Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability
Abstract
1. Introduction
2. Methods of Activity Evaluation and Mechanisms of Action of Antioxidant Peptides
2.1. Chemical Evaluation Methods and Activity Mechanisms
2.1.1. Free Radical Scavenging
2.1.2. Chelation of Metal Ions
2.1.3. Lipid Peroxidation Inhibition (LPI)
2.2. Biological Evaluation Methods and Activity Mechanisms
2.2.1. In Vitro Cell Aspects
2.2.2. In Vivo Animal Aspects
3. Sources of Antioxidant Peptides
3.1. Marine Sources
3.2. Dairy Sources
| Source | Extraction Method(s) | Extraction Tool | Hydrolysate Name/ Peptide Sequence | Activity Evaluation Methods | Ref. |
|---|---|---|---|---|---|
| Silver carp muscle | Enzymatic and SGID | Alcalase + Pepsin and trypsin | LVPVAVF ISTSLPV MYPGIGDR ADLVHVQ |
| [82] |
| Snakehead soup | SGID | Pepsin and trypsin | PGMLGGSPPGLLG-GSPP SDGSNIHFPN |
| [83] |
| Salmon | Chemical synthesis | - | PMRGGGGYHY PMRGGGYHY PMRGGYHY PMRGYHY PMRYHY YHY |
| [84] |
| Mackerel muscle | Enzymatic | Protamex | ALSTWTLQLGSTSF-ASPM LGTLLFIAIPI |
| [85] |
| Barred mackerel gelatine | Enzymatic | Alcalase and actinidin | Fraction 1 Fraction 2 Fraction 3 |
| [86] |
| Rainbow trout frames | Microwave pretreatment assisted (MPA) + enzymatic + SGID; MPA + SGID | Alcalase + Pepsin and trypsin | SGID-MPCE SGID-NPME |
| [87] |
| Jellyfish | Enzymatic | Flavourzyme | Jellyfish flavourzyme hydrolysate |
| [88] |
| Defatted round scad | Enzymatic | Alcalase | ILGATIDNSK |
| [43] |
| Shrimp | Enzymatic | Alcalase | MTTNI MTTNL |
| [89] |
| Skipjack tuna bone | SGID | Pepsin and trypsin | GPDGR GADIVA GAPGPQMV AGPK GAEGFIF |
| [62] |
| Milk casein | Enzymatic | Trypsin | LHSMK |
| [90] |
| Fresh buffalo cheese | Water extraction | Ultrapure water | Water-solution peptides |
| [77] |
| Bovine and caprine sodic caseinate | Enzymatic | Serine protease | Bovine caseinate hydrolysates Caprine caseinate hydrolysates |
| [91] |
| Milk β-casein and κ-casein | Chemical synthesis | - | ARHPHPHLSFM AVPYPQR NPYVPR KVLPVPEK |
| [40] |
| Buffalo casein | Chemical synthesis | - | VLPVPQK |
| [48] |
| Fermented Rubing cheese | Water extraction | Deionized water | YPFPGPIH |
| [92] |
3.3. Animal Sources
3.4. Plant Sources
| Source | Extraction Method(s) | Extraction Tool | Hydrolysate Name/ Peptide Sequence | Activity Evaluation Methods | Ref. |
|---|---|---|---|---|---|
| Spanish dry-cured ham | Chemical synthesis | - | SNAAC |
| [105] |
| Porcine plasma | Enzymetic | Alkaline protease | WGPGVE |
| [148] |
| Yak bones collagen | Chemical synthesis | - | GASGPMGPR GLPGPM |
| [46] |
| Bovine bone collagen | Enzymetic | Thermolysin-like Protease A69 | Bovine bone collagen hydrolysate |
| [101] |
| Dry-cured Xuanwei ham | Chemical synthesis | - | DLEE |
| [29] |
| Pork sarcoplasmic and myofibrillar protein | Microbial fermentation | Lactobacillus plantarum CD101, Staphylococcus simulans NJ201 | Sarcoplasmic protein hydrolysate; myofibrillar protein hydrolysate |
| [149] |
| Chicken breast | Acid extraction, SGID + acid extraction | Porcine pepsin, trypsin, chymotrypsin, porcine pancreatic α-amylase, and porcine pancreatic lipase, HCl (0.01N) | Cooked protein extracts; Cooked protein + SGID extracts |
| [150] |
| Spanish dry-cured ham | Chemical synthesis | - | AEEEYPDL |
| [107] |
| Duck plasma | Enzymetic | Alcalase | LDGP TGVGTK EVGK RCLQ LHDVK KLGA AGGVPAG |
| [151] |
| Chinese dry-cured mutton ham | Salt extraction | Phosphate | MWTD APYMM FWIIE |
| [110] |
| Duck breast | Enzymetic | neutrase | AGPSIVH LLCVAV FLLPH |
| [152] |
| Mutton ham, Xuanwei ham, Jinhua ham | Salt extraction | Phosphate | Mutton ham peptides Xuanwei ham peptides Jinhua ham peptides |
| [114] |
| Source | Extraction Method(s) | Extraction Tool | Hydrolysate Name/ Peptide Sequence | Activity Evaluation Methods | Ref. |
|---|---|---|---|---|---|
| Lotus seed | Enzymetic | Flavourzyme | Lotus seed protein hydrolysates |
| [153] |
| Wheat glutenin | Enzymetic | Flavourenzyme Savinase Subsitilin Savinase | Flavourenzyme treated hydrolysates Savinase treated hydrolysates Subsitilin treated hydrolysates Alcalase treated hydrolysates |
| [154] |
| Soybean isolate protein | Enzymetic | Protease from strain ERMR1:04 | Soybean protein hydrolysates |
| [155] |
| Germinated amaranth | SGID | Pepsin and trypsin | GAD 90 GADW 90 F1 F2 F3 |
| [132] |
| Walnut | Chemical synthesis | - | PPKDW |
| [3] |
| Corn gluten protein | Enzymetic | Alcalase | AGLPM HALGA AGIPM HAIGA |
| [156] |
| Rhizome of white turmetic, turmeric and ginger | SGID | Pepsin and trypsin | HVVV WTLTPLTPA VTYM RGPFH AEPPR GSGLVP KMSPV |
| [144] |
| Watermelon seed | MPA + enzymetic | Alcalase | RDPEER KELEEK DAAGRLQE LDDGRL GFAGDDAPRA |
| [127] |
| Pecan meal | Enzymetic | Alcalase | LAYLQYTDFETR |
| [157] |
| Tartary buckwheat albumin | Enzymetic | Alkaline protease | GEVPW YMENF AFYRW |
| [119] |
| Peony seed dreg | Enzymetic | Alcalase | Peony seed dreg protein hydrolysates |
| [143] |
| Sorghum kafirin | Enzymetic | Papain | Fraction 1 Fraction 2 Fraction 3 |
