Cardioprotective Effect of Taxifolin against Isoproterenol-Induced Cardiac Injury through Decreasing Oxidative Stress, Inflammation, and Cell Death, and Activating Nrf2/HO-1 in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Animals and Treatment
2.3. Assessment of Cardiac Biomarkers
2.4. Investigation of Oxidative Stress Markers and Antioxidants in the Heart
2.5. Investigation of Pro-Inflammatory Cytokines in the Heart
2.6. Histopathological and Immunohistochemical Analysis
2.7. Analysis of Data
3. Results
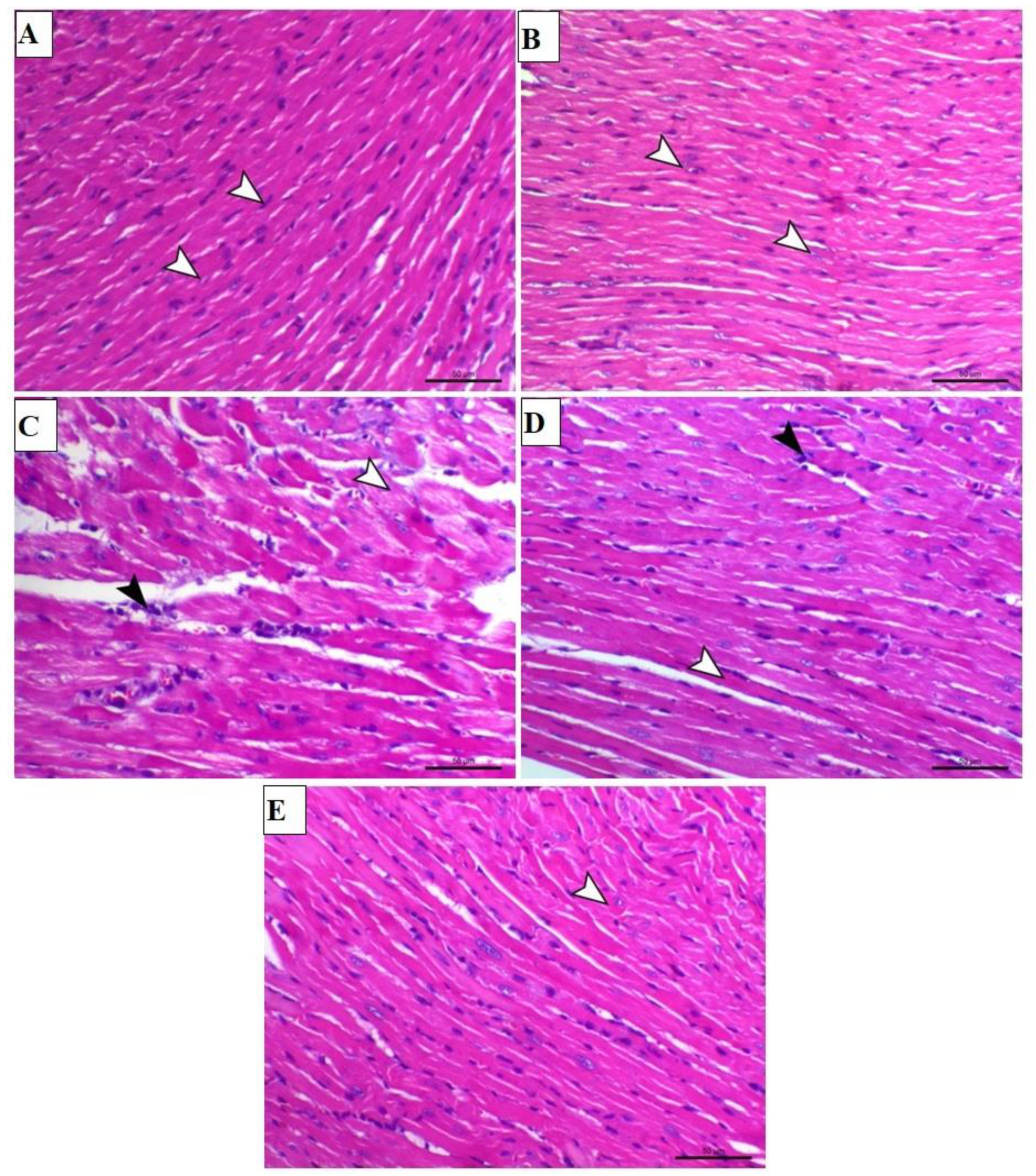
3.1. TAX Prevents ISO-Triggered Myocardial Injury in Mice
3.2. TAX Attenuates Oxidative Stress and Enhances Antioxidants in Heart of ISO-Injected Mice
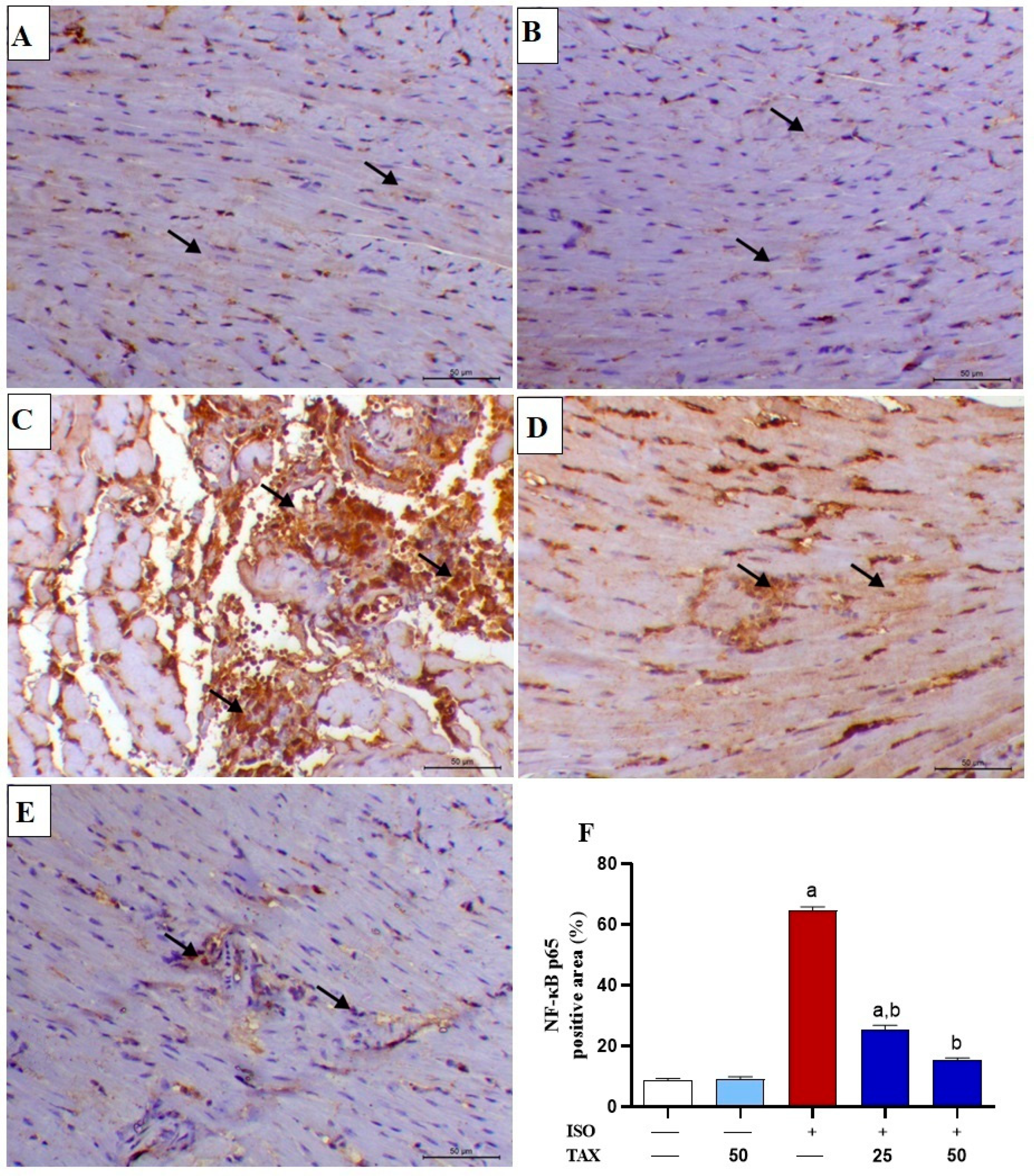
3.3. TAX Mitigates Myocardial Inflammation in ISO-Injected Mice
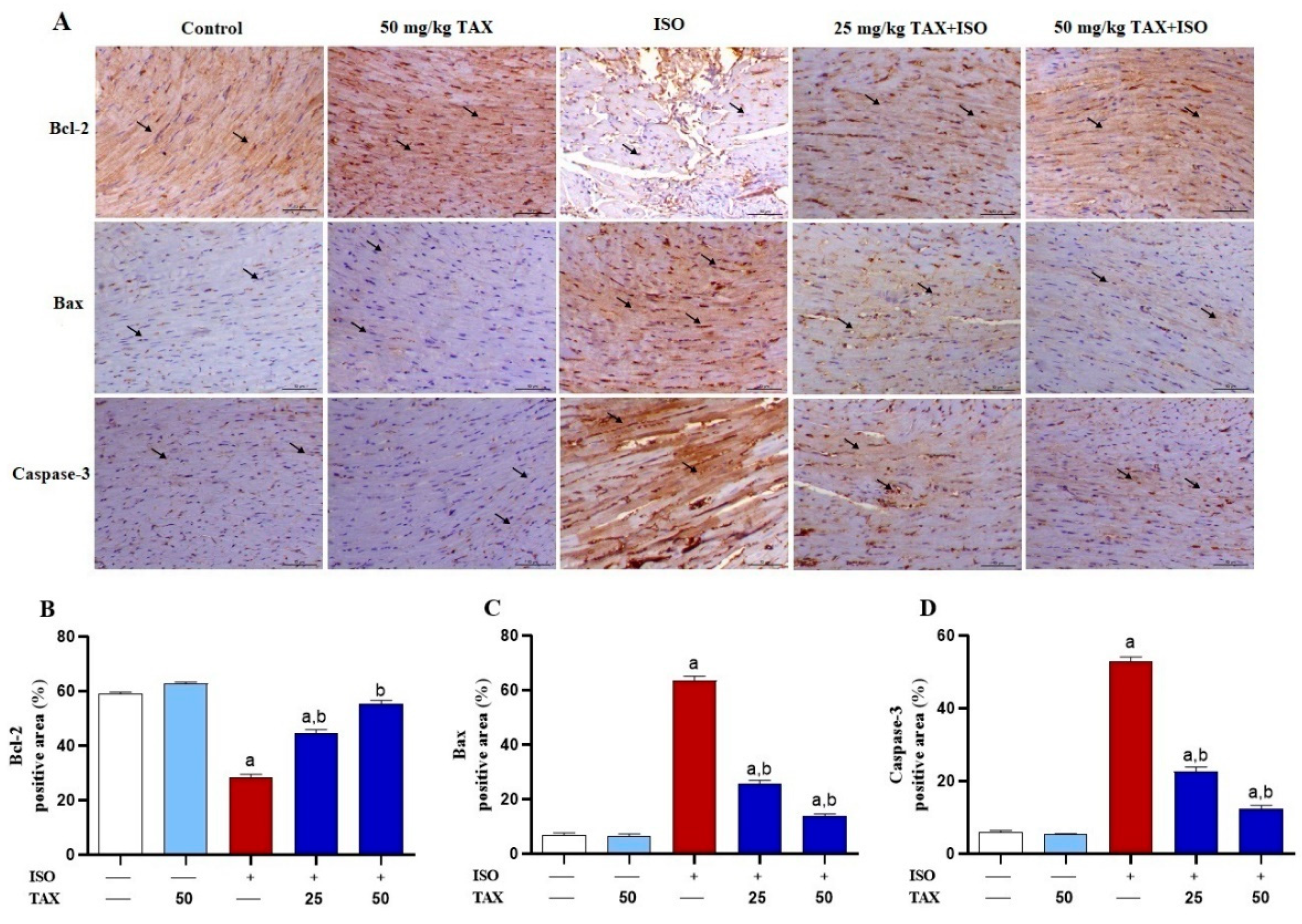
3.4. TAX Prevents Myocardial Apoptosis in ISO-Treated Mice
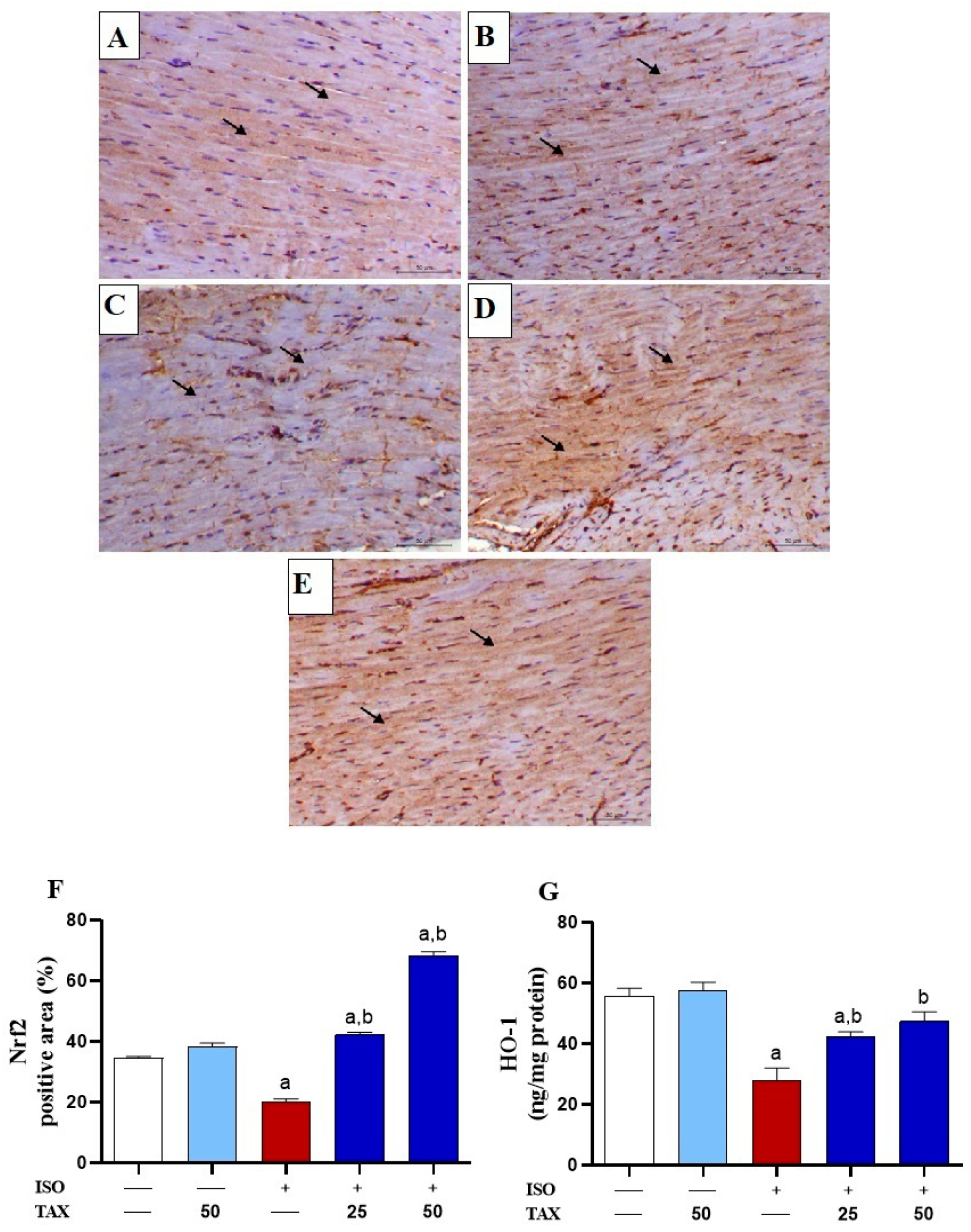
3.5. TAX Activates Cardiac Nrf2/HO-1 in ISO-Injected Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.M.; Savin, S.; Khan, T.; Altay, S. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [Google Scholar] [CrossRef] [Green Version]
- Saglietto, A.; Manfredi, R.; Elia, E.; D’Ascenzo, F.; GM, D.F.; Munzel, T. Cardiovascular disease burden: Italian and global perspectives. Minerva Cardiol. Angiol. 2021, 69, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Lochner, A.; Wang, H.-H.; Wang, S.; Zhu, H.; Ren, J.; Zhou, H. Coronary microvascular injury in myocardial infarction: Perception and knowledge for mitochondrial quality control. Theranostics 2021, 11, 6766. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Pathophysiology of myocardial infarction. Compr. Physiol. 2011, 5, 1841–1875. [Google Scholar]
- Timercan, T.; Şveţ, I.; Pantea, V.; Ambros, A.; Lîsîi, L. Advanced glycation end products in isoproterenol-induced acute myocardial infarction. Med. Pharm. Rep. 2019, 92, 235. [Google Scholar] [CrossRef] [PubMed]
