Proline-Rich Region II (PRR2) Plays an Important Role in Tau–Glycan Interaction: An NMR Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NMR Titration of Tau K18 and Tau 441 with Heparin 7-Mer
2.3. Reconstitution, Overexpression, and Purification of Tau PRR2* and Tau R2* Construct
2.4. NMR Assignment of Tau PRR2* and Tau R2*
2.5. NMR Titration of Tau PRR2* and Tau R2* with Arixtra and Heparin
3. Results
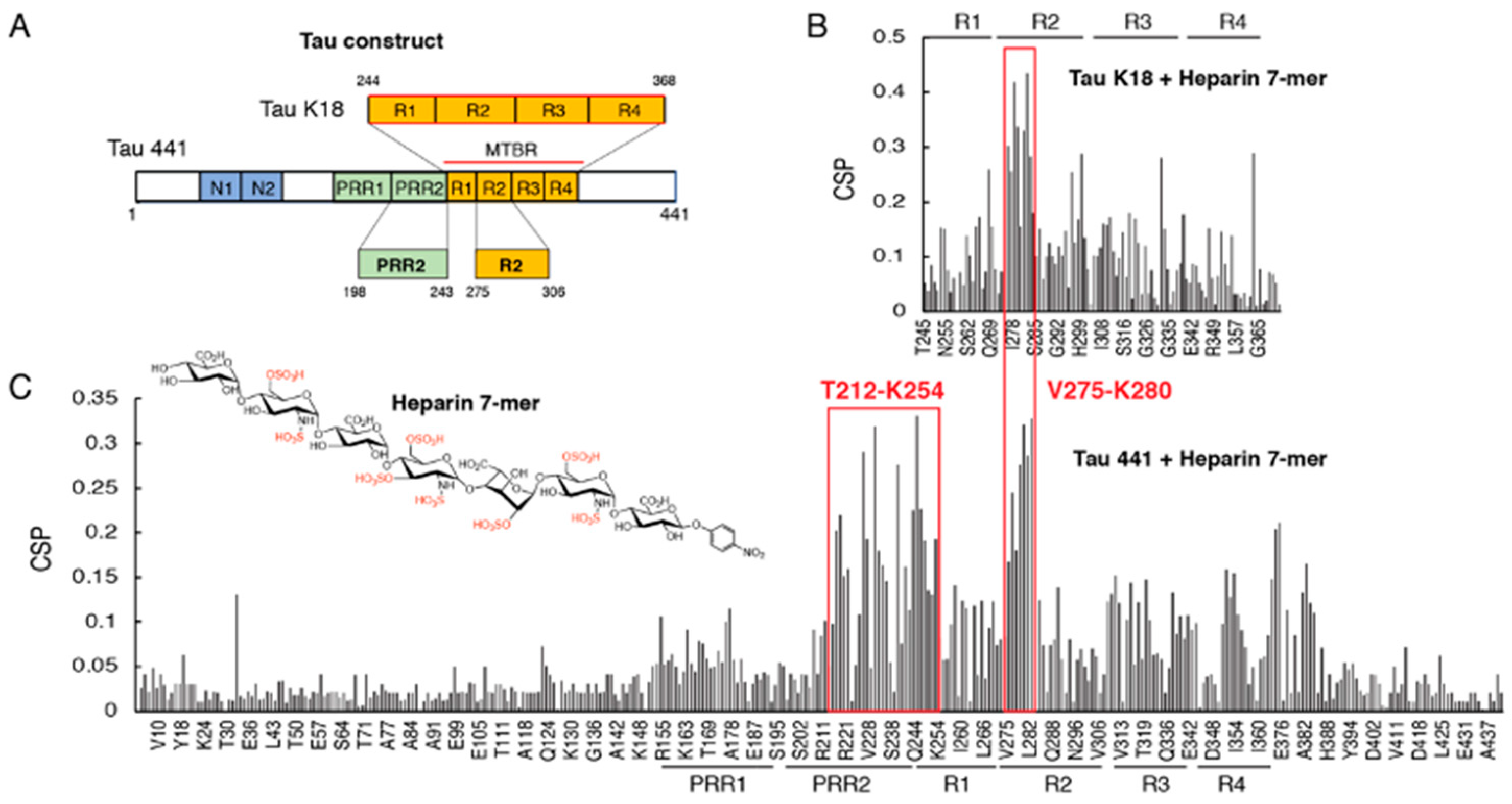
3.1. Proline-Rich Region II (PRR2) Experiences Largest CSP in Full-Length Tau Binding to Heparin 7-Mer
3.2. Design of Tau Constructs R2* and PRR2* and Their NMR Assignments
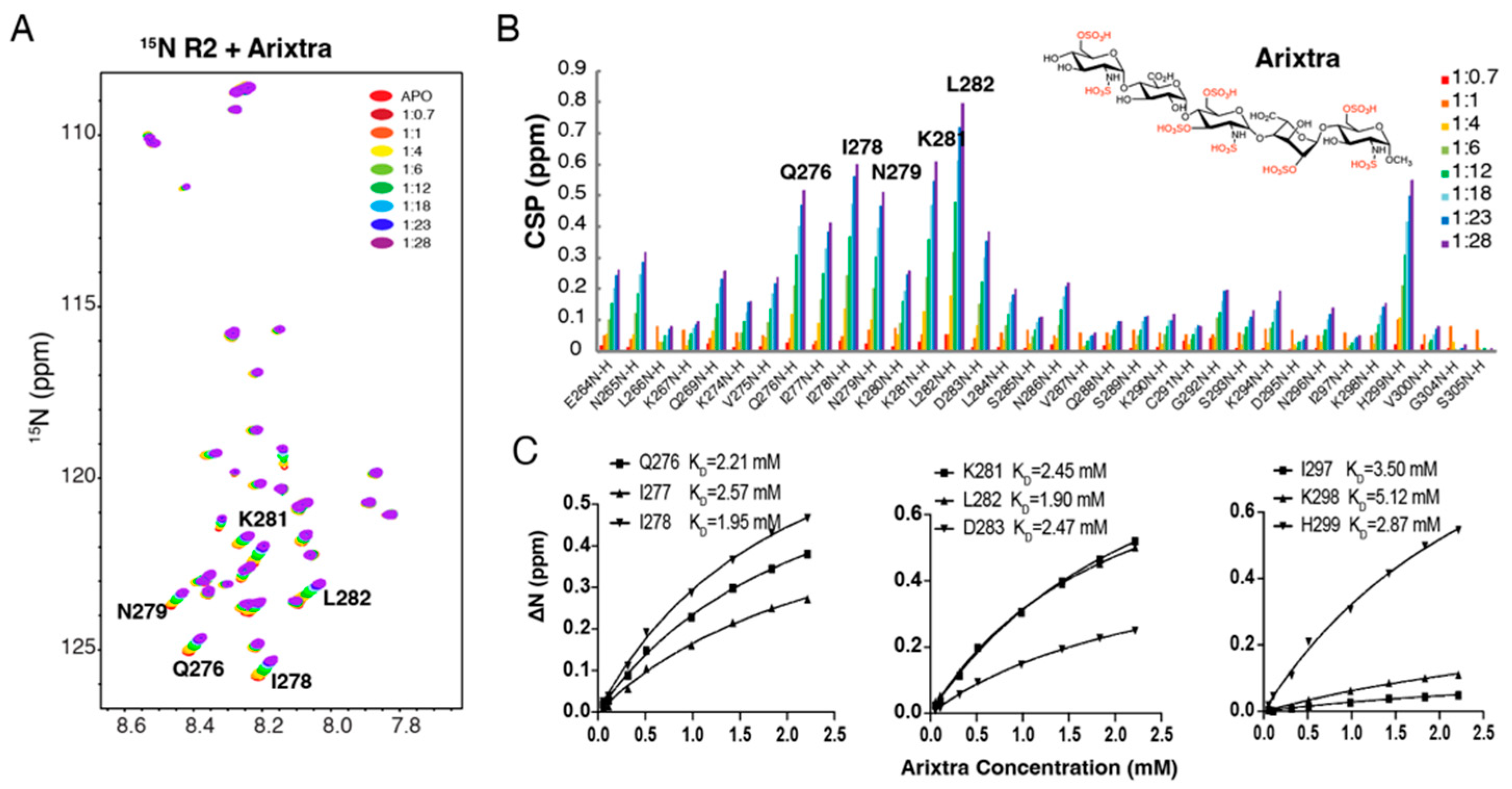
3.3. Characterization of R2*-Arixtra Interaction
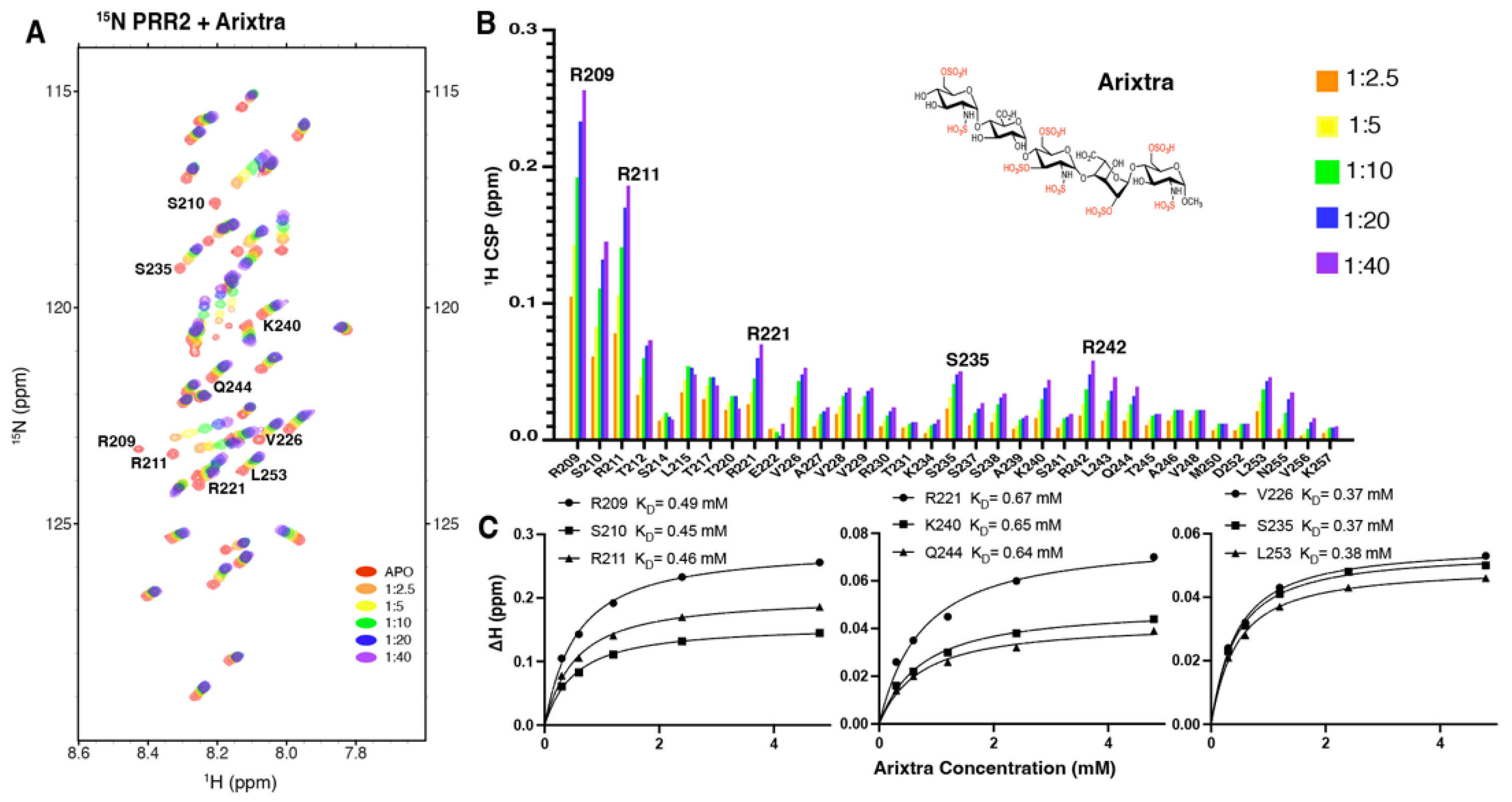
3.4. Characterization of Tau PRR2*-Arixtra Interaction
3.5. Characterization of PRR2*–Heparin and R2*–Heparin Interaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.W.; Herman, M.; Liu, L.; Simoes, S.; Acker, C.M.; Figueroa, H.; Steinberg, J.I.; Margittai, M.; Kayed, R.; Zurzolo, C.; et al. Small Misfolded Tau Species Are Internalized via Bulk Endocytosis and Anterogradely and Retrogradely Transported in Neurons. J. Biol. Chem. 2013, 288, 1856–1870. [Google Scholar] [CrossRef]
- Frost, B.; Jacks, R.L.; Diamond, M.I. Propagation of Tau Misfolding from the Outside to the inside of a Cell. J. Biol. Chem. 2009, 284, 12845–12852. [Google Scholar] [CrossRef]
