CircRNAs in Tumor Radioresistance
Abstract
:1. Introduction
2. Biogenesis and Functional Mechanisms of CircRNAs
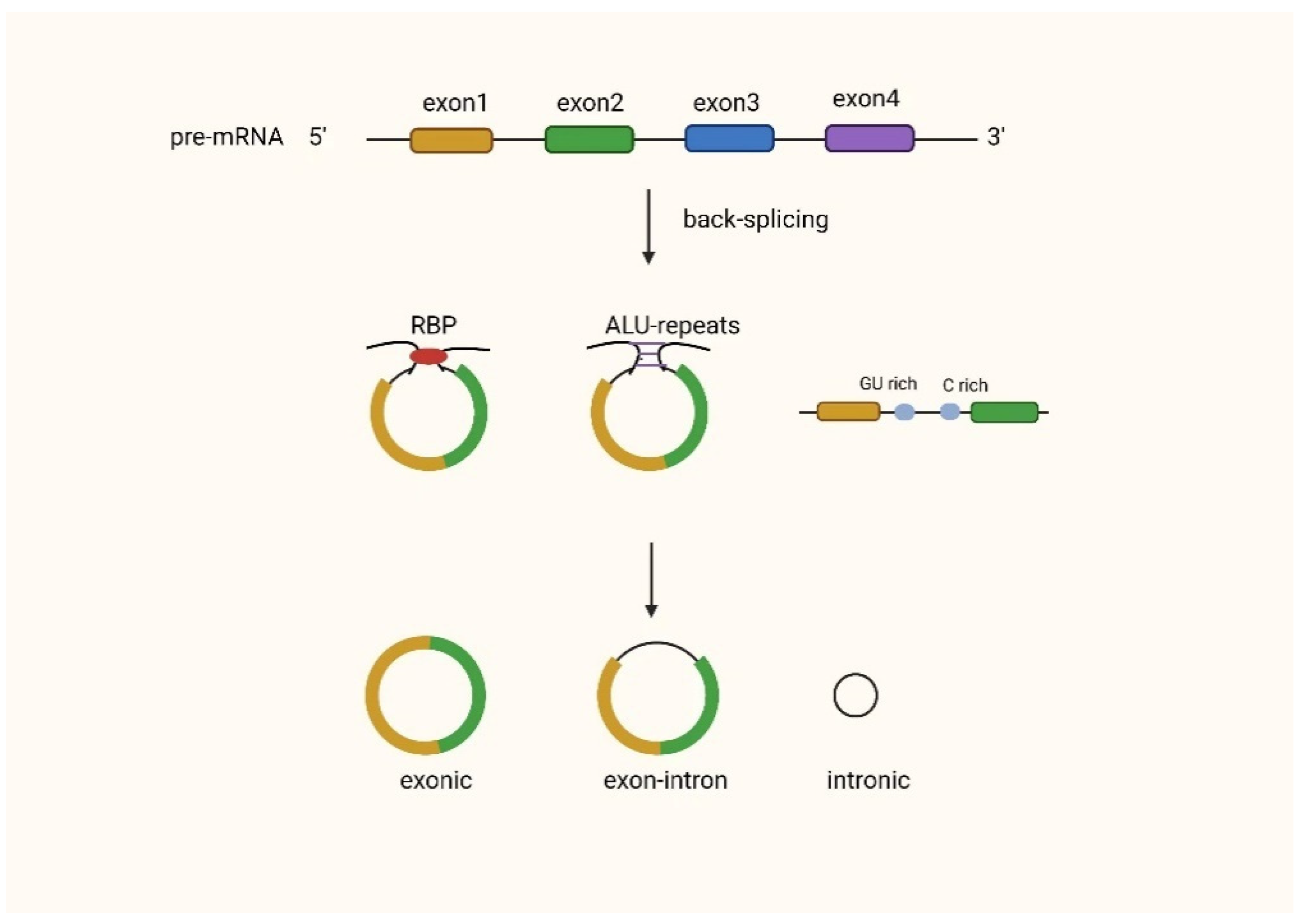
2.1. Biogenesis of CircRNAs
2.2. Functions of CircRNAs
2.2.1. MiRNA Sponges
2.2.2. CircRNAs Regulate Transcription and Translation
2.2.3. CircRNAs Interact with Proteins
2.2.4. Translation of CircRNAs into Proteins
2.2.5. Exosomal CircRNAs
3. Research Progress of CircRNAs Related to Tumor Radioresistance
3.1. Glioma
3.2. Nasopharyngeal Carcinoma
3.3. Non-Small Cell Lung Cancer
3.4. Colon Cancer and Colorectal Cancer
3.5. Esophageal Cancer
3.6. Prostate Cancer
3.7. Materials and Methods Used in CircRNA Studies
4. Conclusions
5. Discussion and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Salzman, J. Detecting circular RNAs: Bioinformatic and experimental challenges. Nat. Rev. Genet. 2016, 17, 679–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef] [Green Version]
- Nigro, J.M.; Cho, K.R.; Fearon, E.R.; Kern, S.E.; Ruppert, J.M.; Oliner, J.D.; Kinzler, K.W.; Vogelstein, B. Scrambled exons. Cell 1991, 64, 607–613. [Google Scholar] [CrossRef]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Starke, S.; Jost, I.; Rossbach, O.; Schneider, T.; Schreiner, S.; Hung, L.H.; Bindereif, A. Exon circularization requires canonical splice signals. Cell Rep. 2015, 10, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Adelman, K.; Egan, E. Non-coding RNA: More uses for genomic junk. Nature 2017, 543, 183–185. [Google Scholar] [CrossRef] [Green Version]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Tian, Y.; Gao, Y.; Dong, X.; Chen, W.; Yuan, X.; Yin, W.; Xu, J.; Chen, K.; et al. CircRNA inhibits DNA damage repair by interacting with host gene. Mol. Cancer 2020, 19, 128. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Perriman, R.; Ares, M., Jr. Circular mRNA can direct translation of extremely long repeating-sequence proteins in vivo. RNA 1998, 4, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.S.; Ai, Y.; Wilusz, J.E. Biogenesis and Functions of Circular RNAs Come into Focus. Trends Cell Biol. 2020, 30, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circRNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef] [PubMed]
- Ghotra, V.P.; Geldof, A.A.; Danen, E.H. Targeted radiosensitization in prostate cancer. Curr. Pharm. Des. 2013, 19, 2819–2828. [Google Scholar] [CrossRef]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Golden, E.B.; Formenti, S.C. Is tumor (R)ejection by the immune system the “5th R” of radiobiology? Oncoimmunology 2014, 3, e28133. [Google Scholar] [CrossRef]
- Steel, G.G.; McMillan, T.J.; Peacock, J.H. The 5Rs of radiobiology. Int. J. Radiat. Biol. 1989, 56, 1045–1048. [Google Scholar] [CrossRef] [Green Version]
- Zaphiropoulos, P.G. Exon skipping and circular RNA formation in transcripts of the human cytochrome P-450 2C18 gene in epidermis and of the rat androgen binding protein gene in testis. Mol Cell Biol. 1997, 17, 2985–2993. [Google Scholar] [CrossRef]
- Surono, A.; Takeshima, Y.; Wibawa, T.; Ikezawa, M.; Nonaka, I.; Matsuo, M. Circular dystrophin RNAs consisting of exons that were skipped by alternative splicing. Hum. Mol. Genet. 1999, 8, 493–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burd, C.E.; Jeck, W.R.; Liu, Y.; Sanoff, H.K.; Wang, Z.; Sharpless, N.E. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010, 6, e1001233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaiou, M. Circular RNAs as Potential Biomarkers and Therapeutic Targets for Metabolic Diseases. Adv. Exp. Med. Biol. 2019, 1134, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, X.O.; Chen, T.; Xiang, J.F.; Yin, Q.F.; Xing, Y.H.; Zhu, S.; Yang, L.; Chen, L.L. Circular intronic long noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Capel, B.; Swain, A.; Nicolis, S.; Hacker, A.; Walter, M.; Koopman, P.; Goodfellow, P.; Lovell-Badge, R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 1993, 73, 1019–1030. [Google Scholar] [CrossRef]
- Wang, P.L.; Bao, Y.; Yee, M.C.; Barrett, S.P.; Hogan, G.J.; Olsen, M.N.; Dinneny, J.R.; Brown, P.O.; Salzman, J. Circular RNA is expressed across the eukaryotic tree of life. PLoS ONE 2014, 9, e90859. [Google Scholar] [CrossRef] [Green Version]