| [158] |
| Finger millet seeds | Enzymetic | Pepsin Trypsin | TSSSLNMAVRGG-LTR STTVGLGISMRSA-SVR |
| [122] |
| Pine nut meal protein | Enzymetic | Alcalase | KWFCT QWFCT |
| [159] |
| Rapeseed | Chemical synthesis | - | WDHHAPQLR |
| [42] |
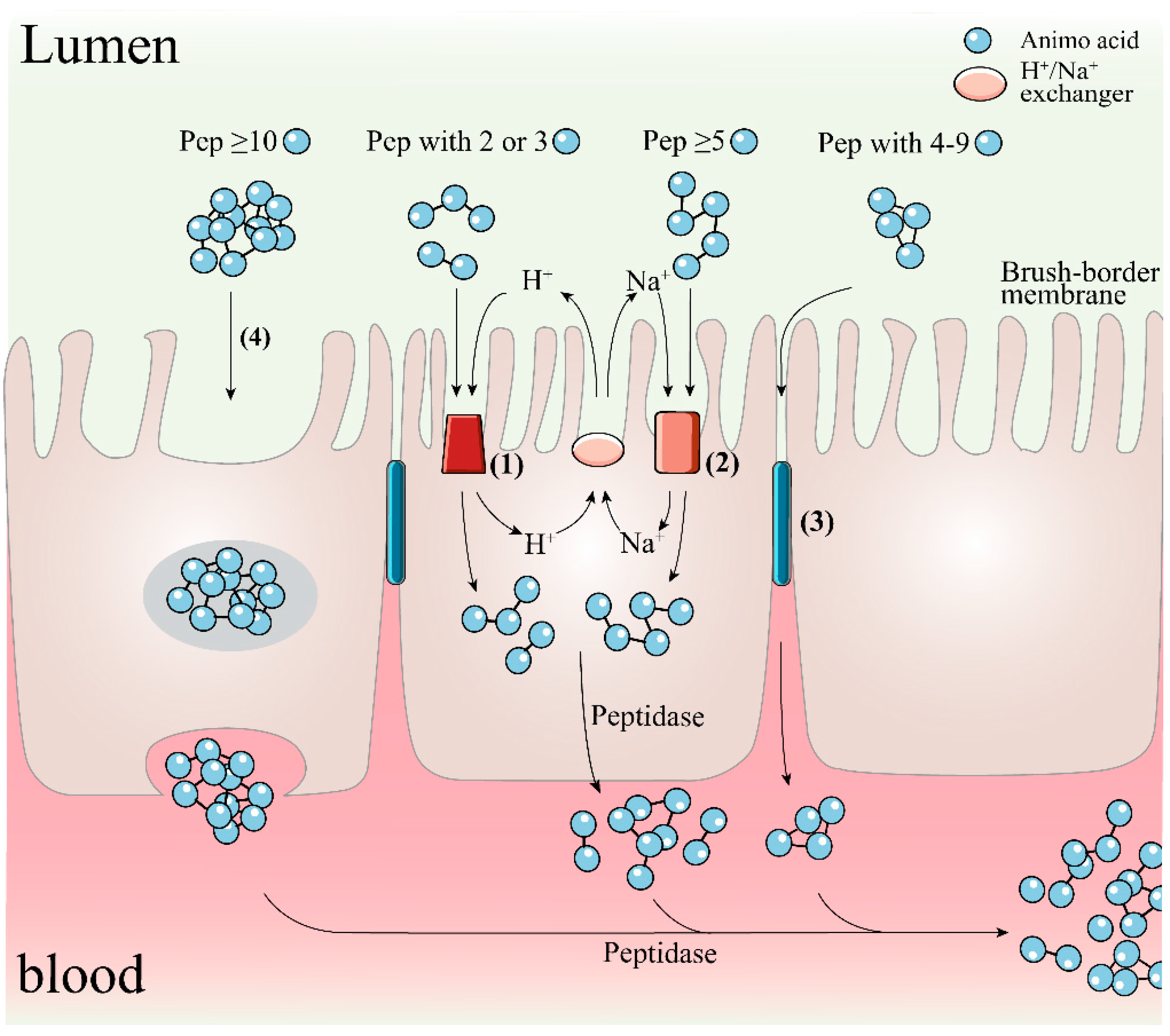
4. Digestive Stability and Bioavailability of Antioxidant Peptides
5. Application of Antioxidant Peptides in Food Systems
6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Non-Communicable Diseases. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 26 June 2022).
- Chen, M.; Ning, P.; Jiao, Y.; Xu, Z.; Cheng, Y. Extraction of antioxidant peptides from rice dreg protein hydrolysate via an angling method. Food Chem. 2021, 337, 3–8. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Luo, W.; Božović, S.; Gong, C.; Ren, J. Characterization and analysis of antioxidant activity of walnut-derived pentapeptide PW5 via nuclear magnetic resonance spectroscopy. Food Chem. 2021, 339, 128047. [Google Scholar] [CrossRef]
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases. Available online: http://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf (accessed on 26 June 2022).
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 3–8. [Google Scholar] [CrossRef]
- Barati, M.; Javanmardi, F.; Mousavi Jazayeri, S.M.H.; Jabbari, M.; Rahmani, J.; Barati, F.; Nickho, H.; Davoodi, S.H.; Roshanravan, N.; Mousavi Khaneghah, A. Techniques, perspectives, and challenges of bioactive peptide generation: A comprehensive systematic review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1488–1520. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Lafarga, T.; Sánchez-Zurano, A.; Villaró, S.; Morillas-España, A.; Acién, G. Industrial production of spirulina as a protein source for bioactive peptide generation. Trends Food Sci. Technol. 2021, 116, 176–185. [Google Scholar] [CrossRef]
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Martini, S.; Gotti, R.; Themelis, T.; Tesini, F.; Gianotti, A.; Gallina Toschi, T.; Babini, E. Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba, L.) seed protein hydrolysates and fortified apple juice. Food Chem. 2020, 330, 127120. [Google Scholar] [CrossRef]
- Al-Shamsi, K.A.; Mudgil, P.; Hassan, H.M.; Maqsood, S. Camel milk protein hydrolysates with improved technofunctional properties and enhanced antioxidant potential in in vitro and in food model systems. J Dairy Sci. 2018, 101, 47–60. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gomez, B.; Barba, F.J.; Mora, L.; Perez-Santaescolastica, C.; Toldra, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Antioxidative and antibacterial peptides derived from bovine milk proteins. Crit. Rev. Food Sci. Nutr. 2018, 58, 726–740. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Antioxidative peptides derived from plants for human nutrition: Their production, mechanisms and applications. Eur. Food Res. Technol. 2020, 246, 853–865. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Munoz-Rugeles, L.; Galano, A.; Raul Alvarez-Idaboy, J. The role of acid-base equilibria in formal hydrogen transfer reactions: Tryptophan radical repair by uric acid as a paradigmatic case. Phys. Chem. Chem. Phys. 2017, 19, 15296–15309. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal-ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Flora, S.J.S. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid. Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Gregersen, S.; Nedamani, E.R.; Olsen, T.H.; Marcatili, P.; Overgaard, M.T.; Andersen, M.L.; Hansen, E.B.; Jacobsen, C. Identification of emulsifier potato peptides by bioinformatics: Application to omega-3 delivery emulsions and release from potato industry side streams. Sci. Rep. 2020, 10, 690. [Google Scholar] [CrossRef]
- Ghelichi, S.; Sørensen, A.M.; García-Moreno, P.J.; Hajfathalian, M.; Jacobsen, C. Physical and oxidative stability of fish oil-in-water emulsions fortified with enzymatic hydrolysates from common carp (Cyprinus carpio) roe. Food Chem. 2017, 237, 1048–1057. [Google Scholar] [CrossRef][Green Version]
- Aguilar-Toala, J.E.; Liceaga, A.M. Cellular antioxidant effect of bioactive peptides and molecular mechanisms underlying: Beyond chemical properties. Int. J. Food Sci. Technol. 2021, 56, 2193–2204. [Google Scholar] [CrossRef]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: Homeostatic and pathological responses in mammalian cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Wong, F.; Xiao, J.; Wang, S.; Ee, K.; Chai, T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Xing, L.; Fu, L.; Hao, Y.; Zhang, W. Dry-cured ham-derived peptide (Asp-Leu-Glu-Glu) exerts cytoprotective capacity in human intestinal epithelial Caco-2 cells. Antioxidants 2021, 10, 1354. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Q.; Han, L.; Ma, X.; Song, R.; Zhao, S.; Zhang, W. Study on the effect of reactive oxygen species-mediated oxidative stress on the activation of mitochondrial apoptosis and the tenderness of yak meat. Food Chem. 2018, 244, 394–402. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Xiao, G.; Vargas-De-La-Cruz, C.; Simal-Gandara, J.; Xiao, J.; Wang, Q. Active sites of peptides Asp-Asp-Asp-Tyr and Asp-Tyr-Asp-Asp protect against cellular oxidative stress. Food Chem. 2022, 366, 130626. [Google Scholar] [CrossRef]
