Evaluation of Halophyte Biopotential as an Unused Natural Resource: The Case of Lobularia maritima
Abstract
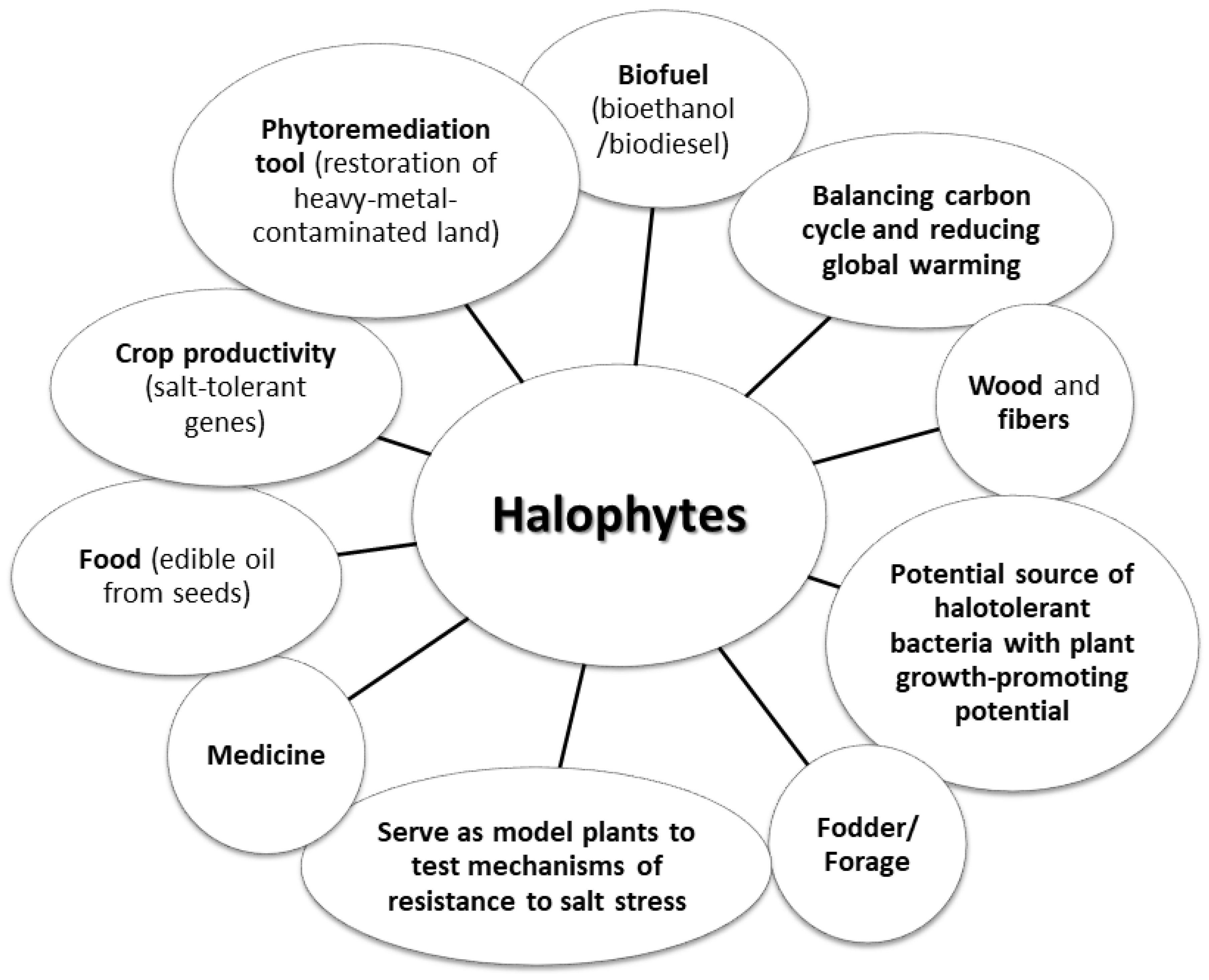
1. Introduction
2. Salt Tolerance in Halophytes
2.1. Lobularia Maritima Genes as Tools for Conferring Environmental Stress Tolerance to Crops
2.2. Halophyte SAP Genes for Abiotic and Biotic Stress Response: A Well-Known Success Story
3. Phytochemical Composition of Halophytes
3.1. Nitrogen-Containing Compounds and the Salt Tolerance of Halophytes
3.2. Active Components of Lobularia Maritima and Its Biological Properties
3.2.1. Antioxidant Activity
3.2.2. Anti-Inflammatory Activity
3.2.3. Antiobesity
3.2.4. Antimicrobial Activity
4. Pharmacological Properties of Halophytes with a Focus on Lobularia maritima (In Vivo Studies)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Roohi, A.; Nazish, B.; Maleeha, M.; Waseem, S. A critical review on halophytes: Salt tolerant plants. J. Med. Plant Res 2011, 5, 7108–7118. [Google Scholar]
- Qasim, M.; Gulzar, S.; Shinwari, Z.K.; Aziz, I.; Khan, M.A. Traditional ethnobotanical uses of halophytes from Hub, Balochistan. Pak. J. Bot. 2010, 42, 1543–1551. [Google Scholar]
- Hameed, A.; Khan, M.A. Halophytes: Biology and economic potentials. Karachi Univ. J. Sci. 2011, 39, 40–44. [Google Scholar]
- Kukula-Koch, W.; Koch, W.; Angelis, A.; Halabalaki, M.; Aligiannis, N. Application of pH-zone refining hydrostatic countercurrent chromatography (hCCC) for the recovery of antioxidant phenolics and the isolation of alkaloids from Siberian barberry herb. Food Chem. 2016, 203, 394–401. [Google Scholar] [CrossRef]
- Gómez, J. Phenotypic selection and response to selection in Lobularia maritima: The importance of direct and correlational components of natural selection. J. Evol. Biol. 2000, 13, 689–699. [Google Scholar] [CrossRef]
- Picó, F.X.; Retana, J. The flowering pattern of the perennial herb Lobularia maritima: An unusual case in the Mediterranean basin. Acta Oecol. 2001, 22, 209–217. [Google Scholar] [CrossRef]
- Huang, R.W.; Liu, D.F.; Zhao, M.; Li, Z.N.; Li, M.Y.; Sue, S.Z. Artificially induced polyploidization in Lobularia maritima (L.) Desv. and its effect on morphological traits. Hortscience 2015, 50, 636–639. [Google Scholar] [CrossRef]
- Huang, L.; Ma, Y.; Jiang, J.; Li, T.; Yang, W.; Zhang, L.; Wu, L.; Feng, L.; Xi, Z.; Xu, X.; et al. A chromosome-scale reference genome of Lobularia maritima, an ornamental plant with high-stress tolerance. Hortic. Res. 2020, 7, 197. [Google Scholar] [CrossRef]
- Mahajan, R.; Kapoor, N.; Bajaj, B.K. Use of genomics to improve stress tolerance. In Plant Genomics for Sustainable Agriculture; Springer: Singapore, 2022; pp. 291–312. [Google Scholar]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Abbas, A.; Fahad, S.; Baloch, M.S.; Ahmad, M.I.; Saud, S.; Song, Y. Targeting salt stress coping mechanisms for stress tolerance in Brassica: A research perspective. Plant Physiol. Biochem. 2021, 158, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Introgression of the SbASR-1 gene cloned from a halophyte Salicornia brachiate enhances salinity and drought endurance in transgenic groundnut (Arachis hypogaea) and acts as a transcription factor. PLoS ONE 2015, 10, e0131567. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Lin, R.; Su, H.; Chen, H.; Luo, M.; Yang, L.; Zhang, M. The functional identification of glycine-rich TtASR from Tetragonia tetragonoides (Pall.) Kuntze involving in plant abiotic stress tolerance. Plant Physiol. Biochem. 2019, 143, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Su, Q.; An, L.J.; Wu, S. Characterization and expression of a vacuolar Na(+)/H(+) antiporter gene from the monocot halophyte Aeluropus littoralis. Plant Physiol. Biochem. 2008, 46, 117–126. [Google Scholar] [CrossRef]
- Ben-Romdhane, W.; Ben Saad, R.; Meynard, D.; Zouari, N.; Mahjoub, A.; Fki, L.; Guiderdoni, E.; Al-Doss, A.; Hassairi, A. Overexpression of AlTMP2 gene from the halophyte grass Aeluropus littoralis in transgenic tobacco enhances tolerance to different abiotic stresses by improving membrane stability and deregulating some stress-related genes. Protoplasma 2018, 255, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Ben Romdhane, W.; Ben Saad, R.; Meynard, D.; Verdeil, J.L.; Azaza, J.; Zouari, N.; Fki, L.; Guiderdoni, E.; Al-Doss, A.; Hassairi, A. Ectopic Expression of Aeluropus littoralis plasma membrane protein gene AlTMP1confers abiotic stress tolerance in transgenic tobacco by improving water status and cation homeostasis. Int. J. Mol. Sci. 2017, 18, 692. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Halima, N.; Ghorbel, M.; Zouari, N.; Ben Romdhane, W.; Guiderdoni, E.; Al-Doss, A.; Hassairi, A. AlSRG1, a novel gene encoding an RRM-type RNA-binding protein (RBP) from Aeluropus littoralis, confers salt and drought tolerance in transgenic tobacco. Environ. Exp. Bot. 2018, 150, 25–36. [Google Scholar] [CrossRef]
- Lv, S.L.; Lian, L.J.; Tao, P.L.; Li, Z.X.; Zhang, K.W.; Zhang, J.R. Overexpression of Thellungiella halophila H(+)-PPase (TsVP) in cotton enhances drought stress resistance of plants. Planta 2009, 229, 899–910. [Google Scholar] [CrossRef]
- Pei, L.; Wang, J.; Li, K.; Li, Y.; Li, B.; Gao, F.; Yang, A. Overexpression of Thellungiella halophila H+-pyrophosphatase gene improves low phosphate tolerance in maize. PLoS ONE 2012, 7, e43501. [Google Scholar] [CrossRef]
