DAR 16-II Primes Endothelial Cells for Angiogenesis Improving Bone Ingrowth in 3D-Printed BCP Scaffolds and Regeneration of Critically Sized Bone Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of DAR 16-II
2.2. Fabrication of the 3D-Printed BCP Scaffold
2.2.1. Calcination and Attrition Milling
2.2.2. Ink Formation
2.2.3. Scaffold Robocasting
2.2.4. Heat Treating the Scaffolds
2.3. Bioassays
2.3.1. Cell Culture
2.3.2. Endothelial Cell Spreading
2.3.3. Endothelial Cell Morphology
2.3.4. Matrigel Assay
2.3.5. Proliferation, MTT Assay
2.3.6. hMSC Differentiation Assay
2.3.7. HMSC/HUVEC Co-Culture
2.3.8. RT2 Profiler PCR Array
2.3.9. Whole Proteome Analysis
2.3.10. In Vivo Study
2.4. SEM and EDS Coupled SEM
2.5. Micro Bicinchoninic Acid (BCA) Assay
2.6. Statistical Analysis
3. Results
3.1. DAR 16-II Drives Morphological Changes in Endothelial Cells, Reminiscent of Angiogenic Activation
3.2. DAR 16-II Switches on a Migratory and Morphogenetic Program in EC
3.3. DAR-16-II-Activated Endothelial Cells Promote MSC Differentiation
3.4. DAR 16-II Effectively Coats Microporous 3D-Printed Scaffolds
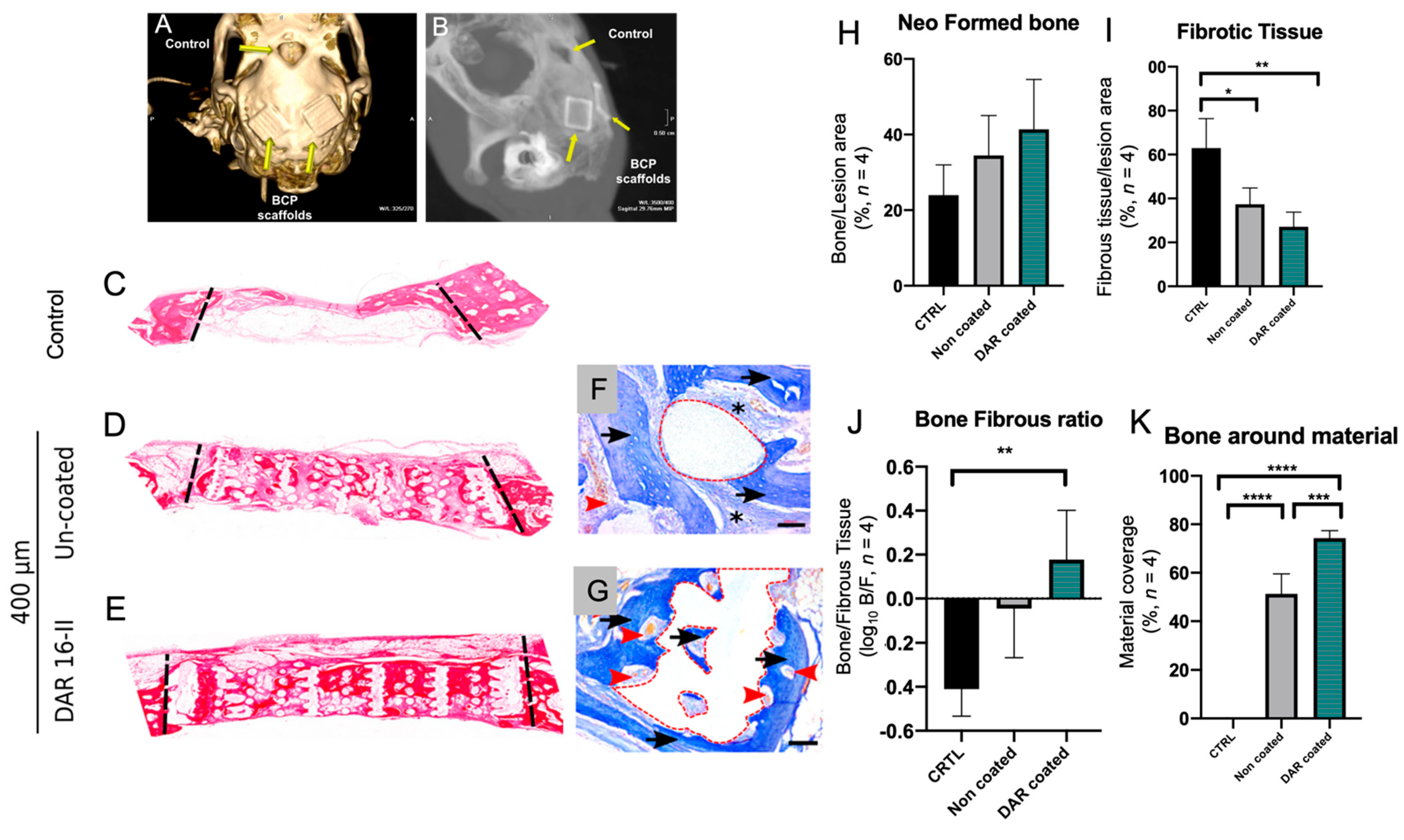
3.5. DAR-16-II-Coated Scaffolds Improve Bone Regeneration and Inhibit Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, Fracture and Bone Repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, N.; Villicana, C.; Yang, F. Immunomodulatory Strategies for Bone Regeneration: A Review from the Perspective of Disease Types. Biomaterials 2022, 286, 121604. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, N.G.; Weijs, W.L.J.; Koole, R.; Rosenberg, A.J.W.P.; Meijer, G.J. Tissue Engineering Strategies for Alveolar Cleft Reconstruction: A Systematic Review of the Literature. Clin. Oral. Investig. 2014, 18, 219–226. [Google Scholar] [CrossRef]
- Zizzari, V.L.; Zara, S.; Tetè, G.; Vinci, R.; Gherlone, E.; Cataldi, A. Biologic and Clinical Aspects of Integration of Different Bone Substitutes in Oral Surgery: A Literature Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 392–402. [Google Scholar] [CrossRef]
- Fishero, B.; Kohli, N.; Das, A.; Christophel, J.; Cui, Q. Current Concepts of Bone Tissue Engineering for Craniofacial Bone Defect Repair. Craniomaxillofacial Trauma Reconstr. 2015, 8, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Idowu, B.; Cama, G.; Deb, S.; Di Silvio, L. In Vitro Osteoinductive Potential of Porous Monetite for Bone Tissue Engineering. J. Tissue Eng. 2014, 5, 204173141453657. [Google Scholar] [CrossRef]
- Wang, M.O.; Vorwald, C.E.; Dreher, M.L.; Mott, E.J.; Cheng, M.-H.; Cinar, A.; Mehdizadeh, H.; Somo, S.; Dean, D.; Brey, E.M.; et al. Evaluating 3D-Printed Biomaterials as Scaffolds for Vascularized Bone Tissue Engineering. Adv. Mater. 2015, 27, 138–144. [Google Scholar] [CrossRef] [Green Version]