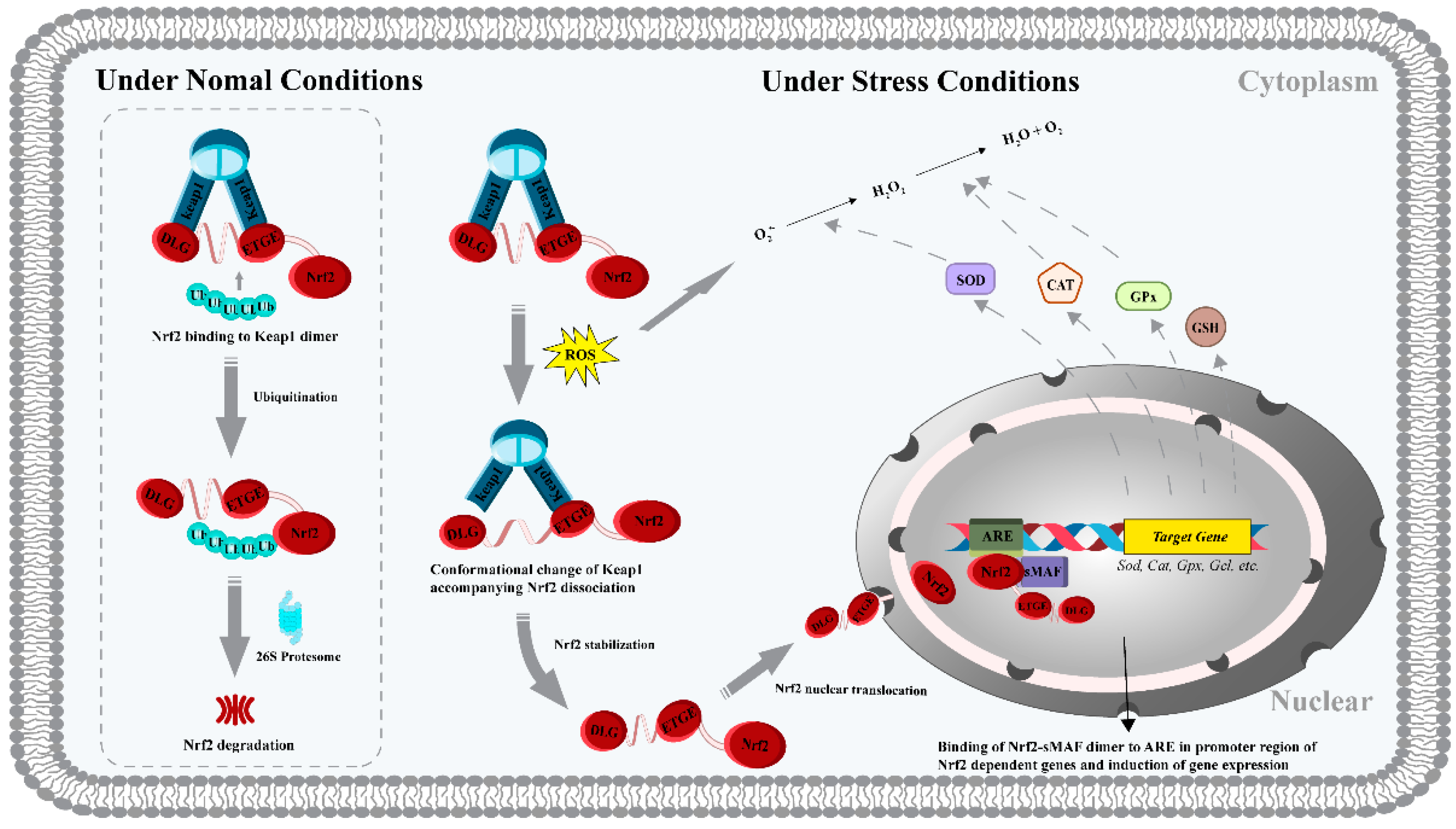
- Lu, M.C.; Ji, J.A.; Jiang, Z.Y.; You, Q.D. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: An update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-kappa B interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 3–8. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The anti-Inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef]
- Han, J.; Huang, Z.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Jim Wang, Z.; Su, X. The novel peptides ICRD and LCGEC screened from tuna roe show antioxidative activity via Keap1/Nrf2-ARE pathway regulation and gut microbiota modulation. Food Chem. 2020, 327, 127094. [Google Scholar] [CrossRef]
- Wu, J.; Sun, B.; Luo, X.; Zhao, M.; Zheng, F.; Sun, J.; Li, H.; Sun, X.; Huang, M. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keapl-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 3–8. [Google Scholar] [CrossRef]
- Li, M.; Ge, Q.; Du, H.; Lin, S. Tricholoma matsutake-derived peptides ameliorate inflammation and mitochondrial dysfunction in RAW264.7 macrophages by modulating the NF-kappa B/COX-2 pathway. Foods 2021, 10, 2680. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Wang, Z.; Yao, Y.; Atungulu, G.G.; Ju, X.; Wang, L. Absorption and metabolism of peptide WDHHAPQLR derived from rapeseed protein and inhibition of HUVEC apoptosis under oxidative stress. J. Agric. Food Chem. 2018, 66, 5178–5189. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Zhou, A.; Miao, J.; Liu, J.; Benjakul, S. A novel antioxidant peptide purified from defatted round scad (Decapterus maruadsi) protein hydrolysate extends lifespan in Caenorhabditis elegans. J. Funct. Foods 2020, 68, 103907. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Liu, C.; Zhou, H.; Wang, X.; Mai, K.; He, G. Effects of dietary raw or Enterococcus faecium fermented soybean meal on growth, antioxidant status, intestinal microbiota, morphology, and inflammatory responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 100, 261–271. [Google Scholar] [CrossRef]
- Shi, H.; Hu, X.; Zheng, H.; Li, C.; Sun, L.; Guo, Z.; Huang, W.; Yu, R.; Song, L.; Zhu, J. Two novel antioxidant peptides derived from Arca subcrenata against oxidative stress and extend lifespan in Caenorhabditis elegans. J. Funct. Foods 2021, 81, 104462. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wang, X.; Wang, Y.; Liao, L.; Zhang, Y.; Fang, B.; Fu, Y. Novel antioxidant peptides from Yak bones collagen enhanced the capacities of antiaging and antioxidant in Caenorhabditis elegans. J. Funct. Foods 2022, 89, 104933. [Google Scholar] [CrossRef]
- Ding, Y.; Ko, S.; Moon, S.; Lee, S. Protective effects of novel antioxidant peptide purified from Alcalase hydrolysate of velvet antler against oxidative stress in chang liver cells in vitro and in a zebrafish model in vivo. Int. J. Mol. Sci. 2019, 20, 5187. [Google Scholar] [CrossRef]
- Mada, S.B.; Reddi, S.; Kumar, N.; Kumar, R.; Kapila, S.; Kapila, R.; Trivedi, R.; Karvande, A.; Ahmad, N. Antioxidative peptide from milk exhibits antiosteopenic effects through inhibition of oxidative damage and bone-resorbing cytokines in ovariectomized rats. Nutrition 2017, 43–44, 21–31. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, H.; Hu, C.; Zeng, J.; Shi, X.; Chen, S.; Zhang, K.; Zheng, W.; Wu, W.; Liu, S. Tilapia skin peptides restore cyclophosphamide-induced premature ovarian failure via inhibiting oxidative stress and apoptosis in mice. Food Funct. 2022, 13, 1668–1679. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Market. Fish Protein Hydrolysate Market Size, By Technology (Acid Hydrolysis, Enzymatic Hydrolysis), By Form (Powder, Paste, Liquid), By Source (Anchovy, Tilapia, Tuna, Sardine, Atlantic Salmon, Crustacean, Molluscs, Codfish), By Application (Animal Feed [Poultry {Broilers, Layers}, Swine, Calves, Aquaculture {Salmon, Trouts, Shrimps}, Equine], Pet Food [Cat, Dog], Food (Flavor Enhancers, Functional Food, Infant Formulation, Nutraceuticals, Sports Nutrition, Protein Supplements, Elderly Food Formulation, Clinicals), Cosmetics [By Protein Type {Collagen (Glycine, Hydroxylysine, Proline), Elastin, Keratin}], Agriculture [Fertilizers, Protection, Elicitor], Pharmaceutical), Industry Analysis Report, Regional Outlook, COVID-19 Impact Analysis, Application Development Potential, Price Trends, Competitive Market Share & Forecast, 2020–2026. Available online: https://www.gminsights.com/fish-protein-hydrolysate-market (accessed on 5 December 2020).