- Bachmayr-Heyda, A.; Reiner, A.T.; Auer, K.; Sukhbaatar, N.; Aust, S.; Bachleitner-Hofmann, T.; Mesteri, I.; Grunt, T.W.; Zeillinger, R.; Pils, D. Correlation of circular RNA abundance with proliferation—exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep. 2015, 5, 8057. [Google Scholar] [CrossRef] [Green Version]
- Conn, S.J.; Pillman, K.A.; Toubia, J.; Conn, V.M.; Salmanidis, M.; Phillips, C.A.; Roslan, S.; Schreiber, A.W.; Gregory, P.A.; Goodall, G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015, 160, 1125–1134. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, C.; Sun, R.; Yang, D.; Liu, Q. Circular Noncoding RNA NR3C1 Acts as a miR-382-5p Sponge to Protect RPE Functions via Regulating PTEN/AKT/mTOR Signaling Pathway. Mol. Ther. 2020, 28, 929–945. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Qin, C.; Li, D.; Zhao, W.; Nie, L.; Cao, J.; Guo, J.; Zhong, T.; Wang, L.; Li, L.; et al. A Novel Long Noncoding RNA, lncR-125b, Promotes the Differentiation of Goat Skeletal Muscle Satellite Cells by Sponging miR-125b. Front. Genet. 2019, 10, 1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arif, K.T.; Okolicsanyi, R.K.; Haupt, L.M.; Griffiths, L.R. A combinatorial in silico approach for microRNA-target identification: Order out of chaos. Biochimie 2021, 187, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Abdelmohsen, K.; Panda, A.C.; Munk, R.; Grammatikakis, I.; Dudekula, D.B.; De, S.; Kim, J.; Noh, J.H.; Kim, K.M.; Martindale, J.L.; et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017, 14, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef] [Green Version]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Conlon, E.G.; Manley, J.L. RNA-binding proteins in neurodegeneration: Mechanisms in aggregate. Genes Dev. 2017, 31, 1509–1528. [Google Scholar] [CrossRef]
- Yang, Z.G.; Awan, F.M.; Du, W.W.; Zeng, Y.; Lyu, J.; Wu, D.; Gupta, S.; Yang, W.; Yang, B.B. The Circular RNA Interacts with STAT3, Increasing Its Nuclear Translocation and Wound Repair by Modulating Dnmt3a and miR-17 Function. Mol. Ther. 2017, 25, 2062–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Du, W.W.; Wu, N.; Yang, W.; Awan, F.M.; Fang, L.; Ma, J.; Li, X.; Zeng, Y.; Yang, Z.; et al. A circular RNA promotes tumorigenesis by inducing c-myc nuclear translocation. Cell Death Differ. 2017, 24, 1609–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armakola, M.; Higgins, M.J.; Figley, M.D.; Barmada, S.J.; Scarborough, E.A.; Diaz, Z.; Fang, X.; Shorter, J.; Krogan, N.J.; Finkbeiner, S.; et al. Inhibition of RNA lariat debranching enzyme suppresses TDP-43 toxicity in ALS disease models. Nat. Genet. 2012, 44, 1302–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Du, W.W.; Wu, Y.; Yang, Z.; Awan, F.M.; Li, X.; Yang, W.; Zhang, C.; Yang, Q.; Yee, A.; et al. A Circular RNA Binds To and Activates AKT Phosphorylation and Nuclear Localization Reducing Apoptosis and Enhancing Cardiac Repair. Theranostics 2017, 7, 3842–3855. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, G.; Yan, X.; Lv, Z.; Yin, H.; Zhang, S.; Song, W.; Li, X.; Li, L.; Du, Z.; et al. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 2018, 19, 218. [Google Scholar] [CrossRef] [Green Version]
- Abe, N.; Matsumoto, K.; Nishihara, M.; Nakano, Y.; Shibata, A.; Maruyama, H.; Shuto, S.; Matsuda, A.; Yoshida, M.; Ito, Y.; et al. Rolling Circle Translation of Circular RNA in Living Human Cells. Sci. Rep. 2015, 5, 16435. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhu, S.; Meng, N.; He, Y.; Lu, R.; Yan, G.R. ncRNA-Encoded Peptides or Proteins and Cancer. Mol. Ther. 2019, 27, 1718–1725. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, N.; Yang, X.; Luo, J.; Yan, S.; Xiao, F.; Chen, W.; Gao, X.; Zhao, K.; Zhou, H.; et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene 2018, 37, 1805–1814. [Google Scholar] [CrossRef]
- Di Timoteo, G.; Dattilo, D.; Centrón-Broco, A.; Colantoni, A.; Guarnacci, M.; Rossi, F.; Incarnato, D.; Oliviero, S.; Fatica, A.; Morlando, M.; et al. Modulation of circRNA Metabolism by m(6)A Modification. Cell Rep. 2020, 31, 107641. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Anderson, D.G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018, 9, 2629. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, X.; Zhang, M.; Yan, S.; Sun, C.; Xiao, F.; Huang, N.; Yang, X.; Zhao, K.; Zhou, H.; et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J. Natl. Cancer Inst. 2018, 110, 304–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; El Andaloussi, S.