- El-Ghannam, A. Bone Reconstruction: From Bioceramics to Tissue Engineering. Expert Rev. Med. Devices 2005, 2, 87–101. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhumiratana, S.; Bernhard, J.C.; Alfi, D.M.; Yeager, K.; Eton, R.E.; Bova, J.; Shah, F.; Gimble, J.M.; Lopez, M.J.; Eisig, S.B.; et al. Tissue-Engineered Autologous Grafts for Facial Bone Reconstruction. Sci. Transl. Med. 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast Precursors, but Not Mature Osteoblasts, Move into Developing and Fractured Bones along with Invading Blood Vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkin, C.; Olsen, B.R. On Bone-Forming Cells and Blood Vessels in Bone Development. Cell Metab. 2010, 12, 314–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Feng, J.; Ho, T.-V.; Grimes, W.; Urata, M.; Chai, Y. The Suture Provides a Niche for Mesenchymal Stem Cells of Craniofacial Bones. Nat. Cell Biol. 2015, 17, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Maes, C.; Goossens, S.; Bartunkova, S.; Drogat, B.; Coenegrachts, L.; Stockmans, I.; Moermans, K.; Nyabi, O.; Haigh, K.; Naessens, M.; et al. Increased Skeletal VEGF Enhances β-Catenin Activity and Results in Excessively Ossified Bones. EMBO J. 2010, 29, 424–441. [Google Scholar] [CrossRef] [Green Version]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of Angiogenesis and Osteogenesis by a Specific Vessel Subtype in Bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Péault, B.; Asatrian, G.; Pham, D.; Hardy, W.R.; James, A.W. Stem Cell Technology for Bone Regeneration: Current Status and Potential Applications. SCCAA 2015, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Sui, B.-D.; Hu, C.-H.; Liu, A.-Q.; Zheng, C.-X.; Xuan, K.; Jin, Y. Stem Cell-Based Bone Regeneration in Diseased Microenvironments: Challenges and Solutions. Biomaterials 2019, 196, 18–30. [Google Scholar] [CrossRef]
- Yu, H.-S.; Won, J.-E.; Jin, G.-Z.; Kim, H.-W. Construction of Mesenchymal Stem Cell–Containing Collagen Gel with a Macrochanneled Polycaprolactone Scaffold and the Flow Perfusion Culturing for Bone Tissue Engineering. BioRes. Open Access 2012, 1, 124–136. [Google Scholar] [CrossRef]
- Colnot, C. Cell Sources for Bone Tissue Engineering: Insights from Basic Science. Tissue Eng. Part B Rev. 2011, 17, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive Calcium Phosphate–Based Glasses and Ceramics and Their Biomedical Applications: A Review. J. Tissue Eng. 2017, 8, 204173141771917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.H.; Harini, B.; Shadamarshan, R.P.K.; Balagangadharan, K.; Selvamurugan, N. Natural and Synthetic Polymers/Bioceramics/Bioactive Compounds-Mediated Cell Signalling in Bone Tissue Engineering. Int. J. Biol. Macromol. 2018, 110, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Buranawat, B.; Di Silvio, L.; Deb, S.; Nannmark, U.; Sennerby, L.; Palmer, R.M. Evaluation of a β-Calcium Metaphosphate Bone Graft Containing Bone Morphogenetic Protein-7 in Rabbit Maxillary Defects. J. Periodontol. 2014, 85, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-H.; Lee, A.K.-X.; Ho, C.-C.; Fang, M.-J.; Kuo, T.-Y.; Shie, M.-Y. The Effects of a 3D-Printed Magnesium-/Strontium-Doped Calcium Silicate Scaffold on Regulation of Bone Regeneration via Dual-Stimulation of the AKT and WNT Signaling Pathways. Biomater. Adv. 2022, 133, 112660. [Google Scholar] [CrossRef]