- Liu, L.; Drouet, V.; Wu, J.W.; Witter, M.P.; Small, S.A.; Clelland, C.; Duff, K. Trans-Synaptic Spread of Tau Pathology in Vivo. PLoS One 2012, 7, e31302. [Google Scholar] [CrossRef]
- De Calignon, A.; Polydoro, M.; Suárez-Calvet, M.; William, C.; Adamowicz, D.H.; Kopeikina, K.J.; Pitstick, R.; Sahara, N.; Ashe, K.H.; Carlson, G.A.; et al. Propagation of Tau Pathology in a Model of Early Alzheimer’s Disease. Neuron 2012, 73, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and Spreading of Tauopathy in Transgenic Mouse Brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Cope, T.E.; Rittman, T.; Borchert, R.J.; Jones, P.S.; Vatansever, D.; Allinson, K.; Passamonti, L.; Vazquez Rodriguez, P.; Bevan-Jones, W.R.; O’Brien, J.T.; et al. Tau Burden and the Functional Connectome in Alzheimer’s Disease and Progressive Supranuclear Palsy. Brain 2018, 141, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Del Tredici, K.; Lee, V.M.Y.; Trojanowski, J.Q. Spreading of Pathology in Neurodegenerative Diseases: A Focus on Human Studies. Nat. Rev. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef]
- Goedert, M.; Masuda-Suzukake, M.; Falcon, B. Like Prions: The Propagation of Aggregated Tau and α-Synuclein in Neurodegeneration. Brain 2016, 140, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Lee, V.M.Y. Cell-to-Cell Transmission of Pathogenic Proteins in Neurodegenerative Diseases. Nat. Med. 2014, 20, 130–138. [Google Scholar] [CrossRef]
