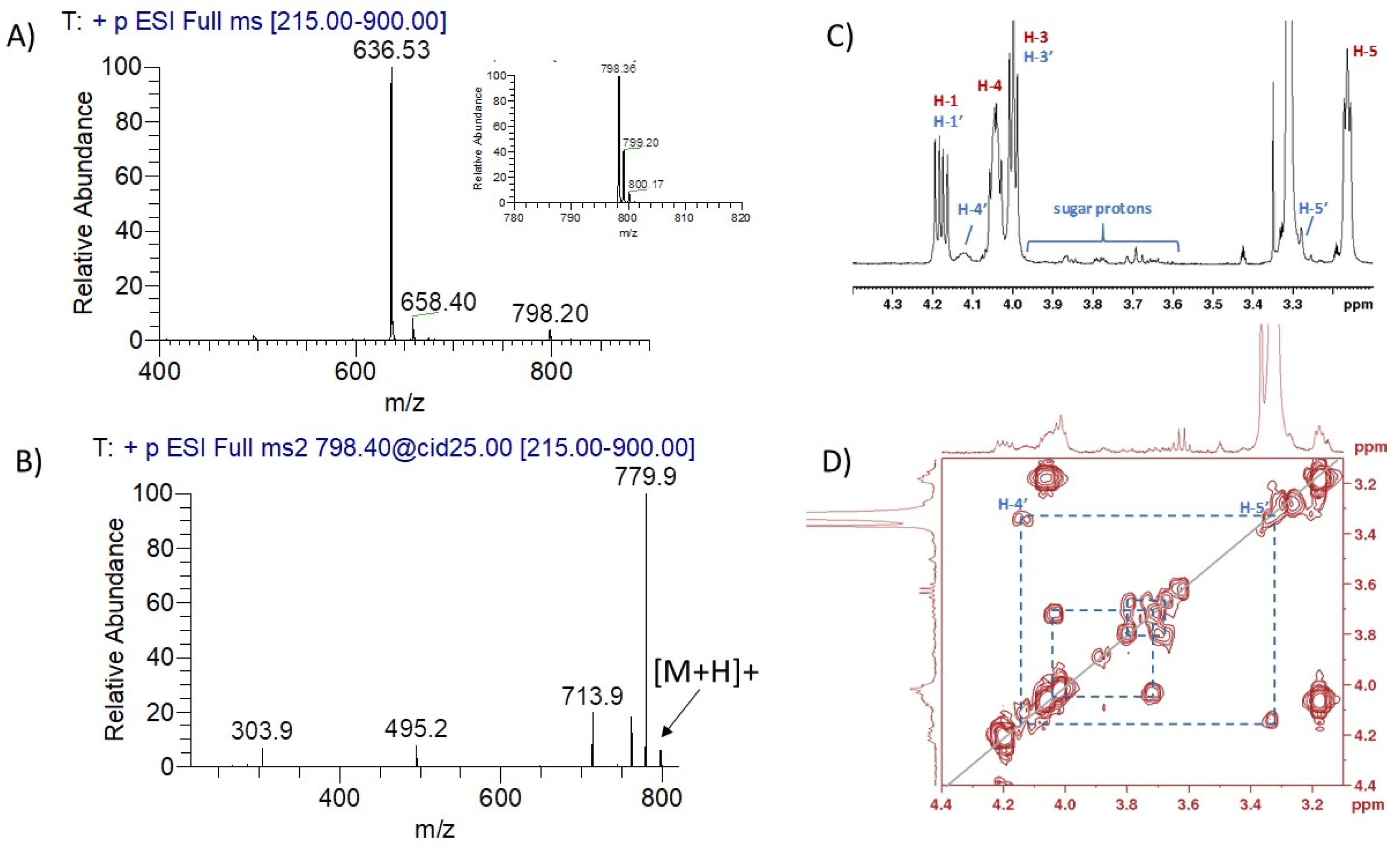
2.3. Characterisation of DMPE-Glucose Adducts by Mass Spectrometry (ESI-MS) and Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3.1. ESI-MS and ESI-MS/MS Analysis
2.3.2. NMR Analysis
2.4. Preparation of Large Unilamelar Vesicles (LUVs)
2.5. Characterisation of Thermotropic Behaviour of Epithelial Models by Spectroscopic Approaches
2.6. Determination of Surface Potential
3. Results
3.1. Synthesis and Structural Characterisation of Glycated Dimyristoyl-Phosphatidylethanolamine (DMPE-Glyc) Adducts
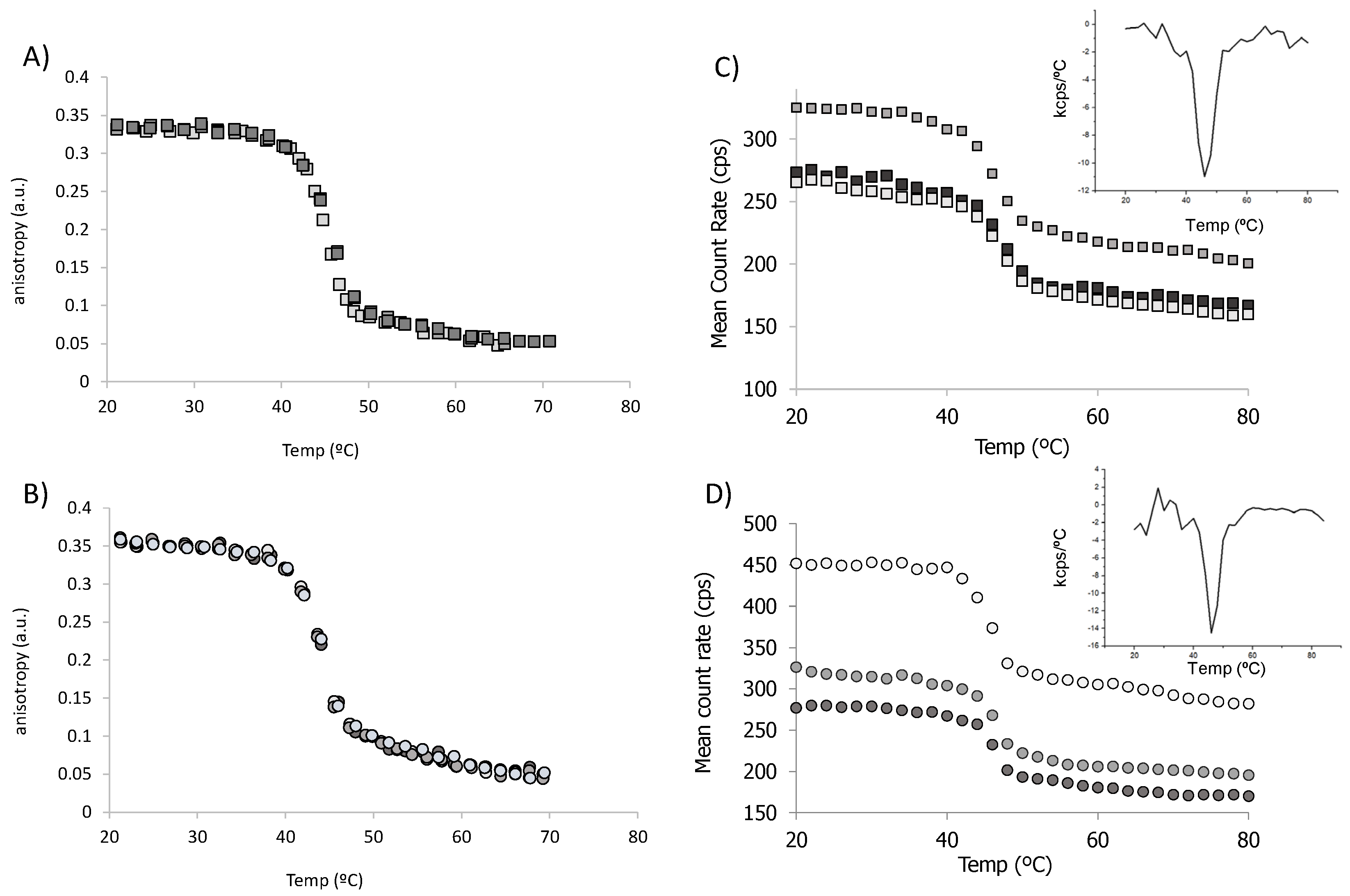
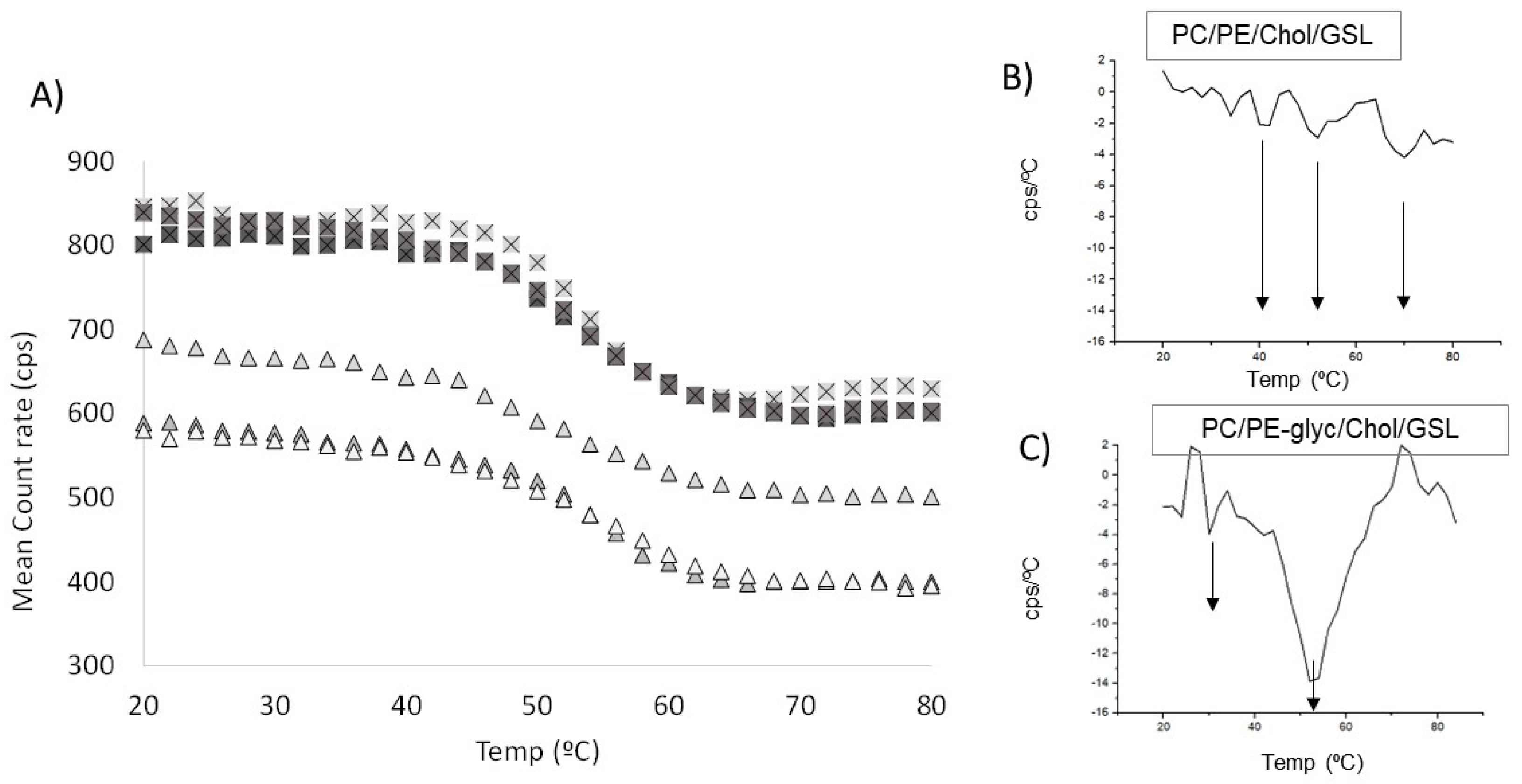
3.2. Development and Thermotropic Characterisation of Epithelial Membrane Model (PL/Chol/GSL) under Normoglycaemic Conditions
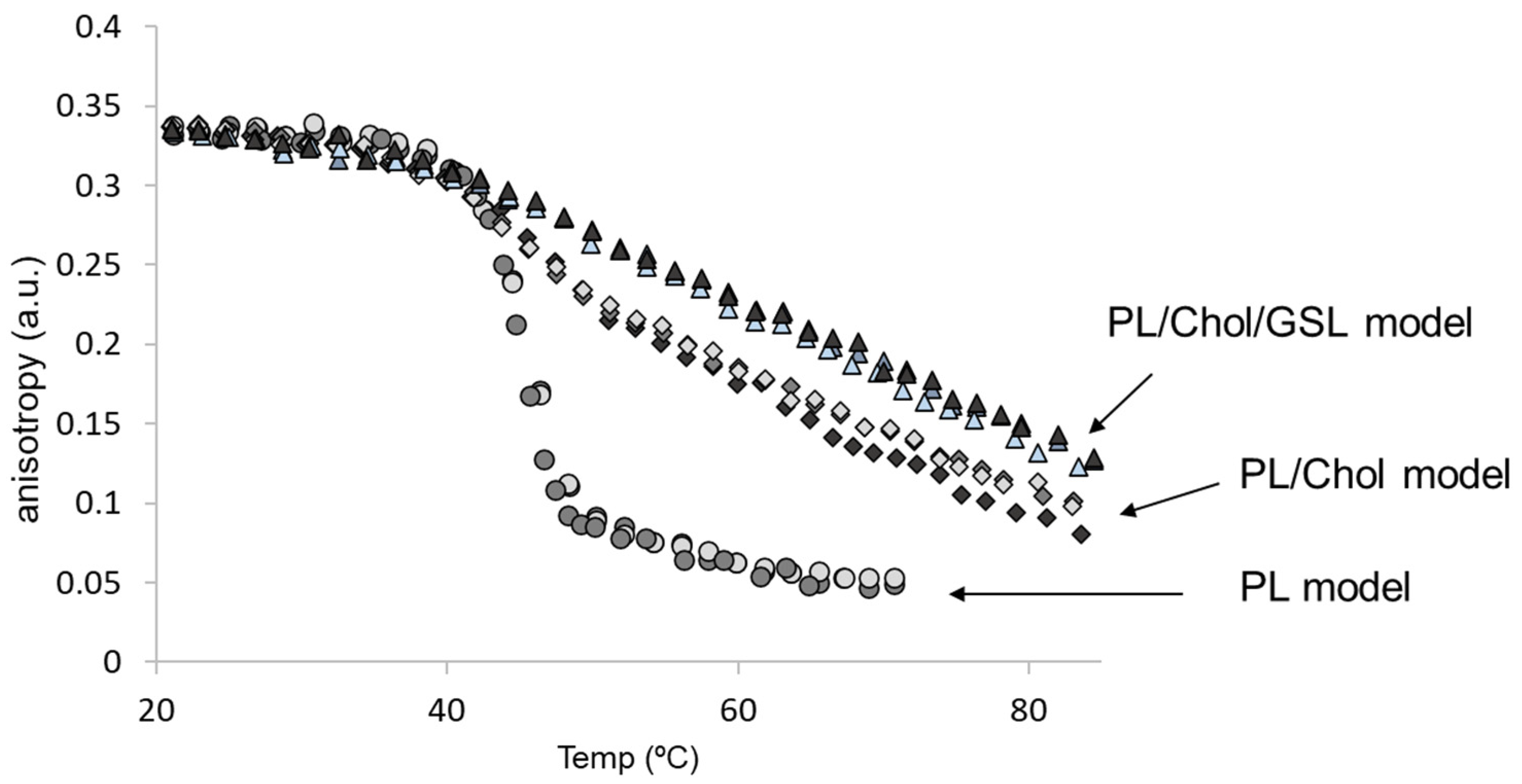
3.3. Effect of Hyperglycaemia on the Properties of Epithelial Membrane Model (PL/Chol/GSL)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webber, S. International Diabetes Federation Report 2021, 10th ed.; GLOBODIAB Consortium: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar] [CrossRef]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics; European Heart Network: Brussels, Belgium, 2017; Volume 34, pp. 3028–3034. [Google Scholar]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.L.; Ramkumar, K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorello, M.L.; Treweeke, A.T.; MacFarlane, D.P.; Megson, I.L. The impact of glucose exposure on bioenergetics and function in a cultured endothelial cell model and the implications for cardiovascular health in diabetes. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Rocha, S.; Oskolkova, O.; de Freitas, V.; Reis, A. (Poly)phenol-Rich Diets in the Management of Endothelial Dysfunction in Diabetes Mellitus: Biological Properties in Cultured Endothelial Cells. Mol. Nutr. Food Res. 2021, 65, 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Feng, L.; Jin, H. Constant or fluctuating hyperglycemias increases cytomembrane stiffness of human umbilical vein endothelial cells in culture: Roles of cytoskeletal rearrangement and nitric oxide synthesis. BMC Cell Biol. 2013, 14, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravandi, A.; Kuksis, A.; Marai, L.; Myher, J.; Steiner, G.; Lewis, G. Kamido, H. Isolation and identification of glycated aminophospholipids from red cells and plasma of diabetic blood. FEBS Lett. 1996, 381, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, K.; Oak, J.