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetta, C.; Ghigo, E.; Silengo, L.; Deregibus, M.C.; Camussi, G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 2013, 44, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Z.; Jiang, P.; Peng, M.; Zhang, X.; Chen, K.; Liu, H.; Bi, H.; Liu, X.; Li, X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 177. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Li, P.; Fan, L.; Wu, M. The Potential Role of circRNA in Tumor Immunity Regulation and Immunotherapy. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Meads, M.B.; Gatenby, R.A.; Dalton, W.S. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat. Rev. Cancer 2009, 9, 665–674. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Zhao, X.; Ding, J. Knockdown of circ_0008344 contributes to radiosensitization in glioma via miR-433-3p/RNF2 axis. J. Biosci. 2021, 46, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Mao, X.; Zhao, H. The circ_VCAN with radioresistance contributes to the carcinogenesis of glioma by regulating microRNA-1183. Medicine 2020, 99, e19171. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Cao, Z.; Du, J.; Liu, T.; Wang, T. Circular RNA circPITX1 knockdown inhibits glycolysis to enhance radiosensitivity of glioma cells by miR-329-3p/NEK2 axis. Cancer Cell Int. 2020, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, H.; Guan, Z. CircRNA_000543 knockdown sensitizes nasopharyngeal carcinoma to irradiation by targeting miR-9/platelet-derived growth factor receptor B axis. Biochem. Biophys. Res. Commun. 2019, 512, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Shuai, M.; Huang, L. High Expression of hsa_circRNA_001387 in Nasopharyngeal Carcinoma and the Effect on Efficacy of Radiotherapy. Onco. Targets Ther. 2020, 13, 3965–3973. [Google Scholar] [CrossRef] [PubMed]
- Shuai, M.; Hong, J.; Huang, D.; Zhang, X.; Tian, Y. Upregulation of circRNA_0000285 serves as a prognostic biomarker for nasopharyngeal carcinoma and is involved in radiosensitivity. Oncol. Lett. 2018, 16, 6495–6501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.C.; Li, Y.; Feng, X.Z.; Li, D.B. Circular RNA circ_0001287 inhibits the proliferation, metastasis, and radiosensitivity of non-small cell lung cancer cells by sponging microRNA miR-21 and up-regulating phosphatase and tensin homolog expression. Bioengineered 2021, 12, 414–425. [Google Scholar] [CrossRef]
- Jin, Y.; Su, Z.; Sheng, H.; Li, K.; Yang, B.; Li, S. Circ_0086720 knockdown strengthens the radiosensitivity of non-small cell lung cancer via mediating the miR-375/SPIN1 axis. Neoplasma 2021, 68, 96–107. [Google Scholar] [CrossRef]
- Huang, M.; Li, T.; Wang, Q.; Li, C.; Zhou, H.; Deng, S.; Lv, Z.; He, Y.; Hou, B.; Zhu, G. Silencing circPVT1 enhances radiosensitivity in non-small cell lung cancer by sponging microRNA-1208. Cancer Biomark. 2021, 31, 263–279. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Liu, X.; Li, F.; Chen, W.; Kuang, Y.; Zhao, X.; Li, L.; Yu, B.; Jin, X.; et al. CircZNF208 enhances the sensitivity to X-rays instead of carbon-ions through the miR-7-5p /SNCA signal axis in non-small-cell lung cancer cells. Cell Signal. 2021, 84, 110012. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.; Tian, Y.; Han, C.; Wang, L.; Ding, B.; Tian, H.; Zhou, C.; Ju, Y.; Peng, A.; et al. Circ_0055625 knockdown inhibits tumorigenesis and improves radiosensitivity by regulating miR-338-3p/MSI1 axis in colon cancer. World J. Surg. Oncol. 2021, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, X.; Lu, X.; Wei, Q.; Chen, M.; Liu, L. Inhibition of hsa_circ_0001313 (circCCDC66) induction enhances the radio-sensitivity of colon cancer cells via tumor suppressor miR-338-3p: Effects of cicr_0001313 on colon cancer radio-sensitivity. Pathol. Res. Pract. 2019, 215, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zhang, J.; Chu, Z.; Liu, P.; Zhang, X.; Li, C.; Gu, X. Circ_0007031 Serves as a Sponge of miR-760 to Regulate the Growth and Chemoradiotherapy Resistance of Colorectal Cancer via Regulating DCP1A. Cancer Manag. Res. 2020, 12, 8465–8479. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Y.; Yang, Y.; Chen, Y.; Liu, H. Circ_0067835 Knockdown Enhances the Radiosensitivity of Colorectal Cancer by miR-296-5p/IGF1R Axis. Onco. Targets Ther. 2021, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Peng, S.; Li, Y.; Guo, T. Circ-MFN2 Positively Regulates the Proliferation, Metastasis, and Radioresistance of Colorectal Cancer by Regulating the miR-574-3p/IGF1R Signaling Axis. Front. Genet. 