- Holmes, B.B.; DeVos, S.L.; Kfoury, N.; Li, M.; Jacks, R.; Yanamandra, K.; Ouidja, M.O.; Brodsky, F.M.; Marasa, J.; Bagchi, D.P.; et al. Heparan Sulfate Proteoglycans Mediate Internalization and Propagation of Specific Proteopathic Seeds. Proc. Natl. Acad. Sci. USA 2013, 110, E3138–E3147. [Google Scholar] [CrossRef]
- Holmes, B.B.; Furman, J.L.; Mahan, T.E.; Yamasaki, T.R.; Mirbaha, H.; Eades, W.C.; Belaygorod, L.; Cairns, N.J.; Holtzman, D.M.; Diamond, M.I. Proteopathic Tau Seeding Predicts Tauopathy in Vivo. Proc. Natl. Acad. Sci. USA 2014, 111, E4376–E4385. [Google Scholar] [CrossRef] [PubMed]
- Rauch, J.N.; Chen, J.J.; Sorum, A.W.; Miller, G.M.; Sharf, T.; See, S.K.; Hsieh-Wilson, L.C.; Kampmann, M.; Kosik, K.S. Tau Internalization Is Regulated by 6-O Sulfation on Heparan Sulfate Proteoglycans (HSPGs). Sci. Rep. 2018, 8, 6382. [Google Scholar] [CrossRef] [PubMed]
- Stopschinski, B.E.; Holmes, B.B.; Miller, G.M.; Manon, V.A.; Vaquer-Alicea, J.; Prueitt, W.L.; Hsieh-Wilson, L.C.; Diamond, M.I. Specific Glycosaminoglycan Chain Length and Sulfation Patterns Are Required for Cell Uptake of Tau versus Alpha-Synuclein and Beta-Amyloid Aggregates. J. Biol. Chem. 2018, 293, 10826–10840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huvent, I.; Lippens, G.; Eliezer, D.; Zhang, A.; Li, Q.; Tessier, P.; Linhardt, R.J.; Zhang, F.; Wang, C. Glycan Determinants of Heparin-Tau Interaction. Biophys. J. 2017, 112, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Y.; Song, X.; Xiao, Y.; Su, G.; Liu, X.; Wang, Z.; Xu, Y.; Liu, J.; Eliezer, D.; et al. 3-O-Sulfation of Heparan Sulfate Enhances Tau Interaction and Cellular Uptake. Angew. Chemie - Int. Ed. 2020, 59, 1818–1827. [Google Scholar] [CrossRef]