- Wong, Z.W.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of the protective role of natural products on isoproterenol-induced myocardial infarction: A review. Biomed. Pharmacother. 2017, 94, 1145–1166. [Google Scholar] [CrossRef]
- Upaganlawar, A.; Gandhi, H.; Balaraman, R. Isoproterenol induced myocardial infarction: Protective role of natural products. J. Pharmacol. Toxicol. 2011, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Von Harsdorf, R.; Li, P.F.; Dietz, R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 1999, 99, 2934–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, B.; Li, Z.; Ji, X.; Huang, J.; Zhang, H.; Jiang, C. The protective role of lutein on isoproterenol-induced cardiac failure rat model through improving cardiac morphology, antioxidant status via positively regulating Nrf2/HO-1 signalling pathway. Pharm. Biol. 2019, 57, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Abdelzaher, W.Y.; Ahmed, S.M.; Welson, N.N.; Alsharif, K.F.; Batiha, G.E.-S.; Labib, D.A.A. Dapsone ameliorates isoproterenol-induced myocardial infarction via Nrf2/HO-1; TLR4/TNF-α signaling pathways and the suppression of oxidative stress, inflammation, and apoptosis in rats. Front. Pharmacol. 2021, 12, 669679. [Google Scholar] [CrossRef]
- Abukhalil, M.H.; Hussein, O.E.; Aladaileh, S.H.; Althunibat, O.Y.; Al-Amarat, W.; Saghir, S.A.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M.; Al-Swailmi, F.K. Visnagin prevents isoproterenol-induced myocardial injury by attenuating oxidative stress and inflammation and upregulating Nrf2 signaling in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22906. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Abduh, M.S.; Abukhalil, M.H.; Aladaileh, S.H.; Hanieh, H.; Mahmoud, A.M. Umbelliferone prevents isoproterenol-induced myocardial injury by upregulating Nrf2/HO-1 signaling, and attenuating oxidative stress, inflammation, and cell death in rats. Biomed. Pharmacother. 2022, 149, 112900. [Google Scholar] [CrossRef]