- Nazish, T.; Javaid, A.; Ali, M.; Zhu, Y.H.; Li, J.; Zhang, H.Y.; Wu, J.; Xiang, C.B.; Wu, S.J.; Alfatih, A. Thellungiella halophila ST5 improves salt tolerance in cotton. J. Cotton Res. 2022, 5, 7. [Google Scholar] [CrossRef]
- Ardie, S.W.; Liu, S.; Takano, T. Expression of the AKT1-type K(+) channel gene from Puccinellia tenuiflora, PutAKT1, enhances salt tolerance in Arabidopsis. Plant Cell Rep. 2010, 29, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Takano, T.; Liu, S. Discovery and characterization of two novel salt-tolerance genes in Puccinellia tenuiflora. Int. J. Mol. Sci. 2014, 15, 16469–16483. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.J.; Wang, Z.J.; Wang, X.H.; Takano, T.; Liu, S.K. A peroxisomal APX from Puccinellia tenuiflora improves the abiotic stress tolerance of transgenic Arabidopsis thaliana through decreasing of H2O2 accumulation. J. Plant Physiol. 2015, 175, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.D.; Hall, N.E.; Gendall, A.R.; Boon, P.I.; James, E.A. Using transcriptomics to identify differential gene expression in response to salinity among Australian Phragmites australisclones. Front. Plant Sci. 2016, 7, 432. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, J.; Li, Q.; Li, S.; Wang, P.; Xiang, F. Cloning and characterization of a Phragmites australis phytochelatin synthase (PaPCS) and achieving Cd tolerance in tall fescue. PLoS ONE 2014, 9, e103771. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Lu, F.; Liu, Z.; Lan, S.; Han, G. Differentially expressed genes related to oxidoreductase activity and glutathione metabolism underlying the adaptation of Phragmites australis from the salt marsh in the Yellow River Delta, China. PeerJ 2020, 8, e10024. [Google Scholar] [CrossRef]
- Shao, Q.; Han, N.; Ding, T.; Zhou, F.; Wang, B. SsHKT1;1 is a potassium transporter of the C3 halophyte Suaeda salsa that is involved in salt tolerance. Funct. Plant Biol. 2014, 41, 790–802. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Wu, L.; Yu, G.; Wang, X.; Ma, H. The SsDREB transcription factor from the succulent halophyte Suaeda salsaenhances abiotic stress tolerance in transgenic Tobacco. Int. J. Genom. 2015, 2015, 875497. [Google Scholar]
- Mishra, A.; Tanna, B. Halophytes: Potential resources for salt stress tolerance genes and promoters. Front. Plant Sci. 2017, 8, 829. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.P. Adaptive mechanisms of halophytes and their potential in improving salinity tolerance in plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Yuan, F.; Guo, J.; Shabala, S.; Wang, B. Reproductive physiology of halophytes: Current standing. Front. Plant Sci. 2018, 9, 1954. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 2016, 124, 39–63. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Uzilday, B.; Ozgur, R.; Yildiztugay, E.; Sekmen, A.H.; Turkan, I. Halophytes as a source of salt tolerance genes and mechanisms: A case study for the Salt Lake area, Turkey. Funct. Plant Biol. 2016, 43, 575–589. [Google Scholar] [CrossRef]
- Taji, T.; Seki, M.; Satou, M.; Sakurai, T.; Kobayashi, M.; Ishiyama, K.; Narusaka, Y.; Narusaka, M.; Zhu, J.K.; Shinozaki, K. Comparative genomics in salt tolerance between Arabidopsis and Arabidopsis-related halophyte salt cress using Arabidopsis microarray. Plant Physiol. 2004, 135, 1697–1709. [Google Scholar] [CrossRef]
- Horie, T.; Karahara, I.; Katsuhara, M. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice 2012, 5, 11. [Google Scholar] [CrossRef]
- Popova, O.V.; Golldack, D. In the halotolerant Lobularia maritima (Brassicaceae) salt adaptation correlates with activation of the vacuolar H(+)-ATPase and the vacuolar Na+/H+ antiporter. J. Plant Physiol. 2007, 164, 1278–1288. [Google Scholar] [CrossRef]
- Popova, O.V.; Yang, O.; Dietz, K.J.; Golldack, D. Differential transcript regulation in Arabidopsis thaliana and the halotolerant Lobularia maritima indicates genes with potential function in plant salt adaptation. Gene 2008, 423, 142–148. [Google Scholar] [CrossRef]
- Golldack, D. Molecular responses of halophytes to high salinity. In Progress in Botany: Genetics Physiology Systematics Ecology; Esser, K., Lüttge, U., Beyschlag, W., Murata, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 219–234. [Google Scholar]
- Ben Hsouna, A.; Ghneim-Herrera, T.; Ben Romdhane, W.; Dabbous, A.; Ben Saad, R.; Brini, F.; Abdelly, C.; Ben Hamed, K. Early effects of salt stress on the physiological and oxidative status of the halophyte Lobularia maritima. Funct. Plant Biol. 2020, 47, 912–924. [Google Scholar] [CrossRef]
- Dassanayake, M.; Oh, D.H.; Haas, J.S.; Hernandez, A.; Hong, H.; Ali, S.; Yun, D.J.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J.; et al. The genome of the extremophile crucifer Thellungiella parvula. Nat. Genet. 2011, 43, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz, A.; Talavera-Mateo, L.; Gonzalez-Melendi, P.; Martinez, M.; Diaz, I.; Santamaria, M.E. Arabidopsi skunitz trypsin inhibitors in defense against spider mites. Front. Plant Sci. 2018, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Zschiesche, W.; Barth, O.; Daniel, K.; Bohme, S.; Rausche, J.; Humbeck, K. The zinc-binding nuclear protein HIPP3 acts as an upstream regulator of the salicylate-dependent plant immunity pathway and of flowering time in Arabidopsis thaliana. New Phytol. 2015, 207, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Pascuan, C.; Frare, R.; Alleva, K.; Ayub, N.D.; Soto, G. mRNA biogenesis-related helicase eIF4AIII from Arabidopsis thaliana is an important factor for abiotic stress adaptation. Plant Cell Rep. 2016, 35, 1205–1208. [Google Scholar] [CrossRef]