-H.; Higuchi, O.; Tsuduki, T.; Oikawa, S.; Otani, H.; Mune, M.; Cai, H.; Miyazawa, T. Ion-trap tandem mass spectrometric analysis of Amadori-glycated phosphatidylethanolamine in human plasma with or without diabetes. J. Lipid. Res. 2005, 46, 2514–2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazawa, T.; Kamiyoshihara, R.; Shimizu, N.; Harigae, T.; Otoki, Y.; Ito, J.; Kato, S.; Miyazawa, T.; Nakagawa, K. Amadori-glycated phosphatidylethanolamine enhances the physical stability and selective targeting ability of liposomes. R. Soc. Open Sci. 2018, 5, 171249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, K.; Fujita, M.; Nakao, M. Lipid components of two different regions of an intestinal epithelial cell membrane of mouse. Biochim. Biophys. Acta 1974, 369, 222–233. [Google Scholar]
- Schulthess, G.; Hauser, H. A unique feature of lipid dynamics in small intestinal brush border membrane. Mol. Memb. Biol. 1995, 12, 105–112. [Google Scholar] [CrossRef]
- Reis, A.; de Freitas, V. When polyphenols meet lipids: Challenges in membrane biophysics and opportunities in epithelial lipidomics. Food Chem. 2020, 333, 127509. [Google Scholar] [CrossRef] [PubMed]
- Ayee, M.A.A.; LeMaster, E.; Shentu, T.P.; Singh, D.K.; Barbera, N.; Soni, D.; Tiruppathi, C.; Subbaiah, P.V.; Berdyshev, E.; Bronova, I.; et al. Molecular-Scale Biophysical Modulation of an Endothelial Membrane by Oxidized Phospholipids. Biol. J. 2017, 112, 325–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanovic, O.; Skulj, S.; Pohl, O.O.; Vazdar, M. Covalent modification of phosphatidylethanolamine by 4-hydroxy-2-nonenal increases sodium permeability across phospholipid bilayer membranes. Free Rad. Biol. Med. 2019, 143, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Weerachatyanukul, W.; Probodh, I.; Kongmanas, K.; Tanphaichitr, N.; Johnston, L.J. Visualizing the localization of sulfoglycolipids in lipid raft domains in model membranes and sperm membrane extracts. Biochem. Biophys. Acta 2007, 1768, 229–310. [Google Scholar] [CrossRef]