- Lin, S.; Yang, G.; Jiang, F.; Zhou, M.; Yin, S.; Tang, Y.; Tang, T.; Zhang, Z.; Zhang, W.; Jiang, X. A Magnesium-Enriched 3D Culture System That Mimics the Bone Development Microenvironment for Vascularized Bone Regeneration. Adv. Sci. 2019, 6, 1900209. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, W.; Qiu, F. Self-Assembling Peptides in Current Nanomedicine: Versatile Nanomaterials for Drug Delivery. CMC 2020, 27, 4855–4881. [Google Scholar] [CrossRef]
- Zhang, S. Discovery and Design of Self-Assembling Peptides. Interface Focus. 2017, 7, 20170028. [Google Scholar] [CrossRef] [Green Version]
- Najafi, H.; Jafari, M.; Farahavar, G.; Abolmaali, S.S.; Azarpira, N.; Borandeh, S.; Ravanfar, R. Recent Advances in Design and Applications of Biomimetic Self-Assembled Peptide Hydrogels for Hard Tissue Regeneration. Bio-Des. Manuf. 2021, 4, 735–756. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Zhang, S. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-37291-4. [Google Scholar]
- Koutsopoulos, S. Self-Assembling Peptide Nanofiber Hydrogels in Tissue Engineering and Regenerative Medicine: Progress, Design Guidelines, and Applications: Self-Assembling Peptides in Tissue Engineering and Regeneration. J. Biomed. Mater. Res. 2016, 104, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieminski, A.L.; Semino, C.E.; Gong, H.; Kamm, R.D. Primary Sequence of Ionic Self-Assembling Peptide Gels Affects Endothelial Cell Adhesion and Capillary Morphogenesis. J. Biomed. Mater. Res. 2008, 87A, 494–504. [Google Scholar] [CrossRef]
- Dou, X.-Q.; Feng, C.-L. Amino Acids and Peptide-Based Supramolecular Hydrogels for Three-Dimensional Cell Culture. Adv. Mater. 2017, 29, 1604062. [Google Scholar] [CrossRef]
- D’Auria, G.; Vacatello, M.; Falcigno, L.; Paduano, L.; Mangiapia, G.; Calvanese, L.; Gambaretto, R.; Dettin, M.; Paolillo, L. Self-Assembling Properties of Ionic-Complementary Peptides: Self-Assembling Peptides. J. Pept. Sci. 2009, 15, 210–219. [Google Scholar] [CrossRef]
- Gambaretto, R.; Tonin, L.; Di Bello, C.; Dettin, M. Self-Assembling Peptides: Sequence, Secondary Structure in Solution and Film Formation. Biopolymers 2008, 89, 906–915. [Google Scholar] [CrossRef]
- Jung, J.P.; Jones, J.L.; Cronier, S.A.; Collier, J.H. Modulating the Mechanical Properties of Self-Assembled Peptide Hydrogels via Native Chemical Ligation. Biomaterials 2008, 29, 2143–2151. [Google Scholar] [CrossRef] [Green Version]
- Zamuner, A.; Cavo, M.; Scaglione, S.; Messina, G.; Russo, T.; Gloria, A.; Marletta, G.; Dettin, M. Design of Decorated Self-Assembling Peptide Hydrogels as Architecture for Mesenchymal Stem Cells. Materials 2016, 9, 727. [Google Scholar] [CrossRef] [Green Version]
- Conconi, M.T.; Ghezzo, F.; Dettin, M.; Urbani, L.; Grandi, C.; Guidolin, D.; Nico, B.; Di Bello, C.; Ribatti, D.; Parnigotto, P.P. Effects on in Vitro and in Vivo Angiogenesis Induced by Small Peptides Carrying Adhesion Sequences. J. Pept. Sci. 2010, 16, 349–357. [Google Scholar] [CrossRef]