- Velusamy, P.; Mohan, T.; Ravi, D.B.; Kishore Kumar, S.; Srinivasan, A.; Chakrapani, L.N.; Singh, A.; Varadharaj, S.; Kalaiselvi, P. Targeting the Nrf2/ARE signalling pathway to mitigate isoproterenol-induced cardiac hypertrophy: Plausible role of hesperetin in redox homeostasis. Oxid. Med. Cell. Longev. 2020, 2020, 9568278. [Google Scholar] [CrossRef]
- Shanmugam, G.; Challa, A.K.; Devarajan, A.; Athmanathan, B.; Litovsky, S.H.; Krishnamurthy, P.; Davidson, C.J.; Rajasekaran, N.S. Exercise mediated Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling. Front. Cardiovasc. Med. 2019, 6, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, G.; Challa, A.K.; Litovsky, S.H.; Devarajan, A.; Wang, D.; Jones, D.P.; Darley-Usmar, V.M.; Rajasekaran, N.S. Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice. Redox Biol. 2019, 27, 101212. [Google Scholar] [CrossRef] [PubMed]
- Allam, M.A.M.; Khowailed, A.A.; Elattar, S.; Mahmoud, A.M. Umbelliferone ameliorates oxidative stress and testicular injury, improves steroidogenesis and upregulates peroxisome proliferator-activated receptor gamma in type 2 diabetic rats. J. Pharm. Pharmacol. 2021, 74, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, Z.; Wang, Y.; Geng, J.; Han, S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol—Induced heart failure in diabetic rats. Drug Dev. Res. 2019, 80, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Cui, Z.-H.; Zhao, Y.-X.; He, T.-T.; Wang, L.; Liang, X.-W. Ferulic acid ameliorates isoproterenol-induced heart failure by decreasing oxidative stress and inhibiting cardiocyte apoptosis via activating Nrf2 signaling pathway in rats. Biol. Pharm. Bull. 2021, 44, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef]
- Coşgun, M.S.; Çoşkun, R.; Celik, A.I. The preventive effect of taxifolin on acrylamide-induced heart damage in rats. Rev. Nutr. 2022, 35. [Google Scholar] [CrossRef]