- Supancic, E.; Carreira, A.C.; Almeida, R.F.M.; Silva, L. Biophysical implications of sphingosine accumulation in membrane properties at neutral and acidic pH. J. Phys. Chem. B 2014, 118, 4858–4866. [Google Scholar] [CrossRef]
- Varela, A.R.; Couto, A.S.; Fedorov, A.; Futerman, A.H.; Prieto, M.; Silva, L.C. Glucosylceramide Reorganizes Cholesterol-Containing Domains in a Fluid Phospholipid Membrane. Biophys. J. 2016, 110, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Varela, A.R.P.; Ventura, A.E.; Carreira, A.C.; Fedorov, A.; Futerman, A.H.; Prieto, M.; Silva, L.C. Pathological levels of glucosylceramide change the biophysical properties of artificial and cell membranes. Phys. Chem. Chem. Phys. 2017, 19, 340–346. [Google Scholar] [CrossRef]
- Zhu, W.; Deng, X.; Peng, J.; Zou, B.; Li, C. A-type ECG and EGCG dimers inhibit 3T3-L1 differentiation by binding to cholesterol in lipid rafts. J. Nutr. Biochem. 2017, 48, 62–73. [Google Scholar] [CrossRef]
- Simões, C.; Simões, V.; Reis, A.; Domingues, P.; Domingues, M.R.M. Oxidation of glycated phosphatidylethanolamines: Evidence of oxidation in glycated polar head identified by LC-MS/MS. Anal. Bioanal. Chem. 2010, 397, 2417–2427. [Google Scholar] [CrossRef]
- He, X.; Zhang, Q. Synthesis, Purification, and Mass Spectrometric Characterization of Stable Isotope-Labeled Amadori-Glycated Phospholipids. ACS Omega 2018, 3, 15725–15733. [Google Scholar] [CrossRef]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochem. Biophys. Acta 1985, 812, 55–65. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Merino-Montero, S.; Montero, M.T.; Hernadez-Borrell, J. Effects of lactose permease of Escherichia coli on the anisotropy and electrostatic surface potential of liposomes. Biophys. Chem. 2006, 119, 101–105. [Google Scholar] [CrossRef]