2021, 12, 671337. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Zhong, J.; Lin, L. Circ-ACAP2 facilitates the progression of colorectal cancer through mediating miR-143-3p/FZD4 axis. Eur. J. Clin. Investig. 2021, 51, e13607. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, X.; Liu, B.; Zhang, P.; Chen, W.; Li, Q. CircRNA CBL.11 suppresses cell proliferation by sponging miR-6778-5p in colorectal cancer. BMC Cancer 2019, 19, 826. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jiang, Z.; Zou, X.; Hao, T. Exosomal circ_IFT80 Enhances Tumorigenesis and Suppresses Radiosensitivity in Colorectal Cancer by Regulating miR-296-5p/MSI1 Axis. Cancer Manag. Res. 2021, 13, 1929–1941. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Wu, H.; Li, P.; Zhao, W.; Yang, X.; Xing, X.; Li, S.; Li, J. Circular RNA PRKCI silencing represses esophageal cancer progression and elevates cell radiosensitivity through regulating the miR-186-5p/PARP9 axis. Life Sci. 2020, 259, 118168. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Wu, R.; Lin, Y. Circular RNA hsa_circ_0000554 promotes progression and elevates radioresistance through the miR-485-5p/fermitin family members 1 axis in esophageal cancer. Anticancer Drugs 2021, 32, 405–416. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Mingyan, E.; Wang, C.; Liu, G.; Shi, M.; Liu, S. CircVRK1 regulates tumor progression and radioresistance in esophageal squamous cell carcinoma by regulating miR-624-3p/PTEN/PI3K/AKT signaling pathway. Int. J. Biol. Macromol. 2019, 125, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, X.; Wen, L.; You, C.; Jin, X.; Liu, J. Hsa_circ_0014879 regulates the radiosensitivity of esophageal squamous cell carcinoma through miR-519-3p/CDC25A axis. Anticancer Drugs 2022, 33, e349–e361. [Google Scholar] [CrossRef]
- Li, H.; Zhi, Y.; Ma, C.; Shen, Q.; Sun, F.; Cai, C. Circ_0062020 Knockdown Strengthens the Radiosensitivity of Prostate Cancer Cells. Cancer Manag. Res. 2020, 12, 11701–11712. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Li, J.; Zhang, J.; Huang, S. Knockdown of Circ_CCNB2 Sensitizes Prostate Cancer to Radiation Through Repressing Autophagy by the miR-30b-5p/KIF18A Axis. Cancer Biother. Radiopharm. 2020, 37, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Zhang, P.; Ren, W.; Yang, F.; Du, C. Circ-ZNF609 Accelerates the Radioresistance of Prostate Cancer Cells by Promoting the Glycolytic Metabolism Through miR-501-3p/HK2 Axis. Cancer Manag. Res. 2020, 12, 7487–7499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dong, W.; Luo, G.; Xie, J.; Liu, J.; Yu, F. Silencing of hsa_circ_0009035 Suppresses Cervical Cancer Progression and Enhances Radiosensitivity through MicroRNA 889-3p-Dependent Regulation of HOXB7. Mol. Cell Biol. 2021, 41, e0063120. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, R.; Deng, C.; Zhou, Z. Circle RNA circABCB10 Modulates PFN2 to Promote Breast Cancer Progression, as Well as Aggravate Radioresistance Through Facilitating Glycolytic Metabolism Via miR-223-3p. Cancer Biother. Radiopharm. 2021, 36, 477–490. [Google Scholar] [CrossRef]
- Lenting, K.; Verhaak, R.; Ter Laan, M.; Wesseling, P.; Leenders, W. Glioma: Experimental models and reality. Acta Neuropathol. 2017, 133, 263–282. [Google Scholar] [CrossRef] [Green Version]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537.e525. [Google Scholar] [CrossRef]
- Rasmussen, B.K.; Hansen, S.; Laursen, R.J.; Kosteljanetz, M.; Schultz, H.; Nørgård, B.M.; Guldberg, R.; Gradel, K.O. Epidemiology of glioma: Clinical characteristics, symptoms, and predictors of glioma patients grade I-IV in the the Danish Neuro-Oncology Registry. J. Neurooncol. 2017, 135, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro. Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, V.M.; Phan, K.; Rovin, R.A. Comparison of operative outcomes of eloquent glioma resection performed under awake versus general anesthesia: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2018, 169, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.P.; Liew, B.S.; Idris, Z.; Rosman, A.K. Fluorescence-Guided versus Conventional Surgical Resection of High Grade Glioma: A Single-Centre, 7-Year, Comparative Effectiveness Study. Malays. J. Med. Sci. 2017, 24, 78–86. [Google Scholar] [CrossRef]