- Ren, L.; Guo, H.-N.; Yang, J.; Guo, X.-Y.; Wei, Y.-S.; Yang, Z. Dissecting efficacy and metabolic characteristic mechanism of taxifolin on renal fibrosis by multivariate approach and ultra-performance liquid chromatography coupled with mass spectrometry-based metabolomics strategy. Front. Pharmacol. 2021, 11, 608511. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, C.; Zuo, B.; Zhang, Y.; Wu, G.; Wang, Y.; Wang, Z. Taxifolin protects rat against myocardial ischemia/reperfusion injury by modulating the mitochondrial apoptosis pathway. PeerJ 2019, 7, e6383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Feng, J.; Kang, Z.; Zhang, S.; Zhang, L.; Zhang, Y.; Li, X.; Tang, Y. Taxifolin protects RPE cells against oxidative stress-induced apoptosis. Mol. Vis. 2017, 23, 520. [Google Scholar] [PubMed]
- Liu, X.; Liu, W.; Ding, C.; Zhao, Y.; Chen, X.; Ling, D.; Zheng, Y.; Cheng, Z. Taxifolin, Extracted from Waste Larix olgensis Roots, Attenuates CCl4-Induced Liver Fibrosis by Regulating the PI3K/AKT/mTOR and TGF-β1/Smads Signaling Pathways. Drug Des. Devel. Ther. 2021, 15, 871. [Google Scholar] [CrossRef]
- Algefare, A.I. Renoprotective and Oxidative Stress-Modulating Effects of Taxifolin against Cadmium-Induced Nephrotoxicity in Mice. Life 2022, 12, 1150. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. [49] Determination of carbonyl content in oxidatively modified proteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 464–478. [Google Scholar]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R., Jr. Quantitation of nitrate and nitrite in extracellular fluids. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 268, pp. 237–246. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Kim, S.-W.; Roh, J.; Park, C.-S. Immunohistochemistry for pathologists: Protocols, pitfalls, and tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Brooks, W.W.; Conrad, C.H. Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: Structural and functional correlates. Comp. Med. 2009, 59, 339–343. [Google Scholar] [PubMed]