- Ricci, J.L.; Clark, E.A.; Murriky, A.; Smay, J.E. Three-Dimensional Printing of Bone Repair and Replacement Materials: Impact on Craniofacial Surgery. J. Craniofacial Surg. 2012, 23, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Teßmar, J.; Vorndran, E. Direct 3D Powder Printing of Biphasic Calcium Phosphate Scaffolds for Substitution of Complex Bone Defects. Biofabrication 2014, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Witek, L.; Shi, Y.; Smay, J. Controlling Calcium and Phosphate Ion Release of 3D Printed Bioactive Ceramic Scaffolds: An in Vitro Study. J. Adv. Ceram. 2017, 6, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Isern, J.; Martín-Antonio, B.; Ghazanfari, R.; Martín, A.M.; López, J.A.; del Toro, R.; Sánchez-Aguilera, A.; Arranz, L.; Martín-Pérez, D.; Suárez-Lledó, M.; et al. Self-Renewing Human Bone Marrow Mesenspheres Promote Hematopoietic Stem Cell Expansion. Cell Rep. 2013, 3, 1714–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharibi, B.; Cama, G.; Capurro, M.; Thompson, I.; Deb, S.; Di Silvio, L.; Hughes, F.J. Gene Expression Responses to Mechanical Stimulation of Mesenchymal Stem Cells Seeded on Calcium Phosphate Cement. Tissue Eng. Part A 2013, 19, 2426–2438. [Google Scholar] [CrossRef] [Green Version]
- Gaetani, M.; Chinnici, C.M.; Carreca, A.P.; Di Pasquale, C.; Amico, G.; Conaldi, P.G. Unbiased and Quantitative Proteomics Reveals Highly Increased Angiogenesis Induction by the Secretome of Mesenchymal Stromal Cells Isolated from Fetal Rather than Adult Skin. J. Tissue Eng. Regen. Med. 2018, 12, e949–e961. [Google Scholar] [CrossRef]
- Tasso, R.; Gaetani, M.; Molino, E.; Cattaneo, A.; Monticone, M.; Bachi, A.; Cancedda, R. The Role of BFGF on the Ability of MSC to Activate Endogenous Regenerative Mechanisms in an Ectopic Bone Formation Model. Biomaterials 2012, 33, 2086–2096. [Google Scholar] [CrossRef]
- Thompson, A.; Schäfer, J.; Kuhn, K.; Kienle, S.; Schwarz, J.; Schmidt, G.; Neumann, T.; Hamon, C. Tandem Mass Tags: A Novel Quantification Strategy for Comparative Analysis of Complex Protein Mixtures by MS/MS. Anal. Chem. 2003, 75, 1895–1904. [Google Scholar] [CrossRef]
- Neto, F.; Klaus-Bergmann, A.; Ong, Y.T.; Alt, S.; Vion, A.-C.; Szymborska, A.; Carvalho, J.R.; Hollfinger, I.; Bartels-Klein, E.; Franco, C.A.; et al. YAP and TAZ Regulate Adherens Junction Dynamics and Endothelial Cell Distribution during Vascular Development. eLife 2018, 7, e31037. [Google Scholar] [CrossRef]
- Veschini, L.; Crippa, L.; Dondossola, E.; Doglioni, C.; Corti, A.; Ferrero, E. The Vasostatin-1 Fragment of Chromogranin A Preserves a Quiescent Phenotype in Hypoxia-driven Endothelial Cells and Regulates Tumor Neovascularization. FASEB J. 2011, 25, 3906–3914. [Google Scholar] [CrossRef]
- Veschini, L.; Belloni, D.; Foglieni, C.; Cangi, M.G.; Ferrarini, M.; Caligaris-Cappio, F.; Ferrero, E. Hypoxia-Inducible Transcription Factor–1 Alpha Determines Sensitivity of Endothelial Cells to the Proteosome Inhibitor Bortezomib. Blood 2007, 109, 2565–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweet, L.; Kang, Y.; Czisch, C.; Witek, L.; Shi, Y.; Smay, J.; Plant, G.W.; Yang, Y. Geometrical versus Random β-TCP Scaffolds: Exploring the Effects on Schwann Cell Growth and Behavior. PLoS ONE 2015, 10, e0139820. [Google Scholar] [CrossRef] [PubMed]