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: A review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. Nutr. 2022, 62, 1242–1269. [Google Scholar] [CrossRef]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, bioactive properties, and potential applications of fish protein hydrolysates: Developments and challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, Q.; Li, L.; Khan, M.N.; Yang, X.; Zhang, Z.; Hu, X.; Chen, S. Inhibitory effect of antioxidant peptides derived from Pinctada fucata protein on ultraviolet-induced photoaging in mice. J. Funct. Foods 2013, 5, 527–538. [Google Scholar] [CrossRef]
- Barzideh, Z.; Latiff, A.A.; Gan, C.; Abedin, M.Z.; Alias, A.K. ACE inhibitory and antioxidant activities of collagen hydrolysates from the Ribbon Jellyfish (Chrysaora sp.). Food Technol. Biotechnol. 2014, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Sayari, N.; Balti, R.; Martinez-Alvarez, O.; Nedjar-Arroume, N.; Moncef, N.; Bougatef, A. Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chem. 2014, 148, 445–452. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; He, Y.; Ren, D.; Kow, F.; Song, L.; Yu, X. Novel antioxidative peptides from the protein hydrolysate of oysters (Crassostrea talienwhanensis). Food Chem. 2014, 145, 991–996. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef]
- Ozogul, F.; Cagalj, M.; Simat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A.A.; Kuley, E.; Rathod, N.B.; Phadke, G.G. Recent developments in valorisation of bioactive ingredients in discard/seafood processing by-products. Trends Food Sci. Technol. 2021, 116, 559–582. [Google Scholar] [CrossRef]
- Yu, D.; Chi, C.; Wang, B.; Ding, G.; Li, Z. Characterization of acid- and pepsin-soluble collagens from spines and skulls of skipjack tuna (Katsuwonus pelamis). Chin. J. Nat. Med. 2014, 12, 712–720. [Google Scholar] [CrossRef]
- Yang, X.R.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Preparation and characterization of gelatin and antioxidant peptides from gelatin hydrolysate of Skipjack Tuna (Katsuwonus pelamis) bone stimulated by in vitro gastrointestinal digestion. Mar. Drugs 2019, 17, 78. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, S.; Li, Y.; Wang, H.; Shi, Y.; Zhang, L.; Tu, Z. Protective effect of antioxidant peptides from grass carp scale gelatin on the H2O2-mediated oxidative injured HepG2 cells. Food Chem. 2022, 373, 131539. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, C.; Zhao, J.; Shi, X.; Sun, M.; Liu, J.; Fu, Y.; Jin, W.; Zhu, B. Separation and characterization of antioxidative and angiotensin converting enzyme inhibitory peptide from Jellyfish Gonad hydrolysate. Molecules 2018, 23, 94. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, Y.; Dong, H.; Su, G.; Zhao, M. Structure-activity relationship of antioxidant dipeptides: Dominant role of Tyr, Trp, Cys and Met residues. J. Funct. Foods 2016, 21, 485–496. [Google Scholar] [CrossRef]
- Wu, R.; Chen, L.; Liu, D.; Huang, J.; Zhang, J.; Xiao, X.; Lei, M.; Chen, Y.; He, H. Preparation of antioxidant peptides from salmon byproducts with bacterial extracellular proteases. Mar. Drugs 2017, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Su, G.; Yang, Q.; Wei, Z.; Wang, J.; Zheng, L.; Zhao, M. Round scad-derived octapeptide WCPFSRSF confers neuroprotection by regulating Akt/Nrf2/NFκB signaling. J. Agric. Food Chem. 2021, 69, 10606–10616. [Google Scholar] [CrossRef] [PubMed]
- Aloglu, H.S. The effect of various heat treatments on the antioxidant capacity of milk before and after simulated gastrointestinal digestion. Int. J. Dairy Technol. 2013, 66, 170–174. [Google Scholar] [CrossRef]
- Mudgil, P.; Baba, W.N.; Kamal, H.; FitzGerald, R.J.; Hassan, H.M.; Ayoub, M.A.; Gan, C.; Maqsood, S. A comparative investigation into novel cholesterol esterase and pancreatic lipase inhibitory peptides from cow and camel casein hydrolysates generated upon enzymatic hydrolysis and in-vitro digestion. Food Chem. 2022, 367, 130661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, B.; Luo, B.; Ni, Y.; Bansal, N.; Zhou, P. Characterization of endogenous peptides from Dromedary and Bactrian camel milk. Eur. Food Res. Technol. 2022, 248, 1149–1160. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Isono, H.; Miyata, T. Potential antioxidant bioactive peptides from camel milk proteins. Anim. Nutr. 2018, 4, 273–280. [Google Scholar] [CrossRef]
- Dharmisthaben, P.; Basaiawmoit, B.; Sakure, A.; Das, S.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Hati, S. Exploring potentials of antioxidative, anti-inflammatory activities and production of bioactive peptides in lactic fermented camel milk. Food Biosci. 2021, 44, 3–8. [Google Scholar] [CrossRef]
- Homayouni-Tabrizi, M.; Shabestarin, H.; Asoodeh, A.; Soltani, M. Identification of two novel antioxidant peptides from camel milk using digestive proteases: Impact on expression gene of superoxide dismutase (SOD) in hepatocellular carcinoma cell line. Int. J. Pept. Res. Ther. 2016, 22, 187–195. [Google Scholar] [CrossRef]
- Hinz, K.; O’Connor, P.M.; Huppertz, T.; Ross, R.P.; Kelly, A.L. Comparison of the principal proteins in bovine, caprine, buffalo, equine and camel milk. J. Dairy Res. 2012, 79, 185–191. [Google Scholar] [CrossRef]