- Azevedo, C.; Betsuyaku, S.; Peart, J.; Takahashi, A.; Noel, L.; Sadanandom, A.; Casais, C.; Parker, J.; Shirasu, K. Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J. 2006, 25, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.J.; Muskett, P.; Kahn, K.; Feys, B.J.; Jones, J.D.; Parker, J.E. Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 2002, 295, 2077–2080. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Li, M.W.; Yung, Y.L.; Wen, C.Q.; Lam, H.M. The unconventional P-loop NTPase OsYchF1 and its regulator OsGAP1 play opposite roles in salinity stress tolerance. Plant Cell Environ. 2013, 36, 2008–2020. [Google Scholar] [CrossRef]
- Dabbous, A.; Ben Saad, R.; Brini, F.; Farhat-Khemekhem, A.; Zorrig, W.; Abdely, C.; Ben Hamed, K. Over-expression of a subunit E1 of a vacuolar H+-ATPase gene (LmVHA-E1) cloned from the halophyte Lobularia maritima improves the tolerance of Arabidopsis thaliana to salt and osmotic stresses. Environ. Exp. Bot. 2017, 137, 128–141. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Romdhane, W.; Bouteraa, M.T.; Jrad, O.; Ben Hsouna, A. Lobularia maritima thioredoxin-h2 gene mitigates salt and osmotic stress damage in tobacco by modeling plant antioxidant system. Plant Growth Regul. 2022, 97, 101–115. [Google Scholar] [CrossRef]
- Vanneste, K.; Maere, S.; Van de Peer, Y. Tangled up in two: A burst of genome duplications at the end of the Cretaceous and the consequences for plant evolution. Philos. Trans. R. Soc. Lond B Biol. Sci. 2014, 369, 20130353. [Google Scholar] [CrossRef]
- Vanneste, K.; Baele, G.; Maere, S.; Van de Peer, Y. Analysis of 41 plant genomes supports a wave of successful genome duplications in association with the Cretaceous-Paleogene boundary. Genome Res. 2014, 24, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Vij, S.; Tyagi, A.K. A20/AN1 zinc-finger domain-containing proteins in plants and animals represent common elements in stress response. Funct. Integr. Genom. 2008, 8, 301–307. [Google Scholar] [CrossRef]
- Giri, J.; Dansana, P.K.; Kothari, K.S.; Sharma, G.; Vij, S.; Tyagi, A.K. SAPs as novel regulators of abiotic stress response in plants. Bioessays 2013, 35, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, R.; Zouari, N.; Ben Ramdhan, W.; Azaza, J.; Meynard, D.; Guiderdoni, E.; Hassairi, A. Improved drought and salt stress tolerance in transgenic tobacco overexpressing a novel A20/AN1 zinc-finger “AlSAP” gene isolated from the halophyte grass Aeluropus littoralis. Plant Mol. Biol. 2010, 72, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, R.; Farhat-Khemekhem, A.; Ben Halima, N.; Ben Hamed, K.; Brini, F.; Saibi, W. The LmSAP gene isolated from the halotolerant Lobularia maritima improves salt and ionic tolerance in transgenic tobacco lines. Funct. Plant Biol. 2017, 45, 378–391. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, M.; Fu, J.; Xuan, N.; Zhu, Y.; Lian, Y.; Jia, Z.; Zheng, J.; Wang, G. Phylogenetic and expression analysis of ZnF-AN1 genes in plants. Genomics 2007, 90, 265–275. [Google Scholar] [CrossRef]
- Vij, S.; Tyagi, A.K. Genome-wide analysis of the stress associated protein (SAP) gene family containing A20/AN1 zinc-finger(s) in rice and their phylogenetic relationship with Arabidopsis. Mol. Genet. Genom. 2006, 276, 565–575. [Google Scholar] [CrossRef]
- Charrier, A.; Planchet, E.; Cerveau, D.; Gimeno-Gilles, C.; Verdu, I.; Limami, A.M.; Lelievre, E. Overexpression of a Medicago truncatula stress-associated protein gene (MtSAP1) leads to nitric oxide accumulation and confers osmotic and salt stress tolerance in transgenic tobacco. Planta 2012, 236, 567–577. [Google Scholar] [CrossRef][Green Version]
- Kang, M.; Abdelmageed, H.; Lee, S.; Reichert, A.; Mysore, K.S.; Allen, R.D. AtMBP-1, an alternative translation product of LOS2, affects abscisic acid responses and is modulated by the E3 ubiquitin ligase AtSAP5. Plant J. 2013, 76, 481–493. [Google Scholar] [CrossRef]
- Kang, M.; Fokar, M.; Abdelmageed, H.; Allen, R.D. Arabidopsis SAP5 functions as a positive regulator of stress responses and exhibits E3 ubiquitin ligase activity. Plant Mol. Biol. 2011, 75, 451–466. [Google Scholar] [CrossRef]
- Stroher, E.; Wang, X.J.; Roloff, N.; Klein, P.; Husemann, A.; Dietz, K.J. Redox-dependent regulation of the stress-induced zinc-finger protein SAP12 in Arabidopsis thaliana. Mol. Plant 2009, 2, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Zahur, M.; Maqbool, A.; Irfan, M.; Jamal, A.; Shahid, N.; Aftab, B.; Husnain, T. Identification and characterization of a novel gene encoding myb-box binding zinc finger protein in Gossypium arboreum. Biol. Plant. 2012, 56, 641–647. [Google Scholar] [CrossRef]