- Li, X.; Zhou, R.; Zheng, P.; Yan, L.; Wu, Y.; Xiao, X.; Dai, G. Cardioprotective effect of matrine on isoproterenol-induced cardiotoxicity in rats. J. Pharm. Pharmacol. 2010, 62, 514–520. [Google Scholar] [CrossRef]
- Nigam, P. Biochemical markers of myocardial injury. Indian J. Clin. Biochem. 2007, 22, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, V.; Upaganlawar, A.; Zalawadia, R.; Balaraman, R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. Eur. J. Pharmacol. 2010, 644, 160–168. [Google Scholar] [CrossRef]
- Shu, Z.; Yang, Y.; Yang, L.; Jiang, H.; Yu, X.; Wang, Y. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct. 2019, 10, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, R.-C.; Yang, Z.-H.; Sun, G.-B.; Wang, M.; Ma, X.-J.; Yang, L.-J.; Sun, X.-B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014, 63, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Singal, P. Potential oxidative pathways of catecholamines in the formation of lipid peroxides and genesis of heart disease. Adv. Exp. Med. Biol. 1980, 191, 421–427. [Google Scholar]
- Kondo, T.; Ogawa, Y.; Sugiyama, S.; Ito, T.; Satake, T.; Ozawa, T. Mechanism of isoproterenol induced myocardial damage. Cardiovasc. Res. 1987, 21, 248–254. [Google Scholar] [CrossRef]
- Ramakrishna, K.; Krishnamurthy, S. Indole-3-carbinol ameliorated the isoproterenol-induced myocardial infarction via multimodal mechanisms in Wistar rats. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Khalil, M.; Ahmmed, I.; Ahmed, R.; Tanvir, E.; Afroz, R.; Paul, S.; Gan, S.H.; Alam, N. Amelioration of isoproterenol-induced oxidative damage in rat myocardium by Withania somnifera leaf extract. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar]