- Hailu, Y.; Hansen, E.B.; Seifu, E.; Eshetu, M.; Ipsen, R.; Kappeler, S. Functional and technological properties of camel milk proteins: A review. J. Dairy Res. 2016, 83, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, R.; Kumari, R.; Singh, S.P.; Sahoo, D.; Rai, A.K. Characterization of native lactic acid bacteria from traditionally fermented chhurpi of Sikkim Himalayan region for the production of chhurpi cheese with enhanced antioxidant effect. Lebensm. Wiss. Technol. 2022, 154, 112801. [Google Scholar] [CrossRef]
- Silva, D.D.D.; Lima, M.D.S.F.; Silva, M.F.D.; Silva, G.R.D.; Campos, J.F.; Albuquerque, W.W.C.; Cavalcanti, M.T.H.; Porto, A.L.F. Bioactive water-soluble peptides from fresh buffalo cheese may be used as product markers. Lebensm. Wiss. Technol. 2019, 108, 97–105. [Google Scholar] [CrossRef]
- Elkhtab, E.; El-Alfy, M.; Shenana, M.; Mohamed, A.; Yousef, A.E. New potentially antihypertensive peptides liberated in milk during fermentation with selected lactic acid bacteria and kombucha cultures. J. Dairy Sci. 2017, 100, 9508–9520. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Hansen, E.B.; Ballegaard, A.R.; Sancho, A.I.; Bøgh, K.L. Comparison of the allergenicity and immunogenicity of camel and cow’s milk-A study in Brown Norway rats. Nutrients 2018, 10, 1903. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Zuo, F.; Ma, H.; Zhang, Y.; Chen, S. Comparison of dipeptidyl peptidase IV-inhibitory activity of peptides from bovine and caprine milk casein by in silico and in vitro analyses. Int. Dairy J. 2016, 53, 37–44. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.W.; Han, X.; Xin, L.; Meng, Z.X.; Gong, P.M.; Cheng, D.Y. Yak milk casein as potential precursor of angiotensin I-converting enzyme inhibitory peptides based on in silico proteolysis. Food Chem. 2018, 254, 340–347. [Google Scholar] [CrossRef]
- Wang, K.; Han, L.; Hong, H.; Pan, J.; Liu, H.; Luo, Y. Purification and identification of novel antioxidant peptides from silver carp muscle hydrolysate after simulated gastrointestinal digestion and transepithelial transport. Food Chem. 2021, 342, 128275. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, G.; Tian, Y.; Kong, F.; Xiong, S.; Zhao, S.; Jia, D.; Manyande, A.; Du, H. Identification of novel antioxidant peptides from snakehead (Channa argus) soup generated during gastrointestinal digestion and insights into the anti-oxidation mechanisms. Food Chem. 2021, 337, 127921. [Google Scholar] [CrossRef]
- Wu, R.; Huang, J.; Huan, R.; Chen, L.; Yi, C.; Liu, D.; Wang, M.; Liu, C.; He, H. New insights into the structure-activity relationships of antioxidative peptide PMRGGGGYHY. Food Chem. 2021, 337, 127678. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Sohn, J.H.; Kim, J.; Choi, J. Identification and characterization of novel antioxidant peptides from mackerel (Scomber japonicus) muscle protein hydrolysates. Food Chem. 2020, 323, 126809. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Kim, Y.; Eun, J. Antioxidant mechanism, antibacterial activity, and functional characterization of peptide fractions obtained from barred mackerel gelatin with a focus on application in carbonated beverages. Food Chem. 2021, 342, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Wickramathilaka, M.; Liceaga, A.M. Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food Chem. 2018, 254, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Upata, M.; Siriwoharn, T.; Makkhun, S.; Yarnpakdee, S.; Regenstein, J.M.; Wangtueai, S. Tyrosinase inhibitory and antioxidant activity of enzymatic protein hydrolysate from Jellyfish (Lobonema smithii). Foods 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, M.; Ding, J.; Zheng, J.; Zhu, B.; Lin, S. Structure-activity relationship and pathway of antioxidant shrimp peptides in a PC12 cell model. J. Funct. Foods 2020, 70, 103978. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, T.; Liang, X.; Yuan, Q.; Zeng, X.; Wu, Z.; Pan, D.; Tao, M.; Guo, Y. Production and transepithelial transportation of casein-derived peptides and identification a novel antioxidant peptide LHSMK. Lebensm. Wiss. Technol. 2021, 151, 112194. [Google Scholar] [CrossRef]
- Nascimento, T.C.E.S.; Molino, J.V.D.; Donado, P.R.S.; Montalvo, G.S.A.; Dos Santos, W.L.; Gomes, J.E.G.; Santos, J.H.P.M.; Da Silva, R.; Sette, L.D.; Pessoa Junior, A.; et al. Antarctic fungus proteases generate bioactive peptides from caseinate. Food Res. Int. 2021, 139, 109944. [Google Scholar] [CrossRef]
- Wei, G.; Zhao, Q.; Wang, D.; Fan, Y.; Shi, Y.; Huang, A. Novel ACE inhibitory, antioxidant and α-glucosidase inhibitory peptides identified from fermented rubing cheese through peptidomic and molecular docking. Lebensm. Wiss. Technol. 2022, 159, 113196. [Google Scholar] [CrossRef]
- OECD-FAO. OECD-FAO Agricultural Outlook 2020–2029. Available online: https://www.fao.org/publications/oecd-fao-agricultural-outlook/2020-2029/en (accessed on 1 September 2022).