- Saad, R.B.; Hsouna, A.B.; Saibi, W.; Hamed, K.B.; Brini, F.; Ghneim-Herrera, T. A stress-associated protein, LmSAP, from the halophyte Lobularia maritima provides tolerance to heavy metals in tobacco through increased ROS scavenging and metal detoxification processes. J. Plant Physiol. 2018, 231, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, R.; Ben Romdhane, W.; Mihoubi, W.; Ben Hsouna, A.; Brini, F. A Lobularia maritima LmSAP protein modulates gibberellic acid homeostasis via its A20 domain under abiotic stress conditions. PLoS ONE 2020, 15, e0233420. [Google Scholar] [CrossRef]
- Giri, J.; Vij, S.; Dansana, P.K.; Tyagi, A.K. Rice A20/AN1 zinc-finger containing stress-associated proteins (SAP1/11) and a receptor-like cytoplasmic kinase (OsRLCK253) interact via A20 zinc-finger and confer abiotic stress tolerance in transgenic Arabidopsis plants. New Phytol. 2011, 191, 721–732. [Google Scholar] [CrossRef]
- Huang, J.; Wang, M.M.; Jiang, Y.; Bao, Y.M.; Huang, X.; Sun, H.; Xu, D.Q.; Lan, H.X.; Zhang, H.S. Expression analysis of rice A20/AN1-type zinc finger genes and characterization of ZFP177 that contributes to temperature stress tolerance. Gene 2008, 420, 135–144. [Google Scholar] [CrossRef]
- Kanneganti, V.; Gupta, A.K. Overexpression of OsiSAP8, a member of stress associated protein (SAP) gene family of rice confers tolerance to salt, drought and cold stress in transgenic tobacco and rice. Plant Mol. Biol. 2008, 66, 445–462. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Vij, S.; Tyagi, A.K. Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc. Natl. Acad. Sci. USA 2004, 101, 6309–6314. [Google Scholar] [CrossRef]
- Zouari, N.; Ben Saad, R.; Legavre, T.; Azaza, J.; Sabau, X.; Jaoua, M.; Masmoudi, K.; Hassairi, A. Identification and sequencing of ESTs from the halophyte grass Aeluropus littoralis. Gene 2007, 404, 61–69. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Ramdhan, W.; Zouari, N.; Azaza, J.; Mieulet, D.; Guiderdoni, E.; Ellouz, R.; Hassairi, A. Marker-free transgenic durum wheat cv. Karim expressing the AlSAP gene exhibits a high level of tolerance to salinity and dehydration stresses. Mol. Breed. 2012, 30, 521–533. [Google Scholar] [CrossRef]
- Ben Saad, R.; Fabre, D.; Mieulet, D.; Meynard, D.; Dingkuhn, M.; AL-DOSS, A.; Guiderdoni, E.; Hassairi, A. Expression of the Aeluropus littoralisAlSAP gene in rice confers broad tolerance to abiotic stresses through maintenance of photosynthesis. Plant Cell Environ. 2012, 35, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Ghneim-Herrera, T.; Selvaraj, M.G.; Meynard, D.; Fabre, D.; Pena, A.; Ben Romdhane, W.; Ben Saad, R.; Ogawa, S.; Rebolledo, M.C.; Ishitani, M.; et al. Expression of the Aeluropus littoralisAlSAPgene enhances rice yield under field drought at the reproductive stage. Front. Plant Sci. 2017, 8, 994. [Google Scholar] [CrossRef] [PubMed]
- Ben Romdhane, W.; Ben Saad, R.; Meynard, D.; Zouari, N.; Tarroum, M.; Ali, A.; Droc, G.; Périn, C.; Morel, J.; Fki, L.; et al. Expression of an A20/AN1 stress-associated protein from Aeluropus littoralis in rice deregulates stress-related genes. J. Plant Growth Regul. 2021, 41, 848–862. [Google Scholar] [CrossRef]
- Ben Saad, R.; Safi, H.; Ben Hsouna, A.; Brini, F.; Ben Romdhane, W. Functional domain analysis of LmSAP protein reveals the crucial role of the zinc-finger A20 domain in abiotic stress tolerance. Protoplasma 2019, 256, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Chaturvedi, A.K.; Mishra, A.; Jha, B. The transcriptional regulatory mechanism of the peroxisomal ascorbate peroxidase (pAPX) gene cloned from an extreme halophyte, Salicornia brachiata. Plant Cell Physiol. 2014, 55, 201–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tiwari, V.; Patel, M.K.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Functional characterization of the Tau class glutathione-S-transferases gene (SbGSTU) promoter of Salicornia brachiata under salinity and osmotic Stress. PLoS ONE 2016, 11, e0148494. [Google Scholar] [CrossRef]
- Sun, Q.; Gao, F.; Zhao, L.; Li, K.; Zhang, J. Identification of a new 130 bp cis-acting element in the TsVP1 promoter involved in the salt stress response from Thellungiella halophila. BMC Plant Biol. 2010, 10, 90. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, Y.; Luo, D.; Zhang, H. Isolating the promoter of a stress-induced gene encoding betaine aldehyde dehydrogenase from the halophyte Atriplex centralasiatica Iljin. Biochim. Biophys. Acta 2002, 1577, 452–456. [Google Scholar] [CrossRef]
- Guo, L.; Yu, Y.; Xia, X.; Yin, W. Identification and functional characterisation of the promoter of the calcium sensor gene CBL1 from the xerophyte Ammopiptanthus mongolicus. BMC Plant Biol. 2010, 10, 18. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Romdhane, W.; Zouari, N.; Ben Hsouna, A.; Harbaoui, M.; Brini, F.; Ghneim-Herrera, T. Characterization of a novel LmSAP gene promoter from Lobularia maritima: Tissue specificity and environmental stress responsiveness. PLoS ONE 2020, 15, e0236943. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Romdhan, W.; Zouari, N.; Azaza, J.; Mieulet, D.; Verdeil, J.L.; Guiderdoni, E.; Hassairi, A. Promoter of the AlSAP gene from the halophyte grass Aeluropus littoralis directs developmental-regulated, stress-inducible, and organ-specific gene expression in transgenic tobacco. Transgenic Res. 2011, 20, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Ben-Saad, R.; Meynard, D.; Ben-Romdhane, W.; Mieulet, D.; Verdeil, J.L.; Al-Doss, A.; Guiderdoni, E.; Hassairi, A. The promoter of the AlSAP gene from the halophyte grass Aeluropus littoralis directs a stress-inducible expression pattern in transgenic rice plants. Plant Cell Rep. 2015, 34, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Said-Al Ahl, H.A.H.; Omer, E.A. Medicinal and aromatic plants production under salt stress. A review. Herba Pol. 2011, 57, 72–87. [Google Scholar]
