The Function and Therapeutic Implications of TNF Signaling in MDSCs
Abstract
:1. Introduction
2. The Physiological Effects of TNF-α and Its Receptors
3. The Introduction of MDSCs
4. The Role of TNF Signaling on MDSCs
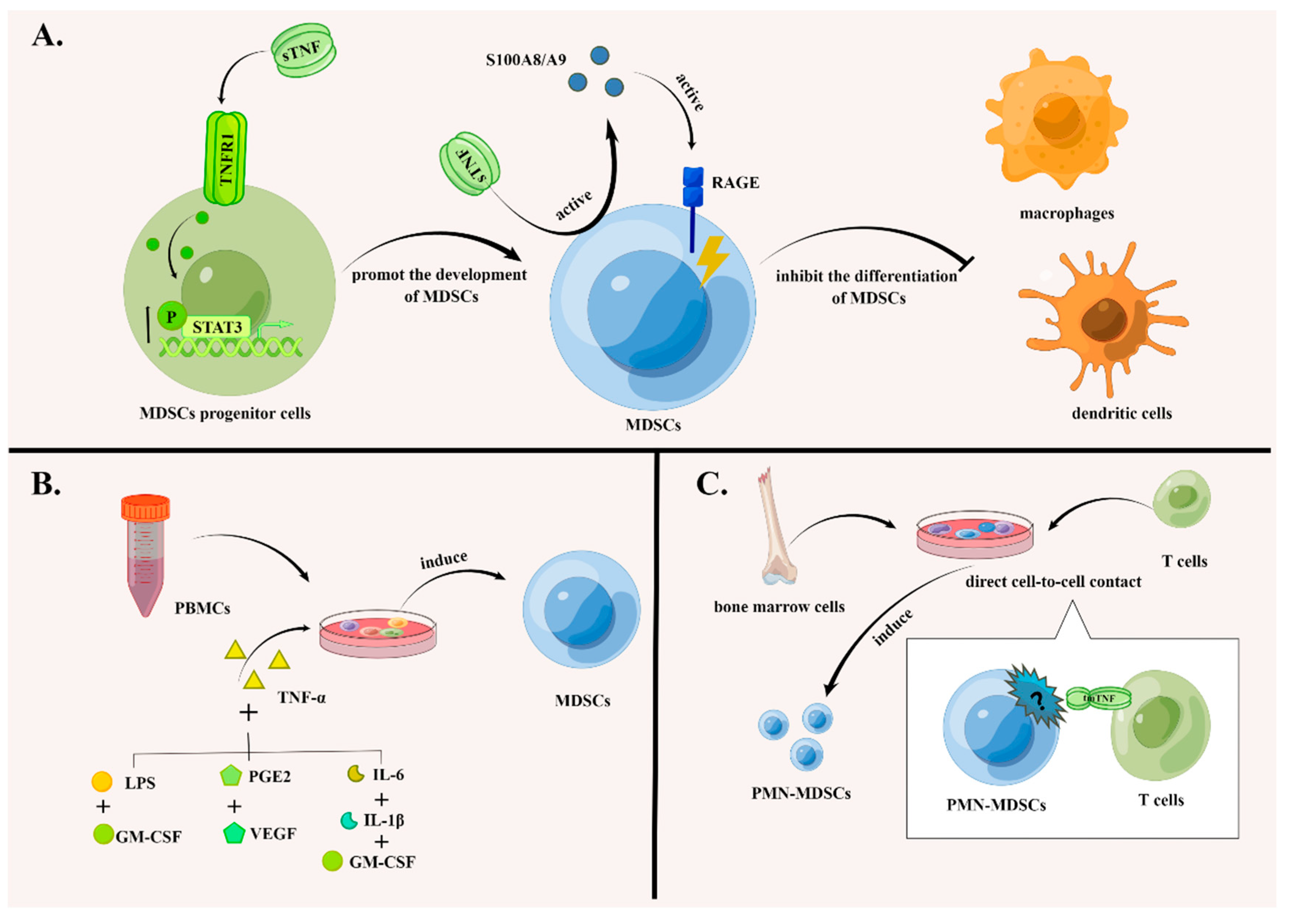
4.1. The Effects of TNF Signaling in the Accumulation of MDSCs
4.1.1. TNF Signaling Promotes the Development of MDSCs and Suppresses Their Differentiation