- Othman, A.I.; Elkomy, M.M.; El-Missiry, M.; Dardor, M. Epigallocatechin-3-gallate prevents cardiac apoptosis by modulating the intrinsic apoptotic pathway in isoproterenol-induced myocardial infarction. Eur. J. Pharmacol. 2017, 794, 27–36. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, J.; Wang, Y.; Lin, T. Remifentanil attenuates cardiac dysfunction, lipid peroxidation and immune disorder in rats with isoproterenol-induced myocardial injury via JNK/NF-KB p65 inhibition. Ann. Transl. Med. 2020, 8, 551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Yi, J. Redox sensing by proteins: Oxidative modifications on cysteines and the consequent events. Antioxid. Redox Signal. 2012, 16, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Najeb, S.M.; Jaccob, A.A.; Al-Moziel, M.S.; Abdulhameed, H.M. Cardioprotective and antioxidant effects of taxifolin and vitamin C against diazinone-induced myocardial injury in rats. Environ. Anal. Health Toxicol. 2022, 37, e2022002. [Google Scholar] [CrossRef]
- Bedir, F.; Kocatürk, H.; Yapanoğlu, T.; Gürsul, C.; Arslan, R.; Mammadov, R.; Çoban, A.; Altuner, D.; Suleyman, H. Protective effect of taxifolin against prooxidant and proinflammatory kidney damage associated with acrylamide in rats. Biomed. Pharmacother. 2021, 139, 111660. [Google Scholar] [CrossRef] [PubMed]
- Ahiskali, I.; Pinar, C.L.; Kiki, M.; Cankaya, M.; Kunak, C.S.; Altuner, D. Effect of taxifolin on methanol-induced oxidative and inflammatory optic nerve damage in rats. Cutan. Ocul. Toxicol. 2019, 38, 384–389. [Google Scholar] [CrossRef]
- Allawadhi, P.; Khurana, A.; Sayed, N.; Kumari, P.; Godugu, C. Isoproterenol—Induced cardiac ischemia and fibrosis: Plant—Based approaches for intervention. Phytother. Res. 2018, 32, 1908–1932. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.D.; Frangogiannis, N.G. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Sukoyan, G.; Golovach, S.; Dolidze, N.; Kezeli, T.; Gongadze, N. Hypertrophic and Inflammatory Markers in Isoproterenol-Induced Cardiac Hypertrophy and its Pharmacological Correction. Cardiovasc. Pharm. Open Access 2017, 6, 225–230. [Google Scholar]