- Kłosowska, K. Plant responses to salinity. Kosmos 2010, 59, 539–549. [Google Scholar]
- Eryılmaz, F. The relationships between salt stress and anthocyanin content in higher plants. Biotechnol. Biotechnol. Equip. 2006, 20, 47–52. [Google Scholar] [CrossRef]
- Bourgou, S.; Kchouk, M.; Bellila, A.; Marzouk, B. Effect of salinity on phenolic composition and biological activity of Nigella sativa. Acta Hortic. 2010, 853, 57–60. [Google Scholar] [CrossRef]
- Kovacik, J.; Klejdus, B.; Hedbavny, J.; Backor, M. Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 2009, 18, 544–554. [Google Scholar] [CrossRef]
- Baatour, O.; Kaddour, R.; Aidi Wannes, W.; Lachaâl, M.; Marzouk, B. Salt effects on the growth, mineral nutrition, essential oil yield and composition of marjoram (Origanum majorana). Acta Physiol. Plant. 2009, 32, 45–51. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.; Hussein, M. Effect of water stress and potassium humate on the productivity of oregano plant using saline and fresh water irrigation. Ozean J. Appl. Sci. 2010, 3, 125–141. [Google Scholar]
- Said-Al, A.; Meawad, A.; Abou-Zeid, E.; Ali, M. Response of different basil varieties to soil salinity. Int. Agrophys. 2010, 24, 183–188. [Google Scholar]
- Baghalian, K.; Haghiry, A.; Naghavi, M.R.; Mohammadi, A. Effect of saline irrigation water on agronomical and phytochemical characters of chamomile (Matricaria recutita L.). Sci. Hortic. 2008, 116, 437–441. [Google Scholar] [CrossRef]
- Neffati, M.; Marzouk, B. Roots volatiles and fatty acids of coriander (Coriandrum sativum L.) grown in saline medium. Acta Physiol. Plant. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Stępień, P.; Kłbus, G. Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biol. Plant. 2006, 50, 610–616. [Google Scholar] [CrossRef]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef]
- Bueno, M.; Cordovilla, M.P. Polyamines in halophytes. Front. Plant Sci. 2019, 10, 439. [Google Scholar] [CrossRef]
- Ben Hassine, A.; Ghanem, M.E.; Bouzid, S.; Lutts, S. Abscisic acid has contrasting effects on salt excretion and polyamine concentrations of an inland and a coastal population of the Mediterranean xero-halophyte species Atriplex halimus. Ann. Bot. 2009, 104, 925–936. [Google Scholar] [CrossRef]
- Reginato, M.A.; Abdala, G.I.; Miersch, O.; Ruiz, O.A.; Moschetti, E.; Luna, V. Changes in the levels of jasmonates and free polyamines induced by Na2SO4 and NaCl in roots and leaves of the halophyte Prosopis strombulifera. Biologia 2012, 67, 689–697. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef]
- Gharbi, E.; Martinez, J.P.; Benahmed, H.; Fauconnier, M.L.; Lutts, S.; Quinet, M. Salicylic acid differently impacts ethylene and polyamine synthesis in the glycophyte Solanum lycopersicum and the wild-related halophyte Solanum chilense exposed to mild salt stress. Physiol. Plant. 2016, 158, 152–167. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Rapparini, F.; Bertazza, G.; Silva, H.; Torrigiani, P.; Biondi, S. Comparing salt-induced responses at the transcript level in a salares and coastal-lowlands landrace of quinoa (Chenopodium quinoa Willd). Environ. Exp. Bot. 2017, 140, 150. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Siahpoosh, M.R.; Roessner, U.; Udvardi, M.; Kopka, J. Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol. Plant. 2008, 132, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, C.; Zhang, L.; Li, L.; Liu, S.; Yu, J.; You, L.; Zhou, D.; Xia, C.; Zhao, J.; et al. Metabolic profiling of cadmium-induced effects in one pioneer intertidal halophyte Suaeda salsa by NMR-based metabolomics. Ecotoxicology 2011, 20, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Cordovilla, M.D. Spermidine pretreatments mitigate the effects of saline stress by improving growth and saline excretion in Frankenia pulverulenta. Agronomy 2021, 11, 1515. [Google Scholar] [CrossRef]
- Bauer, G.A.; Bazzaz, F.A.; Minocha, R.; Long, S.; Magill, A.; Aber, J.; Berntson, G.M. Effects of chronic N additions on tissue chemistry, photosynthetic capacity, and carbon sequestration potential of a red pine (Pinus resinosa Ait.) stand in the NE United States. For. Ecol. Manag. 2004, 196, 173–186. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Sobolev, A.P.; Neelam, A.; Goyal, R.K.; Handa, A.K.; Segre, A.L. Nuclear magnetic resonance spectroscopy-based metabolite profiling of transgenic tomato fruit engineered to accumulate spermidine and spermine reveals enhanced anabolic and nitrogen-carbon interactions. Plant Physiol. 2006, 142, 1759–1770. [Google Scholar] [CrossRef]
- Bor, M.; Özdemir, F. Manipulating metabolic pathways for development of salt-tolerant crops. In Salinity Responses and Tolerance in Plants, Volume 1: Targeting Sensory, Transport and Signaling Mechanisms; Kumar, V., Wani, S.H., Suprasanna, P., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 235–256. [Google Scholar]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araujo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2018, 9, 1945. [Google Scholar] [CrossRef]