- Honda, N.; Yagi, K.; Ding, G.R.; Miyakoshi, J. Radiosensitization by overexpression of the nonphosphorylation form of IkappaB-alpha in human glioma cells. J. Radiat. Res. 2002, 43, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, J.T.; Tsirka, S.E. Advances in immunotherapeutic research for glioma therapy. J. Neurol. 2018, 265, 741–756. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Epstein, T.; Gatenby, R.A.; Brown, J.S. The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. PLoS ONE 2017, 12, e0185085. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Zhi, F.; Peng, Y.; Yang, C.C. MiR-128 promotes the apoptosis of glioma cells via binding to NEK2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8781–8788. [Google Scholar] [CrossRef]
- Sun, J.; Li, B.; Shu, C.; Ma, Q.; Wang, J. Functions and clinical significance of circular RNAs in glioma. Mol. Cancer 2020, 19, 34. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Qiu, Z.; Jiang, Y.; Dong, L.; Yang, W.; Gu, C.; Li, G.; Zhu, Y. Silencing of cZNF292 circular RNA suppresses human glioma tube formation via the Wnt/β-catenin signaling pathway. Oncotarget 2016, 7, 63449–63455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Zhang, S.; Chen, X.; Li, N.; Li, J.; Jia, R.; Pan, Y.; Liang, H. CircNT5E Acts as a Sponge of miR-422a to Promote Glioblastoma Tumorigenesis. Cancer Res. 2018, 78, 4812–4825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; Shi, Z.; Zhao, Y.; Tian, J. CircRNA hsa-circ-0014359 promotes glioma progression by regulating miR-153/PI3K signaling. Biochem. Biophys. Res. Commun. 2019, 510, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.P.; Yip, K.; Bratman, S.V.; Ito, E.; Liu, F.F. Nasopharyngeal Cancer: Molecular Landscape. J. Clin. Oncol. 2015, 33, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Hu, Z.; Wang, Q.; Luo, W.; Li, J.; Duan, L.; Zhu, Y.S.; Luo, D.X. The role of long non-coding RNAs in nasopharyngeal carcinoma: As systemic review. Oncotarget 2017, 8, 16075–16083. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.C.W.; Hui, E.P.; Lo, K.W.; Lam, W.K.J.; Johnson, D.; Li, L.; Tao, Q.; Chan, K.C.A.; To, K.F.; King, A.D.; et al. Nasopharyngeal carcinoma: An evolving paradigm. Nat. Rev. Clin. Oncol. 2021, 18, 679–695. [Google Scholar] [CrossRef]
- Prawira, A.; Oosting, S.F.; Chen, T.W.; Delos Santos, K.A.; Saluja, R.; Wang, L.; Siu, L.L.; Chan, K.K.W.; Hansen, A.R. Systemic therapies for recurrent or metastatic nasopharyngeal carcinoma: A systematic review. Br. J. Cancer 2017, 117, 1743–1752. [Google Scholar] [CrossRef]
- Li, L.N.; Xiao, T.; Yi, H.M.; Zheng, Z.; Qu, J.Q.; Huang, W.; Ye, X.; Yi, H.; Lu, S.S.; Li, X.H.; et al. Retraction: MiR-125b Increases Nasopharyngeal Carcinoma Radioresistance By Targeting A20/NF-κB Signaling Pathway. Mol. Cancer Ther. 2018, 17, 2490. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, Y.; Tan, F.; Liu, L.; Hou, X.; Fan, C.; Tang, L.; Mo, Y.; Wang, Y.; Yan, Q.; et al. Circular RNA circCCNB1 inhibits the migration and invasion of nasopharyngeal carcinoma through binding and stabilizing TJP1 mRNA. Sci. China Life Sci. 2022. [Google Scholar] [CrossRef]
- Mattern, J.; Roghi, C.S.; Hurtz, M.; Knäuper, V.; Edwards, D.R.; Poghosyan, Z. ADAM15 mediates upregulation of Claudin-1 expression in breast cancer cells. Sci. Rep. 2019, 9, 12540. [Google Scholar] [CrossRef]
- Hsu, C.P.; Chuang, H.C.; Lee, M.C.; Tsou, H.H.; Lee, L.W.; Li, J.P.; Tan, T.H. GLK/MAP4K3 overexpression associates with recurrence risk for non-small cell lung cancer. Oncotarget 2016, 7, 41748–41757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, I.; Karedath, T.; Andrews, S.S.; Al-Azwani, I.K.; Mohamoud, Y.A.; Querleu, D.; Rafii, A.; Malek, J.A. Altered expression pattern of circular RNAs in primary and metastatic sites of epithelial ovarian carcinoma. Oncotarget 2016, 7, 36366–36381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tang, Y.; Tang, C.; Cong, H.; Wang, X.; Shen, X.; Ju, S. Diminished LINC00173 expression induced miR-182-5p accumulation promotes cell proliferation, migration and apoptosis inhibition via AGER/NF-κB pathway in non-small-cell lung cancer. Am. J. Transl. Res. 2019, 11, 4248–4262. [Google Scholar]