- Shirsath, A.P.; Henchion, M.M. Bovine and ovine meat co-products valorisation opportunities: A systematic literature review. Trends Food Sci. Technol. 2021, 118, 57–70. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Regenstein, J.; Su, X. In vitro and in vivo anti-oxidation and anti-fatigue effect of monkfish liver hydrolysate. Food Biosci. 2017, 18, 9–14. [Google Scholar] [CrossRef]
- Zou, Y.; Shahidi, F.; Shi, H.; Wang, J.; Huang, Y.; Xu, W.; Wang, D. Values-added utilization of protein and hydrolysates from animal processing by-product livers: A review. Trends Food Sci. Technol. 2021, 110, 432–442. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, P.; Pateiro, M.; Barba, F.J.; Mora, L.; Franco, D.; Toldra, F.; Lorenzo, J.M. Antioxidant and antimicrobial activity of peptides extracted from meat by-products: A review. Food Anal. Methods. 2019, 12, 2401–2415. [Google Scholar] [CrossRef]
- Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 2011–2027. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Wang, Z.; Zhang, X.; Liu, S.; Song, X.; Zhang, Y.; Ding, J.; Chen, X.; Xu, F. Potential of thermolysin-like protease A69 in preparation of bovine collagen peptides with moisture-retention ability and antioxidative activity. Mar. Drugs 2021, 19, 676. [Google Scholar] [CrossRef]
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019, 301, 3–8. [Google Scholar] [CrossRef]
- Offengenden, M.; Chakrabarti, S.; Wu, J. Chicken collagen hydrolysates differentially mediate anti-inflammatory activity and type I collagen synthesis on human dermal fibroblasts. Food Sci. Human Wellness 2018, 7, 138–147. [Google Scholar] [CrossRef]
- Iwaniak, A.; Darewicz, M.; Minkiewicz, P. Peptides derived from foods as supportive diet components in the prevention of metabolic syndrome. Compr. Rev. Food Sci. Food Saf. 2018, 17, 63–81. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Reig, M.; Toldrá, F. Stability of the potent antioxidant peptide SNAAC identified from Spanish dry-cured ham. Food Res. Int. 2018, 105, 873–879. [Google Scholar] [CrossRef]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.; Toldra, F. Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chem. 2013, 138, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Toldra, F. Characterisation of the antioxidant peptide AEEEYPDL and its quantification in Spanish dry-cured ham. Food Chem. 2018, 258, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, W.; Zhou, G.; Xu, X. Identification of antioxidant peptides of Jinhua ham generated in the products and through the simulated gastrointestinal digestion system. J. Sci. Food Agric. 2016, 96, 99–108. [Google Scholar] [CrossRef]
- Xing, L.J.; Hu, Y.Y.; Hu, H.Y.; Ge, Q.F.; Zhou, G.H.; Zhang, W.G. Purification and identification of antioxidative peptides from dry-cured Xuanwei ham. Food Chem. 2016, 194, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, S.; Li, R.; Wang, Y.; Huang, L. Identification and characterization of antioxidant peptides from Chinese dry-cured mutton ham. J. Sci. Food Agric. 2020, 100, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Z.; Zhou, H.; Zhou, K.; Wang, Z.; Ma, X.; Xu, B. The mechanism of peptides formation in dry-cured ham: A review. Food Ferment. Industries 2022, 48, 294–300. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Reig, M.; Toldra, F. Challenges in the quantitation of naturally generated bioactive peptides in processed meats. Trends Food Sci. Technol. 2017, 69, 306–314. [Google Scholar] [CrossRef]
- Toldra, F.; Gallego, M.; Reig, M.; Aristoy, M.; Mora, L. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020, 321, 3–8. [Google Scholar] [CrossRef]
- Wang, J.; Guo, M.; Wang, Q.; Dong, J.; Lu, S.; Lyu, B.; Ma, X. Antioxidant activities of peptides derived from mutton ham, Xuanwei ham and Jinhua ham. Food Res. Int. 2021, 142, 3–8. [Google Scholar] [CrossRef]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments-A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Du, Y.; Esfandi, R.; Willmore, W.G.; Tsopmo, A. Antioxidant activity of oat proteins derived peptides in stressed hepatic HepG2 cells. Antioxidants 2016, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Leung, R.; Venus, C.; Zeng, T.; Tsopmo, A. Structure-function relationships of hydroxyl radical scavenging and chromium-VI reducing cysteine-tripeptides derived from rye secalin. Food Chem. 2018, 254, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, D.; Gao, Y.; Cao, X.; Shen, X. A novel antioxidant peptide derived from wheat germ prevents high glucose-induced oxidative stress in vascular smooth muscle cells in vitro. Food Funct. 2017, 8, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Fei, Y.; Xu, Q.; Lei, T.; Mo, X.; Wang, Z.; Zhang, L.; Mou, X.; Li, H. Isolation and identification of antioxidant peptides from tartary buckwheat albumin (Fagopyrum tataricum Gaertn.) and their antioxidant activities. J Food Sci. 2020, 85, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Liu, X.; Zheng, X.; Wang, X.; He, J. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016, 204, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2019, 120, 697–707. [Google Scholar] [CrossRef]
- Mason, E.; L’Hocine, L.; Achouri, A.; Pitre, M.; Karboune, S. Health promoting bioactive properties of novel hairless canary seed flour after in vitro gastrointestinal digestion. Foods 2020, 9, 932. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras, M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- Xie, J.; Du, M.; Shen, M.; Wu, T.; Lin, L. Physico-chemical properties, antioxidant activities and angiotensin-I converting enzyme inhibitory of protein hydrolysates from Mung bean (Vigna radiate). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.; Li, B.; Zhao, M.; Hou, T. Selenium-containing soybean antioxidant peptides: Preparation and comprehensive comparison of different selenium supplements. Food Chem. 2021, 358, 129888. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Wang, Q.; Liu, Q.; Guo, Y.; Gong, F.; Hao, L.; Wu, H.; Dong, Z. The antioxidant activity of protein fractions from Sacha inchi seeds after a simulated gastrointestinal digestion. Lebensm. Wiss. Technol. 2021, 145, 111356. [Google Scholar] [CrossRef]
- Yang, J.; Hu, L.; Cai, T.; Chen, Q.; Ma, Q.; Yang, J.; Meng, C.; Hong, J. Purification and identification of two novel antioxidant peptides from perilla (Perilla frutescens L. Britton) seed protein hydrolysates. PLoS ONE 2018, 13, e0200021. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tang, X.; Ren, Y.; Wang, E.; Shi, L.; Wu, X.; Wu, H. Novel antioxidant peptides purified from mulberry (Morus atropurpurea Roxb.) leaf protein hydrolysates with hemolysis inhibition ability and cellular antioxidant activity. J. Agric. Food Chem. 2019, 67, 7650–7659. [Google Scholar] [CrossRef]