- Sepulveda-Diaz, J.E.; Alavi Naini, S.M.; Huynh, M.B.; Ouidja, M.O.; Yanicostas, C.; Chantepie, S.; Villares, J.; Lamari, F.; Jospin, E.; Van Kuppevelt, T.H.; et al. HS3ST2 Expression Is Critical for the Abnormal Phosphorylation of Tau in Alzheimer’s Disease-Related Tau Pathology. Brain 2015, 138, 1339–1354. [Google Scholar] [CrossRef]
- Gustke, N.; Trinczek, B.; Biernat, J.; Mandelkow, E.M.; Mandelkow, E. Domains of τ Protein and Interactions with Microtubules. Biochemistry 1994, 33, 9511–9522. [Google Scholar] [CrossRef]
- Butner, K.A.; Kirschner, M.W. Tau Protein Binds to Microtubules through a Flexible Array of Distributed Weak Sites. J. Cell Biol. 1991, 115, 717–730. [Google Scholar] [CrossRef]
- Mandelkow, E.M.E. Biochemistry and Cell Biology of Tau Protein in Neurofibrillary Degeneration. Cold Spring Harb. Perspect. Med. 2012, 2, a006247. [Google Scholar] [CrossRef]
- Mukrasch, M.D.; von Bergen, M.; Biernat, J.; Fischer, D.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. The “Jaws” of the Tau-Microtubule Interaction. J. Biol. Chem. 2007, 282, 12230–12239. [Google Scholar] [CrossRef]
- Sibille, N.; Sillen, A.; Leroy, A.; Wieruszeski, J.M.; Mulloy, B.; Landrieu, I.; Lippens, G. Structural Impact of Heparin Binding to Full-Length Tau as Studied by NMR Spectroscopy. Biochemistry 2006, 45, 12560–12572. [Google Scholar] [CrossRef]