- Willers, H.; Azzoli, C.G.; Santivasi, W.L.; Xia, F. Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer. Cancer J. 2013, 19, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Péchoux, C.L.; Mercier, O.; Belemsagha, D.; Bouaita, R.; Besse, B.; Fadel, E. Role of adjuvant radiotherapy in completely resected non-small-cell lung cancer. EJC Suppl. 2013, 11, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Mao, F.; Shen, T.; Luo, Q.; Ding, Z.; Qian, L.; Huang, J. Plasma miR-145, miR-20a, miR-21 and miR-223 as novel biomarkers for screening early-stage non-small cell lung cancer. Oncol. Lett. 2017, 13, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Hou, J.; Yang, C.; Wang, H.; Wu, S.; Wu, Y.; Zhao, X.; Lu, C. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J. Exp. Clin. Cancer Res. 2019, 38, 62. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Liu, Y.; Wang, Y.; Meng, M.; Wang, K.; Zang, X.; Zhao, S.; Sun, X.; Cui, L.; Pan, L.; et al. MiR-21 and MiR-155 promote non-small cell lung cancer progression by downregulating SOCS1, SOCS6, and PTEN. Oncotarget 2016, 7, 84508–84519. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.F.; Wu, Z.P.; Chen, Y.; Zhu, Q.S.; Hamidi, S.; Navab, R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS ONE 2014, 9, e103698. [Google Scholar] [CrossRef] [PubMed]
- Gkountakos, A.; Sartori, G.; Falcone, I.; Piro, G.; Ciuffreda, L.; Carbone, C.; Tortora, G.; Scarpa, A.; Bria, E.; Milella, M.; et al. PTEN in Lung Cancer: Dealing with the Problem, Building on New Knowledge and Turning the Game Around. Cancers 2019, 11, 1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Cai, Z.; Roberts, T.M. The Mechanisms Underlying PTEN Loss in Human Tumors Suggest Potential Therapeutic Opportunities. Biomolecules 2019, 9, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Wang, T.; She, Y.; Wu, K.; Gu, S.; Li, L.; Dong, C.; Chen, C.; Zhou, Y. N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol. Cancer 2021, 20, 105. [Google Scholar] [CrossRef]
- Chen, S.W.; Zhu, S.Q.; Pei, X.; Qiu, B.Q.; Xiong, D.; Long, X.; Lin, K.; Lu, F.; Xu, J.J.; Wu, Y.B. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol. Cancer 2021, 20, 144. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Appelt, A.L.; Pløen, J.; Harling, H.; Jensen, F.S.; Jensen, L.H.; Jørgensen, J.C.; Lindebjerg, J.; Rafaelsen, S.R.; Jakobsen, A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: A prospective observational study. Lancet Oncol. 2015, 16, 919–927. [Google Scholar] [CrossRef]
- Fu, T.; He, L.; Liu, F. Effect of colorectal resection combined with intraoperative radiofrequency ablation in treating colorectal cancer with liver metastasis and analysis of its prognosis. J. Buon 2020, 25, 2171–2179. [Google Scholar]
- Thompson, M.K.; Poortmans, P.; Chalmers, A.J.; Faivre-Finn, C.; Hall, E.; Huddart, R.A.; Lievens, Y.; Sebag-Montefiore, D.; Coles, C.E. Practice-changing radiation therapy trials for the treatment of cancer: Where are we 150 years after the birth of Marie Curie? Br. J. Cancer 2018, 119, 389–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugase, T.; Takahashi, T.; Serada, S.; Fujimoto, M.; Hiramatsu, K.; Ohkawara, T.; Tanaka, K.; Miyazaki, Y.; Makino, T.; Kurokawa, Y.; et al. SOCS1 Gene Therapy Improves Radiosensitivity and Enhances Irradiation-Induced DNA Damage in Esophageal Squamous Cell Carcinoma. Cancer Res. 2017, 77, 6975–6986. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Gong, W.; Wang, J.; Ji, K.; Wang, Y.; Xu, C.; Liu, Y.; He, N.; Du, L.; Liu, Q. Identification of Circular RNAs Altered in Mouse Jejuna After Radiation. Cell Physiol. Biochem. 2018, 47, 2558–2568. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Peng, X.; Yan, D.; Tang, H.; Huang, F.; Yang, Y.; Peng, Z. Msi-1 is a predictor of survival and a novel therapeutic target in colon cancer. Ann. Surg. Oncol. 2011, 18, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Demer, L.L. Exosomes: Nanosized cellular messages. Circ. Res. 2015, 116, 1281–1283. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Kang, H.; Gao, M.; Jin, L.; Zhang, F.; Chen, D.; Li, M.; Xiao, L. Exosome-transmitted circ_MMP2 promotes hepatocellular carcinoma metastasis by upregulating MMP2. Mol. Oncol. 2020, 14, 1365–1380. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhang, N.; Li, A.; Zhou, B.; Chen, Y.; Chen, S.; Huang, M.; Wu, F.; Zhang, L. Insulin-like growth factor-1 receptor induces immunosuppression in lung cancer by upregulating B7-H4 expression through the MEK/ERK signaling pathway. Cancer Lett. 2020, 485, 14–26. [Google Scholar] [CrossRef]