- Lertsiri, S.; Shiraishi, M.; Miyazawa, T. Identification of Deoxy-D-Fructosyl Phosphatidylethanolamine as a non-enzymic glycation product of phosphatidylethanolamine and its occurrence in human blood plasma and red blood cells. Biosci. Biotechnol. Biochem. 1998, 62, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Annibal, A.; Riemer, T.; Jovanovic, O.; Westphal, D.; Griesser, E.; Pohl, E.E.; Schiller, J.; Hoffmann, R.; Fedorova, M. Structural, biological and biophysical properties of glycated and glycoxidized phosphatidylethanolamines. Free Rad. Biol. Med. 2016, 95, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Kodate, A.; Otoki, Y.; Shimizu, N.; Ito, J.; Kato, S.; Umetsu, N.; Miyazawa, T.; Nakagawa, K. Development of quantitation method for glycated aminophospholipids at the molecular species level in powdered milk and powdered buttermilk. Sci. Rep. 2018, 8, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hollingsworth, R.I. A solvent system for the high-resolution proton nuclear magnetic resonance spectroscopy of membrane lipids. Anal. Biochem. 1995, 225, 242–251. [Google Scholar] [CrossRef]
- Sampaio, J.L.; Gerl, M.J.; Klose, C.; Ejsing, C.S.; Beug, H.; Simons, K.; Shevchenko, A. Membrane lipidome of an epithelial cell line. Proc. Nat. Acad. Sci. USA 2011, 108, 1903–1907. [Google Scholar] [CrossRef] [Green Version]
- Squier, C.A.; Cox, P.; Wertz, P.W. Lipid content and water permeability of skin and oral mucosa. J. Investig. Dermatol. 1991, 96, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Meyer zu Duttingdorf, H.D.; Sallmann, H.P.; Glockenthor, U.; von Engelhardt, W.; Busche, R. Isolation and lipid composition of apical and basolateral membranes of colonic segments of guinea pig. Anal. Biochem. 1999, 269, 45–63. [Google Scholar] [CrossRef]
- Nichols, G.E.; Shiraishi, T.; Young, W.W. Polarity of neutral glycolipids, gangliosides, and sulfated lipids in MDCK epithelial cells. J. Lipid. Res. 1988, 29, 1205–1213. [Google Scholar] [CrossRef]
- Reis, A.; Spickett, C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta 2012, 1818, 2374–2387. [Google Scholar] [CrossRef] [Green Version]
- Mateo, C.R.; Lillo, M.P.; Gonzalez-Rodriguez, J.; Acuña, A.U. Molecular order and fluidity of the plasma membrane of human platelets from time-resolved fluorescence depolarization. Eur. Biophys. J. 1991, 20, 41–52. [Google Scholar] [CrossRef]