- Barre, P.; Eliezer, D. Structural Transitions in Tau K18 on Micelle Binding Suggest a Hierarchy in the Efficacy of Individual Microtubule-Binding Repeats in Filament Nucleation. Protein Sci. 2013, 22, 1037–1048. [Google Scholar] [CrossRef]
- Qi, H.; Despres, C.; Prabakaran, S.; Cantrelle, F.-X.; Chambraud, B.; Gunawardena, E.; Lippens, G.; Smet-Nocca, C.; Landrieu, I. Tau Protein. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1523, pp. 179–214. ISBN 978-1-4939-6596-0. [Google Scholar]
- Xu, Y.; Cai, C.; Chandarajoti, K.; Hsieh, P.-H.; Li, L.; Pham, T.Q.; Sparkenbaugh, E.M.; Sheng, J.; Key, N.S.; Pawlinski, R.; et al. Homogeneous Low-Molecular-Weight Heparins with Reversible Anticoagulant Activity. Nat. Chem. Biol. 2014, 10, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.; Xu, Y.; Keire, D.A.; Liu, J. Chemoenzymatic Synthesis and Structural Characterization of 2-O-Sulfated Glucuronic Acid-Containing Heparan Sulfate Hexasaccharides. Glycobiology 2014, 24, 681–692. [Google Scholar] [CrossRef]
- Goddard, T.; Kneller, D.G. Sparky 3. Available online: https://www.cgl.ucsf.edu/home/sparky/ (accessed on 30 May 2008).