- Salisbury, T.B.; Tomblin, J.K. Insulin/Insulin-like growth factors in cancer: New roles for the aryl hydrocarbon receptor, tumor resistance mechanisms, and new blocking strategies. Front. Endocrinol. 2015, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Chen, L.; Zhou, Y.; Wang, Q.; Zheng, Z.; Xu, B.; Wu, C.; Zhou, Q.; Hu, W.; Wu, C.; et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol. Cancer 2019, 18, 47. [Google Scholar] [CrossRef] [Green Version]
- Domper Arnal, M.J.; Ferrández Arenas, Á.; Lanas Arbeloa, Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 2015, 21, 7933–7943. [Google Scholar] [CrossRef]
- Kato, H.; Nakajima, M. Treatments for esophageal cancer: A review. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 330–335. [Google Scholar] [CrossRef]
- Vendrely, V.; Launay, V.; Najah, H.; Smith, D.; Collet, D.; Gronnier, C. Prognostic factors in esophageal cancer treated with curative intent. Dig Liver Dis. 2018, 50, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhang, E.; He, R.; Wang, X. Newly developed strategies for improving sensitivity to radiation by targeting signal pathways in cancer therapy. Cancer Sci. 2013, 104, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ginther, C.; Kim, J.; Mosher, N.; Chung, S.; Slamon, D.; Vadgama, J.V. Expression of Wnt3 activates Wnt/β-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol. Cancer Res. 2012, 10, 1597–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagni, C.; Almeida, L.O.; Balan, T.; Martins, M.T.; Rosselli-Murai, L.K.; Papagerakis, P.; Castilho, R.M.; Squarize, C.H. PTEN Mediates Activation of Core Clock Protein BMAL1 and Accumulation of Epidermal Stem Cells. Stem Cell Rep. 2017, 9, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Haddadi, N.; Lin, Y.; Travis, G.; Simpson, A.M.; Nassif, N.T.; McGowan, E.M. PTEN/PTENP1: ‘Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol. Cancer 2018, 17, 37. [Google Scholar] [CrossRef]
- Kamran, S.C.; D’Amico, A.V. Radiation Therapy for Prostate Cancer. Hematol. Oncol. Clin. N. Am. 2020, 34, 45–69. [Google Scholar] [CrossRef]
- Chang, L.; Graham, P.H.; Hao, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. Emerging roles of radioresistance in prostate cancer metastasis and radiation therapy. Cancer Metastasis. Rev. 2014, 33, 469–496. [Google Scholar] [CrossRef]
- Sita, T.L.; Petras, K.G.; Wafford, Q.E.; Berendsen, M.A.; Kruser, T.J. Radiotherapy for cranial and brain metastases from prostate cancer: A systematic review. J. Neurooncol. 2017, 133, 531–538. [Google Scholar] [CrossRef]
- Xin, Y.; Jiang, F.; Yang, C.; Yan, Q.; Guo, W.; Huang, Q.; Zhang, L.; Jiang, G. Role of autophagy in regulating the radiosensitivity of tumor cells. J. Cancer Res. Clin. Oncol. 2017, 143, 2147–2157. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Zhang, Z.; Zhang, L.; Liang, X.; Sun, L.; Zhong, D. High kinesin family member 18A expression correlates with poor prognosis in primary lung adenocarcinoma. Thorac. Cancer 2019, 10, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.T.; Zhong, F.K. Kinesin Family Member 18A (KIF18A) Contributes to the Proliferation, Migration, and Invasion of Lung Adenocarcinoma Cells In Vitro and In Vivo. Dis. Markers 2019, 2019, 6383685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Zhou, D.; Zhi, H.; Wang, P.; Gao, Y.; Guo, M.; Yue, M.; Wang, Y.; Shen, W.; et al. Inferences of individual drug responses across diverse cancer types using a novel competing endogenous RNA network. Mol. Oncol. 2018, 12, 1429–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Liu, X.; Xie, P.; Wang, P.; Liu, M.; Zhan, Y.; Wang, H.; Feng, Y.; Li, Y. Circular RNA circ_0074026 indicates unfavorable prognosis for patients with glioma and facilitates oncogenesis of tumor cells by targeting miR-1304 to modulate ERBB4 expression. J. Cell Physiol. 2020, 235, 4688–4697. [Google Scholar] [CrossRef]
- Lv, X.; Wang, M.; Qiang, J.; Guo, S. Circular RNA circ-PITX1 promotes the progression of glioblastoma by acting as a competing endogenous RNA to regulate miR-379-5p/MAP3K2 axis. Eur. J. Pharmacol. 2019, 863, 172643. [Google Scholar] [CrossRef]