- Kaiser, R.D.; London, E. Location of diphenylhexatriene (DPH) and its derivatives within membranes: Comparison of different fluorescence quenching analyses of membrane depth. Biochemistry 1999, 38, 2610. [Google Scholar] [CrossRef] [Green Version]
- Michel, N.; Fabiano, A.S.; Polidori, A.; Jack, R.; Pucci, B. Determination of phase transition temperatures of lipids by light scattering. Chem. Phys. Lipids 2006, 139, 11–19. [Google Scholar] [CrossRef]
- Lopes, S.; Neves, C.S.; Eaton, P.; Gameiro, P. Cardiolipin, a key component to mimic the E. coli bacterial membrane in model systems revealed by dynamic light scattering and steady-state fluorescence anisotropy. Anal. Bioanal. Chem. 2010, 398, 1357–1366. [Google Scholar] [CrossRef]
- Lopes, S.C.; Ivanova, G.; de Castro, B.; Gameiro, P. Revealing cardiolipins influence in the construction of a significant mitochondrial membrane model. Biochem. Biophys. Acta 2018, 1860, 2465–2477. [Google Scholar] [CrossRef]
- Sot, J.; Ibarguren, M.; Busto, J.V.; Montes, L.-R.; Goñi, F.M.; Alonso, A. Cholesterol displacement by ceramide in sphingomyelin-containing liquid-ordered domains, and generation of gel regions in giant lipidic vesicles. FEBS Lett. 2008, 582, 3230–3236. [Google Scholar] [CrossRef] [Green Version]
- Cyboran-Mikołajczyk, S.; Żyłka, R.; Jurkiewicz, P.; Pruchnik, H.; Oszmiański, J.; Hof, M.; Kleszczyńska, H. Interaction of procyanidin B3 with membrane lipids—Fluorescence, DSC and FTIR studies. Biochem. Biophys. Acta 2017, 1859, 1362–1371. [Google Scholar] [CrossRef]
- Redondo-Morata, L.; Gianotti, M.I.; Sanz, F. Influence of cholesterol on the phase transition of lipid bilayers: A temperature-controlled force spectroscopy study. Langmuir 2012, 28, 12851–12860. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Chen, W.; Dusa, F.; Witos, J.; Ruokonen, S.K.; Wiedmer, S.K. Determination of the Main Phase Transition Temperature of Phospholipids by Nanoplasmonic Sensing. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- De Meyer, F.; Smit, B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Nat. Acad. Sci. USA 2009, 106, 3654–3658. [Google Scholar] [CrossRef] [Green Version]