- Mutlu, F.; Bozcuk, S. Effects of salinity on the contents of polyamines and some other compounds in sunflower plants differing in salt tolerance. Russ. J. Plant Physiol. 2005, 52, 29–34. [Google Scholar] [CrossRef]
- De-Yun, M.; Lin-Lin, H.O.U.; Sha, Y.; Jing-Jing, M.; Feng, G.U.O.; Xin-Guo, L.I.; Wan Shu-Bo, a. Exogenous polyamines alleviating salt stress on peanuts (Arachis hypogaea) grown in pots. Chin. J. Plant Ecol. 2015, 39, 1209–1215. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tahara, M.; Yamada, Y.; Mitsudomi, Y.; Koga, K. Characterization of the polyamine biosynthetic pathways and salt stress response in Brachypodium distachyon. J. Plant Growth Regul. 2018, 37, 625–634. [Google Scholar] [CrossRef]
- Hernándiz, A.E.; Aucique-Perez, C.E.; Ćavar Zeljković, S.; Štefelová, N.; Salcedo Sarmiento, S.; Spíchal, L.; De Diego, N. Priming with small molecule-based biostimulants to improve abiotic stress tolerance in Arabidopsis thaliana. Plants 2022, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Cavar Zeljkovic, S.; Aucique-Perez, C.E.; Stefelova, N.; De Diego, N. Optimizing growing conditions for hydroponic farming of selected medicinal and aromatic plants. Food Chem. 2022, 375, 131845. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Hfaiedh, M.; Ben Slima, S.; Ben Romdhane, W.; Akacha, B.B.; Bouterra, M.T.; Dhifi, W.; Mnif, W.; Brini, F.; Ben Saad, R.; et al. Antioxidant and hepatoprotective effects of novel heteropolysaccharide isolated from Lobularia maritima on CCl4-induced liver injury in rats. Food Sci. Nutr. 2022, 10, 2271–2284. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Argentieri, M.P.; Avato, P.; Conforti, F. Lobularia maritima (L.) Desv. Aerial Parts Methanolic Extract: In Vitro Screening of Biological Activity. Plants 2020, 9, 89. [Google Scholar] [CrossRef]
- Kouidhi, S.; Zidi, O.; Abdelwahed, S.; Souissi, Y.; Trabelsi, N.; Redissi, A.; Hamdi, M.; Trabelsi, E.; Amara, Y.; Bhiri, T.; et al. Investigation of the chemical composition and antioxidant and antimicrobial activities of Lobularia maritima: Potent therapeutic applications. J. Chem. 2021, 2021, 1981680. [Google Scholar] [CrossRef]
- Tatsuzawa, F.; Usuki, R.; Toki, K.; Saito, N.; Shinoda, K.; Shigihara, A.; Honda, T. Acylated pelargonidin 3-sambubioside-5-glucosides from the red-purple flowers of Lobularia maritima. J. Jpn. Soc. Hortic. Sci. 2010, 79, 84–90. [Google Scholar] [CrossRef][Green Version]
- Ben Hsouna, A.; Dhibi, S.; Dhifi, W.; Ben Saad, R.; Brini, F.; Hfaidh, N.; Mnif, W. Essential oil from halophyte Lobularia maritima: Protective effects against CCl4-induced hepatic oxidative damage in rats and inhibition of the production of proinflammatory gene expression by lipopolysaccharide-stimulated RAW 264.7 macrophages. RSC Adv. 2019, 9, 36758–36770. [Google Scholar] [CrossRef]
- Asrar, H.; Hussain, T.; Qasim, M.; Nielsen, B.L.; Gul, B.; Khan, M.A. Salt induced modulations in antioxidative defense system of Desmostachya bipinnata. Plant Physiol. Biochem. 2020, 147, 113–124. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Dhibi, S.; Dhifi, W.; Ben Saad, R.; Brini, F.; Hfaidh, N.; Almeida, J.; Mnif, W. Lobularia maritima leave extract, a nutraceutical agent with antioxidant activity, protects against CCl4-induced liver injury in mice. Drug Chem. Toxicol. 2022, 45, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Asmaa, D.; Samira, M. Phytochemical screening and comparative analysis of bioactive phenolic compounds composition of Lobularia maritima L. grown in two different locations in the western Algeria. J. Biochem. Int. 2018, 9–16. [Google Scholar]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.D.R.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.J.C.; Barros de Oliveira, D. Plants as sources of anti-inflammatory agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Aravindaram, K.; Yang, N.S. Anti-inflammatory plant natural products for cancer therapy. Planta Med. 2010, 76, 1103–1117. [Google Scholar] [CrossRef]
- Yun, J.W. Possible anti-obesity therapeutics from nature-a review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef]
- Owusu, E.; Ahorlu, M.M.; Afutu, E.; Akumwena, A.; Asare, G.A. Antimicrobial activity of selected medicinal plants from a Sub-Saharan african country against bacterial pathogens from post-operative Wound Infections. Med. Sci. 2021, 9, 23. [Google Scholar] [CrossRef]
- Kurhekar, J.V. Tannins-antimicrobial chemical components. Int. J. Technol. Sci. 2016, 9, 5–9. [Google Scholar]