- Diment, L.E.; Thompson, M.S.; Bergmann, J.H.M. Clinical Efficacy and Effectiveness of 3D Printing: A Systematic Review. BMJ Open 2017, 7, e016891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Song, T.; Chen, X.; Wang, M.; Yang, X.; Xiao, Y.; Zhang, X. Osteoinductivity of Porous Biphasic Calcium Phosphate Ceramic Spheres with Nanocrystalline and Their Efficacy in Guiding Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3722–3736. [Google Scholar] [CrossRef] [PubMed]
- Fellah, B.H.; Josselin, N.; Chappard, D.; Weiss, P.; Layrolle, P. Inflammatory Reaction in Rats Muscle after Implantation of Biphasic Calcium Phosphate Micro Particles. J. Mater. Sci. Mater. Med. 2007, 18, 287–294. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Chen, Y.; Cai, H.; Yang, X.; Zhu, X.; Fan, Y.; Zhang, X. Roles of Calcium Phosphate-Mediated Integrin Expression and MAPK Signaling Pathways in the Osteoblastic Differentiation of Mesenchymal Stem Cells. J. Mater. Chem. B 2016, 4, 2280–2289. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Tavakol, S.; Mousavi, S.M.M.; Tavakol, B.; Hoveizi, E.; Ai, J.; Sorkhabadi, S.M.R. Mechano-Transduction Signals Derived from Self-Assembling Peptide Nanofibers Containing Long Motif of Laminin Influence Neurogenesis in In-Vitro and In-Vivo. Mol. Neurobiol. 2017, 54, 2483–2496. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing Materials to Direct Stem-Cell Fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfayez, E.; Veschini, L.; Dettin, M.; Zamuner, A.; Gaetani, M.; Carreca, A.P.; Najman, S.; Ghanaati, S.; Coward, T.; Di Silvio, L. DAR 16-II Primes Endothelial Cells for Angiogenesis Improving Bone Ingrowth in 3D-Printed BCP Scaffolds and Regeneration of Critically Sized Bone Defects. Biomolecules 2022, 12, 1619. https://doi.org/10.3390/biom12111619
Alfayez E, Veschini L, Dettin M, Zamuner A, Gaetani M, Carreca AP, Najman S, Ghanaati S, Coward T, Di Silvio L. DAR 16-II Primes Endothelial Cells for Angiogenesis Improving Bone Ingrowth in 3D-Printed BCP Scaffolds and Regeneration of Critically Sized Bone Defects. Biomolecules. 2022; 12(11):1619. https://doi.org/10.3390/biom12111619
Chicago/Turabian StyleAlfayez, Eman, Lorenzo Veschini, Monica Dettin, Annj Zamuner, Massimiliano Gaetani, Anna P. Carreca, Stevo Najman, Shahram Ghanaati, Trevor Coward, and Lucy Di Silvio. 2022. "DAR 16-II Primes Endothelial Cells for Angiogenesis Improving Bone Ingrowth in 3D-Printed BCP Scaffolds and Regeneration of Critically Sized Bone Defects" Biomolecules 12, no. 11: 1619. https://doi.org/10.3390/biom12111619
APA StyleAlfayez, E., Veschini, L., Dettin, M., Zamuner, A., Gaetani, M., Carreca, A. P., Najman, S., Ghanaati, S., Coward, T., & Di Silvio, L. (2022). DAR 16-II Primes Endothelial Cells for Angiogenesis Improving Bone Ingrowth in 3D-Printed BCP Scaffolds and Regeneration of Critically Sized Bone Defects. Biomolecules, 12(11), 1619. https://doi.org/10.3390/biom12111619