- Neves, A.R.; Nunes, C.; Reis, S. New Insights on the Biophysical Interaction of Resveratrol with Biomembrane Models: Relevance for Its Biological Effects. J. Phys. Chem. B 2015, 119, 11664–11672. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ando, J. Vascular endothelial cell membranes differentiate between stretch and shear stress through transitions in their lipid phases. Am. J. Physiol. 2015, 309, H1178–H1185. [Google Scholar] [CrossRef]
- Dawaliby, R.; Trubbia, C.; Delporte, C.; Noyon, C.; Ruysschaert, J.-M.; Van Antwerpen, P.; Govaerts, C. Phosphatidylethanolamine is a key regulator of membrane fluidity in eukaryotic cells. J. Biol. Chem. 2016, 291, 3658–3667. [Google Scholar] [CrossRef] [Green Version]
- Engberg, O.; Yasuda, T.; Hautala, V.; Matsumori, N.; Nyholm, T.K.; Murata, M.; Slotte, J.P. Lipid Interactions and Organization in Complex Bilayer Membranes. Biophys. J. 2016, 110, 1563–1573. [Google Scholar] [CrossRef] [Green Version]
- Veatch, S.L.; Keller, S.L. Seeing spots: Complex phase behavior in simple membranes. Biochem. Biophys. Acta 2005, 1746, 172–185. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.M.; Silva, L.C.; Fedorov, A.; de Almeida, R.F.M.; Prieto, M. Cholesterol-rich fluid membranes solubilize ceramide domains: Implications for the structure and dynamics of mammalian intracellular and plasma membranes. J. Biol. Chem. 2009, 284, 22978–22987. [Google Scholar] [CrossRef] [Green Version]
- Westerlund, B.; Slotte, J.P. How the molecular features of glycosphingolipids affect domain formation in fluid membranes. Biochem. Biophys. Acta 2009, 1788, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Saxena, K.; Duclos, R.I.; Zimmermann, P.; Schmidt, R.R.; Shipley, G.G. Structure and properties of totally synthetic galacto- and gluco- cerebrosides. J. Lipid. Res. 1999, 40, 839–849. [Google Scholar] [CrossRef]
- Niu, S.L.; Litman, B.J. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: Effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 2002, 83, 3408–3415. [Google Scholar] [CrossRef] [Green Version]