- Nirumand, M.C.; Hajialyani, M.; Rahimi, R.; Farzaei, M.H.; Zingue, S.; Nabavi, S.M.; Bishayee, A. Dietary Plants for the Prevention and Management of Kidney Stones: Preclinical and Clinical Evidence and Molecular Mechanisms. Int. J. Mol. Sci. 2018, 19, 765. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.E.; Nafie, M.S.; Khodeer, D.M.; El-Tanahy, A.H.H.; Abdel-Kader, M.S.; Badr, J.M.; Abdelhameed, R.F.A. Rubia tinctorum root extracts: Chemical profile and management of type II diabetes mellitus. RSC Adv. 2020, 24, 24159–24168. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.A.; Wahab, S.; Abullais, S.S.; Das, G.; Hani, U.; Ahmad, W.; Amir, M.; Ahmad, A.; Kandasamy, G.; Vasudevan, R. Pharmacological Efficacy of Tamarix aphylla: A Comprehensive Review. Plants 2021, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, M.; Boukhris, M. Flore Succincte et Illustrée des Zones Arides et Sahariennes de Tunisie; Associations pour la protection de la nature et de l’environnement: Sfax, Tunisia, 1998. [Google Scholar]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Dominiak, J.; Luczkiewicz, M.; Ekiert, H. Verbena officinalis (Common Vervain)—A Review on the Investigations of This Medicinally Important Plant Species. Planta Med. 2020, 86, 1241–1257. [Google Scholar] [CrossRef]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants used against cancer-an extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 2020, 73, 347–377. [Google Scholar] [CrossRef]
- McCutcheon, A.R.; Roberts, T.E.; Gibbons, E.; Ellis, S.M.; Babiuk, L.A.; Hancock, R.E.W.; Tower, G.H.N. Antiviral screening of British Colombian medicinal plants. J. Ethnopharmacol. 1995, 49, 101–110. [Google Scholar] [CrossRef]
- Candela, R.G.; Rosselli, S.; Bruno, M.; Fontana, G. A Review of the Phytochemistry, Traditional Uses and Biological Activities of the Essential Oils of Genus Teucrium. Planta Med. 2021, 87, 432–479. [Google Scholar] [CrossRef]
- Satiyavati, G.C.; Raina, M.K.; Sharma, M. Medicinal Plants of India; Indian Council of Medical Research: New Dehli, India, 1996.
- Ravi, S.K.; Ramesh, B.N.; Mundugaru, R.; Vincent, B. Multiple pharmacological activities of Caesalpinia crista against aluminium-induced neurodegeneration in rats: Relevance for Alzheimer’s disease. Environ. Toxicol. Pharmacol. 2018, 58, 202–211. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove Plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Chen, P.S.; Li, J.H.; Liu, T.Y.; Lin, T.C. Folk medicine Terminalia catappa and its major tannin component, punicalagin, are effective against bleomycin-induced genotoxicity in Chinese hamster ovary cells. Cancer Lett. 2000, 152, 115–122. [Google Scholar] [CrossRef]
- Yeh, C.B.; Yu, Y.L.; Lin, C.W.; Chiou, H.L.; Hsieh, M.J.; Yang, S.F. Terminalia catappa attenuates urokinase-type plasminogen activator expression through Erk pathways in Hepatocellular carcinoma. BMC Complement. Altern. Med. 2014, 14, 141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guil-Guerrero, J.L.; Torija Isana, M.E.; Gimenez Martinez, J.J. Composicion nutricional del hinojo marino (Crithmum maritimum L.). Alimentaria 1996, 34, 65–72. [Google Scholar]
- Fuochi, V.; Barbagallo, I.; Distefano, A.; Puglisi, F.; Palmeri, R.; Di Rosa, M.; Giallongo, C.; Longhitano, L.; Fontana, P.; Sferrazzo, G.; et al. Biological properties of Cakile maritima Scop. (Brassicaceae) extracts. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Murshid, S.S.A.; Atoum, D.; Abou-Hussein, D.R.; Abdallah, H.M.; Hareeri, R.H.; Almukadi, H.; Edrada-Ebel, R. Genus Salsola: Chemistry, Biological Activities and Future Prospective-A Review. Plants 2022, 11, 714. [Google Scholar] [CrossRef]
- Seca, A.M.; Grigore, A.; Pinto, D.C.; Silva, A.M. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Abu Ghdeib, S.I. Antifungal activity of plant extracts against dermatophytes. Mycoses 1999, 42, 665–672. [Google Scholar] [CrossRef]
- Taïbi, K.; Aït Abderrahim, L.; Boussaid, M.; Taibi, F.; Achir, M.; Souana, K.; Benaissa, T.; Farhi, K.H.; Naamani, F.Z.; Nait Said, K. Unraveling the ethnopharmacological potential of medicinal plants used in Algerian traditional medicine for urinary diseases. Eur. J. Integr. Med. 2021, 44, 101339. [Google Scholar] [CrossRef]
- Renkema, J.M.; Smith, D. Effects of sweet alyssum flowers and their volatile compounds on Drosophila suzukii(Matsumura) in the laboratory. J. Appl. Entomol. 2020, 144, 968–971. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Han, X.; Cong, J.; Wang, S.; He, S.; Wei, D.; Zhang, Y.; Qin, J.; Sampietro, D.A. Insecticidal and repellent efficacy of the essential oil from Lobularia maritima and trans-3-pentenenitrile against insect pests of stored grains. Int. J. Food Prop. 2020, 23, 1125–1135. [Google Scholar] [CrossRef]


| Use | Species |
|---|---|
| Crops | - Lobularia maritima - Aeluropus littoralis - Populus euphratica - Karelinia caspica - Suaeda salsa - Kalidium foliatum - Puccinellia tenuiflora |
| Food | - Lobularia maritima - Suaeda fruticosa - Arthrocnemum macrostachyum - Halopyrum mucronatum - Cressa cretica - Haloxylon stocksii - Alhaji maurorum |
| Medicine | - Lobularia maritima - Enicostema verticillatum - Haloxylon stocksii - Parkinsonia aculeata |
| Fodder/forage | - Aeluropus logopoides - Atriplex stocksii - Chenopodium album - Panicum turgidum - Desmostachya bipinnata - Salvadora persica - Sporobolus helvolus - Tamarix indica - Urochondra setulosa |
| Biofuel | - Desmostachya bipinnata - Phragmites karka - Halopyrum mucronatum - Panicum turgidum - Typha domingensis |
| Compound | Key Biological Properties | |
|---|---|---|