- Li, M.; Dong, L.; Du, H.; Bao, Z.; Lin, S. Potential mechanisms underlying the protective effects of Tricholoma matsutake singer peptides against LPS-induced inflammation in RAW264.7 macrophages. Food Chem. 2021, 353, 129452. [Google Scholar] [CrossRef]
- Sandoval-Sicairos, E.S.; Milán-Noris, A.K.; Luna-Vital, D.A.; Milán-Carrillo, J.; Montoya-Rodríguez, A. Anti-inflammatory and antioxidant effects of peptides released from germinated amaranth during in vitro simulated gastrointestinal digestion. Food Chem. 2021, 343, 128394. [Google Scholar] [CrossRef]
- Silva Do Nascimento, E.; Anaya, K.; de Oliveira, J.M.C.; de Lacerda, J.T.J.G.; Miller, M.E.; Dias, M.; Mendes, M.A.; de Azevedo Lima Pallone, J.; Weis Arns, C.; Juliano, M.A.; et al. Identification of bioactive peptides released from in vitro gastrointestinal digestion of yam proteins (Dioscorea cayennensis). Food Res. Int. 2021, 143, 110286. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, M.; Wu, S.; Liao, X.; Wang, J.; Wu, Q.; Zhuang, M.; Ding, Y. A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Res. Int. 2020, 134, 109230. [Google Scholar] [CrossRef]
- Kimatu, B.M.; Zhao, L.; Biao, Y.; Ma, G.; Yang, W.; Pei, F.; Hu, Q. Antioxidant potential of edible mushroom (Agaricus bisporus) protein hydrolysates and their ultrafiltration fractions. Food Chem. 2017, 230, 58–67. [Google Scholar] [CrossRef]
- Farzaneh, P.; Khanahamadi, M.; Ehsani, M.R.; Sharifan, A. Bioactive properties of Agaricus bisporus and Terfezia claveryi proteins hydrolyzed by gastrointestinal proteases. Lebensm. Wiss. Technol. 2018, 91, 322–329. [Google Scholar] [CrossRef]
- Kaprasob, R.; Khongdetch, J.; Laohakunjit, N.; Selamassakul, O.; Kaisangsri, N. Isolation and characterization, antioxidant, and antihypertensive activity of novel bioactive peptides derived from hydrolysis of King Boletus mushroom. Lebensm. Wiss. Technol. 2022, 160, 113287. [Google Scholar] [CrossRef]
- Hu, R.; Chen, G.; Li, Y. Production and characterization of antioxidative hydrolysates and peptides from corn gluten meal using papain, ficin, and bromelain. Molecules 2020, 25, 4091. [Google Scholar] [CrossRef]
- Ding, J.; Liang, R.; Yang, Y.; Sun, N.; Lin, S. Optimization of pea protein hydrolysate preparation and purification of antioxidant peptides based on an in silico analytical approach. Lebensm. Wiss. Technol. 2020, 123, 109126. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef] [PubMed]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aadil, R.M. An update on functional, nutraceutical and industrial applications of watermelon by-products: A comprehensive review. Trends Food Sci. Technol. 2021, 114, 275–291. [Google Scholar] [CrossRef]
- Moayedi, A.; Mora, L.; Aristoy, M.C.; Safari, M.; Hashemi, M.; Toldrá, F. Peptidomic analysis of antioxidant and ACE-inhibitory peptides obtained from tomato waste proteins fermented using Bacillus subtilis. Food Chem. 2018, 250, 180–187. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, J.; Thakur, K.; Zhang, J.; Mocan, A.; Wei, Z. Purification and identification of an antioxidative peptide from peony (Paeonia suffruticosa Andr.) seed dreg. Food Chem. 2019, 285, 266–274. [Google Scholar] [CrossRef]
- Sompinit, K.; Lersiripong, S.; Reamtong, O.; Pattarayingsakul, W.; Patikarnmonthon, N.; Panbangred, W. In vitro study on novel bioactive peptides with antioxidant and antihypertensive properties from edible rhizomes. Lebensm. Wiss. Technol. 2020, 134, 110227. [Google Scholar] [CrossRef]
- García, M.C.; Endermann, J.; González-García, E.; Marina, M.L. HPLC-Q-TOF-MS identification of antioxidant and antihypertensive peptides recovered from cherry (Prunus cerasus L.) subproducts. J Agric Food Chem. 2015, 63, 1514–1520. [Google Scholar] [CrossRef]
- Yesiltas, B.; García-Moreno, P.J.; Gregersen, S.; Olsen, T.H.; Jones, N.C.; Hoffmann, S.V.; Marcatili, P.; Overgaard, M.T.; Hansen, E.B.; Jacobsen, C. Antioxidant peptides derived from potato, seaweed, microbial and spinach proteins: Oxidative stability of 5% fish oil-in-water emulsions. Food Chem. 2022, 385, 132699. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dias, F.F.G.; Leite Nobrega De Moura Bell, J.M.; Barile, D. A complete workflow for discovering small bioactive peptides in foods by LC-MS/MS: A case study on almonds. Food Chem. 2022, 369, 130834. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhan, J.; Hu, L.; Yuan, C.; Takaki, K.; Ying, X.; Hu, Y. Identification of a new antioxidant peptide from porcine plasma by in vitro digestion and its cytoprotective effect on H2O2 induced HepG2 model. J. Funct. Foods 2021, 86, 104679. [Google Scholar] [CrossRef]
- Yu, D.; Feng, M.; Sun, J.; Xu, X.; Zhou, G. Protein degradation and peptide formation with antioxidant activity in pork protein extracts inoculated with Lactobacillus plantarum and Staphylococcus simulans. Meat. Sci. 2020, 160, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Toldrá, F.; Zhou, F.; Gallego, M.; Zhao, M.; Mora, L. Effect of cooking and in vitro digestion on the peptide profile of chicken breast muscle and antioxidant and alcohol dehydrogenase stabilization activity. Food Res. Int. 2020, 136, 109459. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Dong, X.; Zhang, Y.; Zhou, X.; Huang, M.; Zhou, G. Purification and identification of antioxidant peptides from duck plasma proteins. Food Chem. 2020, 319, 3–8. [Google Scholar] [CrossRef]
- Li, T.; Shi, C.; Zhou, C.; Sun, X.; Ang, Y.; Dong, X.; Huang, M.; Zhou, G. Purification and characterization of novel antioxidant peptides from duck breast protein hydrolysates. Lebensm. Wiss. Technol. 2020, 125, 109215. [Google Scholar] [CrossRef]
- Yu, Y.; Lai, S.; Chang, C.; Chen, W.; Wu, S.; Lu, C. Peptidomic analysis of low molecular weight antioxidative peptides prepared by lotus (Nelumbo nucifera Gaertn.) seed protein hydrolysates. Lebensm. Wiss. Technol. 2021, 144, 111138. [Google Scholar] [CrossRef]
- Bozkurt, F.; Bekiroglu, H.; Dogan, K.; Karasu, S.; Sagdic, O. Technological and bioactive properties of wheat glutenin hydrolysates prepared with various commercial proteases. Lebensm. Wiss. Technol. 2021, 149, 111787. [Google Scholar] [CrossRef]
- Mukhia, S.; Kumar, A.; Kumar, R. Generation of antioxidant peptides from soy protein isolate through psychrotrophic Chryseobacterium sp. derived alkaline broad temperature active protease. Lebensm. Wiss. Technol. 2021, 143, 111152. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Lin, S.; Cheng, S. Contribution of specific amino acid and secondary structure to the antioxidant property of corn gluten proteins. Food Res. Int. 2018, 105, 836–844. [Google Scholar] [CrossRef]
- Hu, F.; Ci, A.; Wang, H.; Zhang, Y.; Zhang, J.; Thakur, K.; Wei, Z. Identification and hydrolysis kinetic of a novel antioxidant peptide from pecan meal using Alcalase. Food Chem. 2018, 261, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shen, Y.; Xu, J.; Qi, G.; Chen, G.; Wang, W.; Sun, X.; Li, Y. Antioxidant and anticancer effects in human hepatocarcinoma (HepG2) cells of papain-hydrolyzed sorghum kafirin hydrolysates. J. Funct. Foods 2019, 58, 374–382. [Google Scholar] [CrossRef]