- Bakht, O.; Pathak, P.; London, E. Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): Identification of multiple raft-stabilization mechanisms. Biophys. J. 2007, 93, 4307–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, J.W.; Visser, N.V.; Kouptsova, O.; Visser, A.J.W. Oxidation of unsaturated phospholipids in membrane bilayer mixtures is accompanied by membrane fluidity changes. Biochem. Biophys. Acta 2000, 1487, 61–73. [Google Scholar] [CrossRef]
- Wong-Ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.-M.; Tieleman, D.P.; Monticelli, L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandelia, H.; Mouritsen, O.G. Lipid gymnastics: Evidence of complete acyl chain reversal in oxidized phospholipids from molecular simulations. Biophys. J. 2009, 96, 2734–2743. [Google Scholar] [CrossRef] [Green Version]
- Jurkiewicz, P.; Olżyńska, A.; Cwiklik, L.; Conte, E.; Jungwirth, P.; Megli, F.M.; Hof, M. Biophysics of lipid bilayers containing oxidatively modified phospholipids: Insights from fluorescence and EPR experiments and from MD simulations. Biochem. Biophys. Acta 2012, 1818, 2388–2402. [Google Scholar] [CrossRef] [Green Version]
- Allen, H.G.; Allen, J.C.; Boyd, L.C.; Alston-Mills, B.P.; Fenner, G.P. Determination of membrane lipid differences in insulin resistant diabetes mellitus type 2 in whites and blacks. Nutrition 2006, 22, 1096–1102. [Google Scholar] [CrossRef]
- Breimer, M.E.; Hansson, G.C.; Karlsson, K.A.; Larson, G.; Leffler, H. Glycosphingolipid composition of epithelial cells isolated along the villus axis of small intestine of a single human individual. Glycobiology 2012, 22, 1721–1730. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.; de Freitas, V.; Sanchez-Quesada, J.L.; Barros, A.S.; Diaz, S.O.; Leite-Moreira, A. Lipidomics in Cardiovascular Diseases. In Systems Medicine; Elsevier: Amsterdam, The Netherlands, 2021; Volume 3, pp. 454–467. [Google Scholar] [CrossRef]
- Yang, J.; Yang, S.; Gao, X.; Yuan, Y.J. Integrative investigation of lipidome and signal pathways in human endothelial cells under oxidative stress. Mol. Biosyst. 2011, 7, 2428–2440. [Google Scholar] [CrossRef]