- Li, Y.; Feng, L.; Li, G.; An, J.; Zhang, S.; Li, J.; Liu, J.; Ren, J.; Yang, L.; Qi, Z. Resveratrol prevents ISO-induced myocardial remodeling associated with regulating polarization of macrophages through VEGF-B/AMPK/NF-kB pathway. Int. Immunopharmacol. 2020, 84, 106508. [Google Scholar] [CrossRef]
- Freund, C.; Schmidt-Ullrich, R.; Baurand, A.; Dunger, S.; Schneider, W.; Loser, P.; El-Jamali, A.; Dietz, R.; Scheidereit, C.; Bergmann, M.W. Requirement of nuclear factor-κB in angiotensin II–and isoproterenol-induced cardiac hypertrophy in vivo. Circulation 2005, 111, 2319–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.K.; Malik, S.; Narayanan, S.P.; Mutneja, E.; Sahu, A.K.; Bhatia, J.; Arya, D.S. Role of MAPK/NF-κB pathway in cardioprotective effect of Morin in isoproterenol induced myocardial injury in rats. Mol. Biol. Rep. 2019, 46, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhazykbayeva, S.; Pabel, S.; Mügge, A.; Sossalla, S.; Hamdani, N. The molecular mechanisms associated with the physiological responses to inflammation and oxidative stress in cardiovascular diseases. Biophys. Rev. 2020, 12, 947–968. [Google Scholar] [CrossRef]
- Prince, P.S.M.; Hemalatha, K.L. A molecular mechanism on the antiapoptotic effects of zingerone in isoproterenol induced myocardial infarcted rats. Eur. J. Pharmacol. 2018, 821, 105–111. [Google Scholar] [CrossRef]
- Jin, H.; Liu, A.D.; Holmberg, L.; Zhao, M.; Chen, S.; Yang, J.; Sun, Y.; Chen, S.; Tang, C.; Du, J. The role of sulfur dioxide in the regulation of mitochondrion-related cardiomyocyte apoptosis in rats with isopropylarterenol-induced myocardial injury. Int. J. Mol. Sci. 2013, 14, 10465–10482. [Google Scholar] [CrossRef]