- Yang, R.; Li, X.; Lin, S.; Zhang, Z.; Chen, F. Identification of novel peptides from 3 to 10 kDa pine nut (Pinus koraiensis) meal protein, with an exploration of the relationship between their antioxidant activities and secondary structure. Food Chem. 2017, 219, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Sanchón, J.; Recio, I.; Hernández-Ledesma, B. Transepithelial transport of lunasin and derived peptides: Inhibitory effects on the gastrointestinal cancer cells viability. J. Food Compost. Anal. 2018, 68, 101–110. [Google Scholar] [CrossRef]
- Zhu, B.; He, H.; Hou, T. A comprehensive review of corn protein-derived bioactive peptides: Production, characterization, bioactivities, and transport pathways. Compr Rev Food Sci Food Saf. 2019, 18, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Abeer, M.M.; Trajkovic, S.; Brayden, D.J. Measuring the oral bioavailability of protein hydrolysates derived from food sources: A critical review of current bioassays. Biomed. Pharmacother. 2021, 144, 112275. [Google Scholar] [CrossRef]
- Antosova, Z.; Mackova, M.; Kral, V.; Macek, T. Therapeutic application of peptides and proteins: Parenteral forever? Trends Biotechnol. 2009, 27, 628–635. [Google Scholar] [CrossRef]
- Vij, R.; Reddi, S.; Kapila, S.; Kapila, R. Transepithelial transport of milk derived bioactive peptide VLPVPQK. Food Chem. 2016, 190, 681–688. [Google Scholar] [CrossRef]
- Singh, U.; Kaur, D.; Mishra, V.; Krishania, M. Combinatorial approach to prepare antioxidative protein hydrolysate from corn gluten meal with dairy whey: Preparation, kinetics, nutritional study and cost analysis. Lebensm. Wiss. Technol. 2022, 153, 112437. [Google Scholar] [CrossRef]
- Wang, B.; Li, B. Effect of molecular weight on the transepithelial transport and peptidase degradation of casein-derived peptides by using Caco-2 cell model. Food Chem. 2017, 218, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Yin, R.; Howell, K.; Zhang, P. Activity and bioavailability of food protein-derived angiotensin-I-converting enzyme-inhibitory peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1150–1187. [Google Scholar] [CrossRef] [PubMed]
- Vig, B.S.; Stouch, T.R.; Timoszyk, J.K.; Quan, Y.; Wall, D.A.; Smith, R.L.; Faria, T.N. Human PepT1 pharmacophore distinguishes between dipeptide transport and binding. J. Med. Chem. 2006, 49, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, L.; Zhang, T.; Yu, Z.; Liu, J. Hydrolysis and transepithelial transport of two corn gluten derived bioactive peptides in human Caco-2 cell monolayers. Food Res. Int. 2018, 106, 475–480. [Google Scholar] [CrossRef]
- Xu, F.; Wang, L.; Ju, X.; Zhang, J.; Yin, S.; Shi, J.; He, R.; Yuan, Q. Transepithelial transport of YWDHNNPQIR and its metabolic fate with cytoprotection against oxidative stress in human intestinal Caco-2 cells. J. Agric. Food Chem. 2017, 65, 2056–2065. [Google Scholar] [CrossRef]
- Feng, M.; Betti, M. Transepithelial transport efficiency of bovine collagen hydrolysates in a human Caco-2 cell line model. Food Chem. 2017, 224, 242–250. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Shen, X.; Li, T.; Li, X.; Wang, F.; Liu, Y.; Wu, J. Dual cryoprotective and antioxidant effects of silver carp (Hypophthalmichthys molitrix) protein hydrolysates on unwashed surimi stored at conventional and ultra-low frozen temperatures. Lebensm. Wiss. Technol. 2022, 153, 112563. [Google Scholar] [CrossRef]
- Lin, J.; Hong, H.; Zhang, L.; Zhang, C.; Luo, Y. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Fogliano, V. Food matrix interaction and bioavailability of bioactive peptides: Two faces of the same coin? J Funct. Foods 2017, 35, 9–12. [Google Scholar] [CrossRef]
- Lacou, L.; Leonil, J.; Gagnaire, V. Functional properties of peptides: From single peptide solutions to a mixture of peptides in food products. Food Hydrocoll. 2016, 57, 187–199. [Google Scholar] [CrossRef]
- Przybylski, R.; Firdaous, L.; Châtaigné, G.; Dhulster, P.; Nedjar, N. Production of an antimicrobial peptide derived from slaughterhouse by-product and its potential application on meat as preservative. Food Chem. 2016, 211, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Han, X.; Synowiecki, J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem. 1995, 53, 285–293. [Google Scholar] [CrossRef]
- Nasri, R.; Younes, I.; Jridi, M.; Trigui, M.; Bougatef, A.; Nedjar-Arroume, N.; Dhulster, P.; Nasri, M.; Karra-Châabouni, M. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: Effect on meat lipid oxidation. Food Res. Int. 2013, 54, 552–561. [Google Scholar] [CrossRef]

| Source | Extraction Tool | Hydrolysate/ Peptide | Product | Effects | Ref. |
|---|---|---|---|---|---|
| Silver carp | Protamex | Silver carp protein hydrolysate (2 and 4%, w/w) | Surimi | Delayed the formation of MDA and unfavorable flavor volatiles; inhibited the oxidation of free sulfhydryl and the formation of carbonyls in myofibrillar proteins | [173] |
| Bovine | Pepsin | TSKYR (0.1 and 0.5%, w/w) | Ground beef | 0.5% TSKYR provided similar protection against lipid oxidation as 0.1 and 0.5% BHT | [177] |
| Bighead carp | Flavourzyme Alcalase Neutral protease Papain | Bighead carp gill protein hydrolysate (1 and 2%, w/w) | Surimi | Increased sulfhydryl and salt-soluble protein concentrations, enhanced Ca2+-ATPase activity, reduced disulfide bonds, carbonyls and hydrophobicity, and improved gel strength and texture | [174] |
| Barred mackerel | Alcalase and actinidin | Barred mackerel gelatine hydrolysate (<3 kDa) | Carbonated beverage | No adverse effects on emulsification activity and stability, foam expansion and stability, color, and flavor | [86] |
| Faba bean seed | Pepsin Trypsin Alcalase | Faba bean hydrolysate (1%, w/v) | Apple juice | No adverse effects on organoleptic acceptability | [9] |
| Capelin | Alcalase Neutrase Papain | Capelin protein hydrolysate (0.5–3.0%, w/w) | Ground pork | Inhibited 17.7–60.4% of TBARS production; increased cooking yield (up to 4% at 3.0%) | [178] |
| Goby | Grey triggerfish proteases | Goby protein hydrolysate (0.01–0.2%, w/w) | Turkey meat sausage | 0.02% and 0.04% hydrolysate showed higher MDA inhibition capacity than 0.2% vitamin C | [179] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. https://doi.org/10.3390/biom12111622
Zhu Y, Lao F, Pan X, Wu J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules. 2022; 12(11):1622. https://doi.org/10.3390/biom12111622
Chicago/Turabian StyleZhu, Yongsheng, Fei Lao, Xin Pan, and Jihong Wu. 2022. "Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability" Biomolecules 12, no. 11: 1622. https://doi.org/10.3390/biom12111622
APA StyleZhu, Y., Lao, F., Pan, X., & Wu, J. (2022). Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules, 12(11), 1622. https://doi.org/10.3390/biom12111622