- Colombo, S.; Melo, T.; Martínez-López, M.; Carrasco, M.J.; Domingues, M.R.; Pérez-Sala, D.; Domingues, P. Phospholipidome of endothelial cells shows a different adaptation response upon oxidative, glycative and lipoxidative stress. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hirata, T.; Yamamoto, K.; Ikeda, K.; Arita, M. Functional lipidomics of vascular endothelial cells in response to laminar shear stress. FASEB J. 2021, 35, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Liposome (mol%) | t1 (°C) | t2 (°C) | t3 (°C) | t4 (°C) | |
|---|---|---|---|---|---|
| DPH anisotropy | DPPC/DSPC (0.65:0.35) | 44.3 ± 1.1 | |||
| DPPC/DSPC/DMPE (0.50:0.28:0.22) | 43.8 ± 0.5 | ||||
| DPPC/DSPC/DMPE/Chol (0.37:0.21:0.16:0.26) | 46.9 ± 0.7 | 64.6 ± 0.1 | 76.4 ± 0.4 | ||
| DPPC/DSPC/DMPE/Chol/GSL (0.28:0.15:0.16:0.24:0.17) | 46.7 ± 2.6 | 52.3 ± 3.0 | 64.2 ± 1.2 | 77.5 ± 1.7 | |
| Dynamic Light Scattering (DLS) | DPPC/DSPC (0.64:0.36) | 48.0 ± 0.8 | |||
| DPPC/DSPC/DMPE (0.49:0.28:0.24) | 46.5 ± 0.9 | ||||
| DPPC/DSPC/DMPE/Chol (0.37:0.21:0.17:0.28) | 46.0 ± 1.6 | 59.3 ± 3.4 | 70.7 ± 1.9 | ||
| DPPC/DSPC/DMPE/Chol/GSL (0.29:0.15:0.15:0.25:0.15) | 44.7 ± 3.4 | 54.7 ± 0.9 | 65.7 ± 4.5 |
| Epithelial Model | Transition Temp (°C) | Anisotropy (rs, a.u.) | Size (nm) PDI | Surface Potential (ζ, mV) |
|---|---|---|---|---|
| 44.7 ± 3.4 | ||||
| Normoglycaemia 1 | 54.7 ± 0.9 | 0.320 ± 0.005 | 118.6 ± 5.70 | −1.00 ± 0.13 |
| 65.7 ± 4.5 | 0.08 ± 0.01 | |||
| 39.3 ± 0.9 | ||||
| Hyperglycaemia 2 | 53.8 ± 0.4 | 0.308 ± 0.002 | 123.5 ± 3.20 | −0.88 ± 0.22 |
| 0.09 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, A.; Teixeira, J.P.F.; Silva, A.M.G.; Ferreira, M.; Gameiro, P.; de Freitas, V. Modelling Hyperglycaemia in an Epithelial Membrane Model: Biophysical Characterisation. Biomolecules 2022, 12, 1534. https://doi.org/10.3390/biom12101534
Reis A, Teixeira JPF, Silva AMG, Ferreira M, Gameiro P, de Freitas V. Modelling Hyperglycaemia in an Epithelial Membrane Model: Biophysical Characterisation. Biomolecules. 2022; 12(10):1534. https://doi.org/10.3390/biom12101534
Chicago/Turabian StyleReis, Ana, Joana P. F. Teixeira, Ana M. G. Silva, Mariana Ferreira, Paula Gameiro, and Victor de Freitas. 2022. "Modelling Hyperglycaemia in an Epithelial Membrane Model: Biophysical Characterisation" Biomolecules 12, no. 10: 1534. https://doi.org/10.3390/biom12101534