| Phenolic compounds | TP (mg GAE/g): - flowers 60.845 - leaves 147.451 - stems 307.873 - roots 368.150 | - classified as primary antioxidants - eliminate radicals through direct reactions, scavenging, or reduction of free radicals (e.g., hydroxyl, superoxide, peroxide, and alcoxyl radicals) to less reactive compounds - chelate transition metal cations (e.g., Cu2+ and Fe2+) - inhibit the activity of many enzymes involved in free-radical generation (e.g., xanthine oxidase, protein kinase and lipoxygenase) - exhibit anti-inflammatory, antibacterial, antifungal, antiviral, antiallergic, anticancer, anticoagulant, and astringent properties |
| TF (mg CE/g): - leaves 0.432 - roots 0.346 - flowers 0.088 - stems 0.021 | ||
| TC (mg CE/g): - stems 0.310 - flowers 0.303 - leaves0.195 - roots 0.109 | ||
| Fatty acids | e.g., capric, lauric, palmitic, myristic, stearic acid | - important cell membrane components - precursors of eicosanoids (PG, PGI, TX, LT), tissue hormones with a broad spectrum of activity - exhibit anti-inflammatory and antiallergic effects - activate metabolic processes and cell division |
| Phytosterols | e.g., β-sitosterol | - play structural roles in cell membranes - reduce cholesterol and LDL-C plasma levels - exert antiatherogenic effects |
| Terpenoids | e.g., neophytadiene, betulin aldehyde, β-amyrin | - exhibit effective activity against various bacterial, fungal, and yeast strains - exhibit anti-inflammatory activity - exert anticancer effects |
| Essential oil compounds | e.g., linalool, benzyl alcohol, 1-phenyl butanone, 1-terpineol | - exhibit antiseptic, antimicrobial, antifungal, anti-inflammatory, immunostimulatory, neuroprotective, and antioxidant properties |
| Macromolecules | e.g., proteins, polysaccharides | - important organic components of the body - exhibit antioxidant properties through a variety of mechanisms, including free-radicals scavenging, electron or hydrogen transfer reduction, transition-metal-chelating activity, ferric reducing power, and prevention of LPO |
| Species | Botanical Family | Properties | Reference |
|---|---|---|---|
| Rubia tinctorum | Rubiaceae | Diuretic action; treatment of type II diabetes mellitus | [134,135] |
| Tamarix gllica | Tamarixaceae | Astringent, antibacterial, anti-inflammatory, wound-healing, and diuretic properties | [136] |
| Limoniastrum monopetalum | Plumbaginaceae | Cardioprotective, antidysenteric, antioxidant, antidiarrheal properties | [137,138] |
| Verbena officinalis | Vebenaceae | Analgesic, anti-inflammatory, anticancer, neuroprotective, and anticonvulsant activity | [139] |
| Plantago lanceolata, P. major, P. ovata | Plantaginaceae | Anticancer, anti-infectious, anti-inflammatory action in hepatitis; treatment of cold, cough, and digestive disorders | [140,141] |
| Teucrium genus | Labiatae | Antispasmodic, hypoglycemic, anti-inflammatory, analgesic properties | [142] |
| Caesalpinia crista | Leguminosae | Treatment of headaches, cough, asthma, neurodegenerative diseases, and upset stomach | [143,144] |
| Terminalia catappa | Combretaceae | Preventing hepatoma, hepatitis, fever, and diarrhea | [145,146,147] |
| Cakile maritima | Brassicaceae | Diuretic, antiscorbutic, anti-inflammatory, purgative and digestive properties | [148,149] |
| Salsola kali | Amaranthaceae | Hypotensive, hypoglycemic, anticancer, procognitive, antiviral, antimicrobial, hepatoprotective properties | [150] |
| Inula viscosa | Asteraceae | Antiseptic, antiscabies, antipyretic, anticancer, anti-inflammatory agent | [151,152] |
| Compound | Organism | Administration | Dose | Duration | Action Mode | Reference |
|---|---|---|---|---|---|---|
| Heterpolysaccharide | Male Wistar albino rats | i.p. | 250 mg/kg b.w. | 15 days | Anti-inflammatory, detoxifying ↓ ALT ↓ AST ↓ ALP ↓ LDH ↑ SOD ↑ CAT ↑ GPx ↑ IL-10 ↓ TGF-β1 ↓TNF-α | [118] |
| Methanolic extract from the leaves | Male Wistar albino rats | p.o. | 100–500 mg/kg b.w. | 30 days | Anti-inflammatory and detoxifying ↓(AST/GOT) ↓ (ALT/GPT) ↑ SOD ↑ CAT ↑ GPx | [124] |
| Whole plant infusion | Traditional medicine | p.o. | One cup on an empty stomach | 30 days | Treatment of urinary problems Antiradical, anti-inflammatory, diuretic properties | [153] |
| Essential oil | Male albino Wistar rats | i.p. | 250 mg/kg b.w. | 15 days | Anti-inflammatory properties, antioxidant properties ↓ IL-1β ↓IL-6 ↓ TNF-α ↓iNOS ↓COX-2 ↓Inflammatory cytokines | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Hsouna, A.; Michalak, M.; Kukula-Koch, W.; Ben Saad, R.; ben Romdhane, W.; Zeljković, S.Ć.; Mnif, W. Evaluation of Halophyte Biopotential as an Unused Natural Resource: The Case of Lobularia maritima. Biomolecules 2022, 12, 1583. https://doi.org/10.3390/biom12111583
Ben Hsouna A, Michalak M, Kukula-Koch W, Ben Saad R, ben Romdhane W, Zeljković SĆ, Mnif W. Evaluation of Halophyte Biopotential as an Unused Natural Resource: The Case of Lobularia maritima. Biomolecules. 2022; 12(11):1583. https://doi.org/10.3390/biom12111583
Chicago/Turabian StyleBen Hsouna, Anis, Monika Michalak, Wirginia Kukula-Koch, Rania Ben Saad, Walid ben Romdhane, Sanja Ćavar Zeljković, and Wissem Mnif. 2022. "Evaluation of Halophyte Biopotential as an Unused Natural Resource: The Case of Lobularia maritima" Biomolecules 12, no. 11: 1583. https://doi.org/10.3390/biom12111583
APA StyleBen Hsouna, A., Michalak, M., Kukula-Koch, W., Ben Saad, R., ben Romdhane, W., Zeljković, S. Ć., & Mnif, W. (2022). Evaluation of Halophyte Biopotential as an Unused Natural Resource: The Case of Lobularia maritima. Biomolecules, 12(11), 1583. https://doi.org/10.3390/biom12111583