- Yu, D.; Li, Y.; Ming, Z.; Wang, H.; Dong, Z.; Qiu, L.; Wang, T. Comprehensive circular RNA expression profile in radiation-treated HeLa cells and analysis of radioresistance-related circRNAs. Peer J. 2018, 6, e5011. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, Y.; Zheng, Q.; Bao, C.; He, J.; Chen, B.; Lyu, D.; Zheng, B.; Xu, Y.; Long, Z.; et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017, 388, 208–219. [Google Scholar] [CrossRef]
- Wang, Z.; Su, M.; Xiang, B.; Zhao, K.; Qin, B. Circular RNA PVT1 promotes metastasis via miR-145 sponging in CRC. Biochem. Biophys. Res. Commun. 2019, 512, 716–722. [Google Scholar] [CrossRef]
- Zhong, R.; Chen, Z.; Mo, T.; Li, Z.; Zhang, P. Potential Role of circPVT1 as a proliferative factor and treatment target in esophageal carcinoma. Cancer Cell Int. 2019, 19, 267. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Zhang, L.Y.; Du, W.Z. Circular RNA circ-PVT1 contributes to paclitaxel resistance of gastric cancer cells through the regulation of ZEB1 expression by sponging miR-124-3p. Biosci. Rep. 2019, 39, BSR20193045. [Google Scholar] [CrossRef]
- Zheng, F.; Xu, R. CircPVT1 contributes to chemotherapy resistance of lung adenocarcinoma through miR-145-5p/ABCC1 axis. Biomed. Pharm. 2020, 124, 109828. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Jafari Ghods, F. Circular RNA in Saliva. Adv. Exp. Med. Biol. 2018, 1087, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, E.W.; Luo, D.; Seo, J.; Singh, N.N.; Singh, R.N. Human Survival Motor Neuron genes generate a vast repertoire of circular RNAs. Nucleic Acids Res. 2019, 47, 2884–2905. [Google Scholar] [CrossRef] [Green Version]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453. [Google Scholar] [CrossRef]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct Target Ther. 2021, 6, 185. [Google Scholar] [CrossRef]

| Cancer | Name of CircRNAs | Alteration | Target | Mechanism | References |
|---|---|---|---|---|---|
| glioma | circ-0008344 | + | miR-433-3p/RNF2 | enhance apoptosis and DNA damage | [62] |
| circ-VCAN | + | miR-1183 | enhance apoptosis | [63] | |
| circ-PITX1 | + | miR-329-3p/NEK2 | inhibit glycolysis | [64] | |
| nasopharyngeal carcinoma | circ-000543 | + | miR-9/PDGFRB | EMT | [65] |
| circ-001387 | + | Not mentioned | biomarker | [66] | |
| circ-0000285 | + | Not mentioned | biomarker | [67] | |
| non-small cell lung cancer | circ-0001287 | − | miR-21/PTEN | regulate cell cycle progression and DNA damage | [68] |
| circ-0086720 | + | miR-375/SPIN1 | enhance apoptosis | [69] | |
| circ-PVT1 | + | miR-1208 | EMT | [70] | |
| circ-ZNF208 | + | miR-7-5p/SNCA | regulate apoptosis | [71] | |
| colon cancer | circ-0055625 | + | miR-338-3p/MSI1 | regulate apoptosis | [72] |
| circ-CCDC66 | + | miR-338-3p | enhance apoptosis | [73] | |
| colorectal cancer | circ-0007031 | + | miR-760/DCP1A | regulate cell cycle | [74] |
| circ-0067835 | + | miR-296-5p/IGF1R | regulate cell cycle progression and DNA damage | [75] | |
| circ-MFN2 | + | miR-574-3p/IGF1R | regulate cell cycle progression | [76] | |
| circ-ACAP2 | + | miR-143-3p/FZD4 | regulate apoptosis | [77] | |
| circ-CBL.11 | + | miR-6778-5p | DNA damage | [78] | |
| circ-IFT80 | + | miR-296-5p/MSI1 | regulate cell cycle progression | [79] | |
| esophageal cancer | circ-PRKCI | + | miR-186-5p/PARP9 | regulate cell cycle progression | [80] |
| circ-0000554 | + | miR-485-5p/FERMT1 | regulate apoptosis | [81] | |
| circ-VRK1 | − | miR-624-3p/PTEN/PI3K/AKT | EMT | [82] | |
| circ-0014879 | + | miR-519-3p/CDC25A | regulate cell cycle progression and DNA damage | [83] | |
| prostate cancer | circ-0062020 | + | miR-615-5p/TRIP13 | enhance apoptosis | [84] |
| circ-CCNB2 | + | miR-30b-5p/KIF18A | repressing autophagy | [85] | |
| circ-ZNF609 | + | miR-501-3p/HK2 | inhibit glycolysis | [86] | |
| cervical cancer | circ-0009035 | + | miR-889-3p/HOXB7 | regulate apoptosis | [87] |
| breast cancer | circ-ABCB10 | + | miR-223-3p | inhibit glycolysis | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Gao, J.; Lin, F.; Wang, T.; Huo, S.; Wu, J.; Zhou, Q.; Zhang, C. CircRNAs in Tumor Radioresistance. Biomolecules 2022, 12, 1586. https://doi.org/10.3390/biom12111586
Gao Y, Gao J, Lin F, Wang T, Huo S, Wu J, Zhou Q, Zhang C. CircRNAs in Tumor Radioresistance. Biomolecules. 2022; 12(11):1586. https://doi.org/10.3390/biom12111586
Chicago/Turabian StyleGao, Yining, Jiawen Gao, Fei Lin, Ting Wang, Sitong Huo, Jiefang Wu, Qi Zhou, and Chao Zhang. 2022. "CircRNAs in Tumor Radioresistance" Biomolecules 12, no. 11: 1586. https://doi.org/10.3390/biom12111586