- Chen, W.; Deng, W.; Chen, S. Inactivation of Nf-kb Pathway by Taxifolin Attenuates Sepsis-Induced Acute Lung Injury. Curr. Top. Nutraceutical Res. 2020, 18, 176–182. [Google Scholar]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The role of Nrf2 in cardiovascular function and disease. Oxidative Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ichikawa, T.; Villacorta, L.; Janicki, J.S.; Brower, G.L.; Yamamoto, M.; Cui, T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Chai, Y.; Lin, H.; Chen, C.; Zhao, M.; Xiong, W.; Zhuang, J.; Fan, X. Dihydroquercetin activates AMPK/Nrf2/HO-1 signaling in macrophages and attenuates inflammation in LPS-induced endotoxemic mice. Front. Pharmacol. 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obeidat, H.M.; Althunibat, O.Y.; Alfwuaires, M.A.; Aladaileh, S.H.; Algefare, A.I.; Almuqati, A.F.; Alasmari, F.; Aldal’in, H.K.; Alanezi, A.A.; Alsuwayt, B.; et al. Cardioprotective Effect of Taxifolin against Isoproterenol-Induced Cardiac Injury through Decreasing Oxidative Stress, Inflammation, and Cell Death, and Activating Nrf2/HO-1 in Mice. Biomolecules 2022, 12, 1546. https://doi.org/10.3390/biom12111546
Obeidat HM, Althunibat OY, Alfwuaires MA, Aladaileh SH, Algefare AI, Almuqati AF, Alasmari F, Aldal’in HK, Alanezi AA, Alsuwayt B, et al. Cardioprotective Effect of Taxifolin against Isoproterenol-Induced Cardiac Injury through Decreasing Oxidative Stress, Inflammation, and Cell Death, and Activating Nrf2/HO-1 in Mice. Biomolecules. 2022; 12(11):1546. https://doi.org/10.3390/biom12111546
Chicago/Turabian StyleObeidat, Heba M., Osama Y. Althunibat, Manal A. Alfwuaires, Saleem H. Aladaileh, Abdulmohsen I. Algefare, Afaf F. Almuqati, Fawaz Alasmari, Hammad Khalifeh Aldal’in, Abdulkareem A. Alanezi, Bader Alsuwayt, and et al. 2022. "Cardioprotective Effect of Taxifolin against Isoproterenol-Induced Cardiac Injury through Decreasing Oxidative Stress, Inflammation, and Cell Death, and Activating Nrf2/HO-1 in Mice" Biomolecules 12, no. 11: 1546. https://doi.org/10.3390/biom12111546