- Nakamura, K.; Greenwood, A.; Binder, L.; Bigio, E.H.; Denial, S.; Nicholson, L.; Zhou, X.Z.; Lu, K.P. Proline Isomer-Specific Antibodies Reveal the Early Pathogenic Tau Conformation in Alzheimer’s Disease. Cell 2012, 149, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.J.; Wulf, G.; Zhou, X.Z.; Davies, P.; Lu, K.P. The Prolyl Isomerase Pin1 Restores the Function of Alzheimer-Associated Phosphorylated Tau Protein. Nature 1999, 399, 784–788. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray, A.; Yan, L.; Gibson, J.M.; Liu, J.; Eliezer, D.; Lippens, G.; Zhang, F.; Linhardt, R.J.; Zhao, J.; Wang, C. Proline-Rich Region II (PRR2) Plays an Important Role in Tau–Glycan Interaction: An NMR Study. Biomolecules 2022, 12, 1573. https://doi.org/10.3390/biom12111573
Murray A, Yan L, Gibson JM, Liu J, Eliezer D, Lippens G, Zhang F, Linhardt RJ, Zhao J, Wang C. Proline-Rich Region II (PRR2) Plays an Important Role in Tau–Glycan Interaction: An NMR Study. Biomolecules. 2022; 12(11):1573. https://doi.org/10.3390/biom12111573
Chicago/Turabian StyleMurray, Anqesha, Lufeng Yan, James M. Gibson, Jian Liu, David Eliezer, Guy Lippens, Fuming Zhang, Robert J. Linhardt, Jing Zhao, and Chunyu Wang. 2022. "Proline-Rich Region II (PRR2) Plays an Important Role in Tau–Glycan Interaction: An NMR Study" Biomolecules 12, no. 11: 1573. https://doi.org/10.3390/biom12111573
APA StyleMurray, A., Yan, L., Gibson, J. M., Liu, J., Eliezer, D., Lippens, G., Zhang, F., Linhardt, R. J., Zhao, J., & Wang, C. (2022). Proline-Rich Region II (PRR2) Plays an Important Role in Tau–Glycan Interaction: An NMR Study. Biomolecules, 12(11), 1573. https://doi.org/10.3390/biom12111573