4.1.2. TNF Signaling Promotes the Induction of MDSCs In Vitro
4.1.3. TNF Signaling Inhibits the Apoptosis of MDSCs
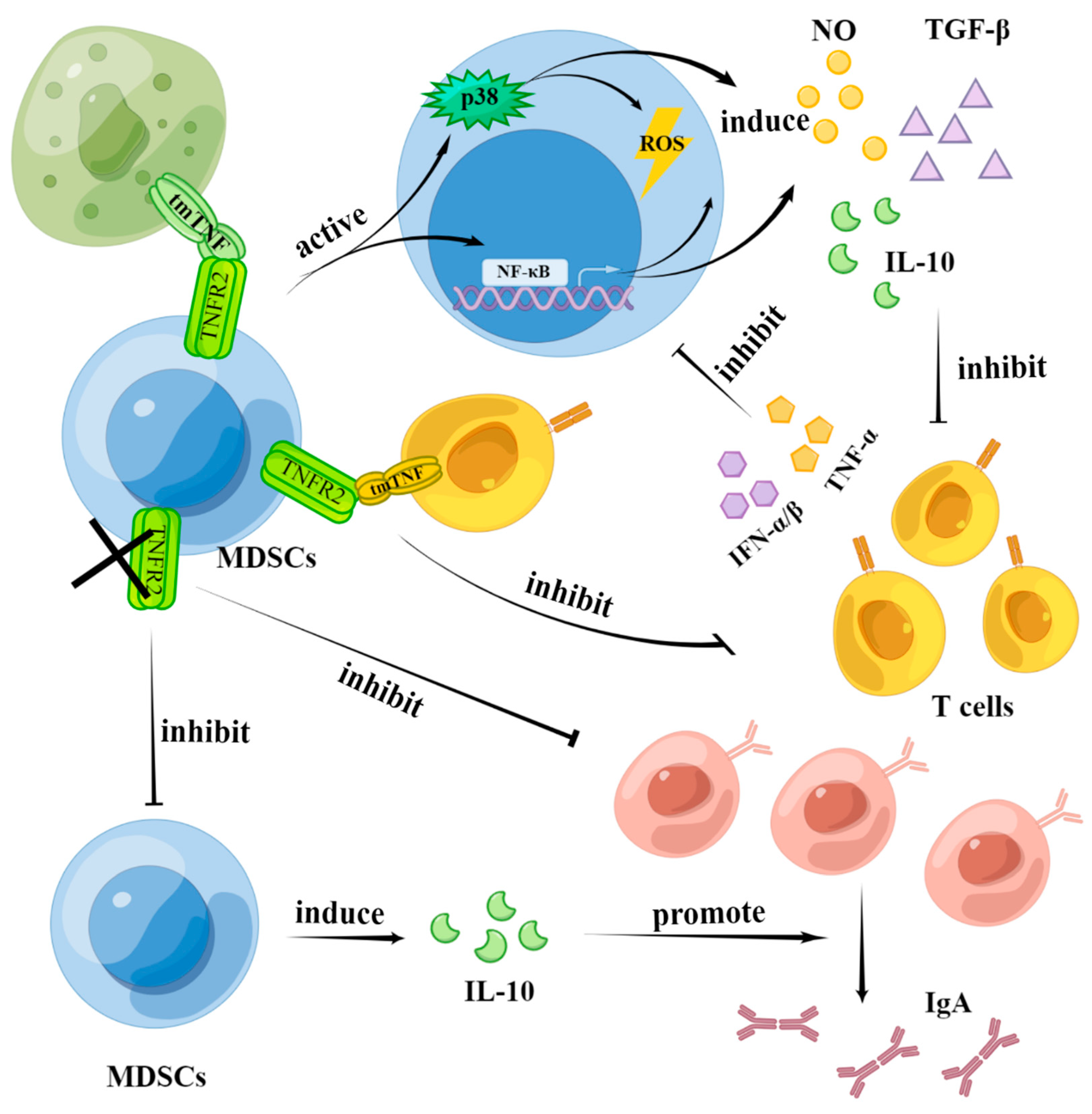
4.2. The Effects of TNF-α Signaling in the Function of MDSCs
4.2.1. MDSCs Suppress the Effect of T Cells through tmTNFα-TNFR2 Signaling
4.2.2. MDSCs Enhance the Activation and Proliferation of B Cells through TNFR2 Signaling
4.2.3. T Cells Affect the Function of MDSCs through TNF Signaling
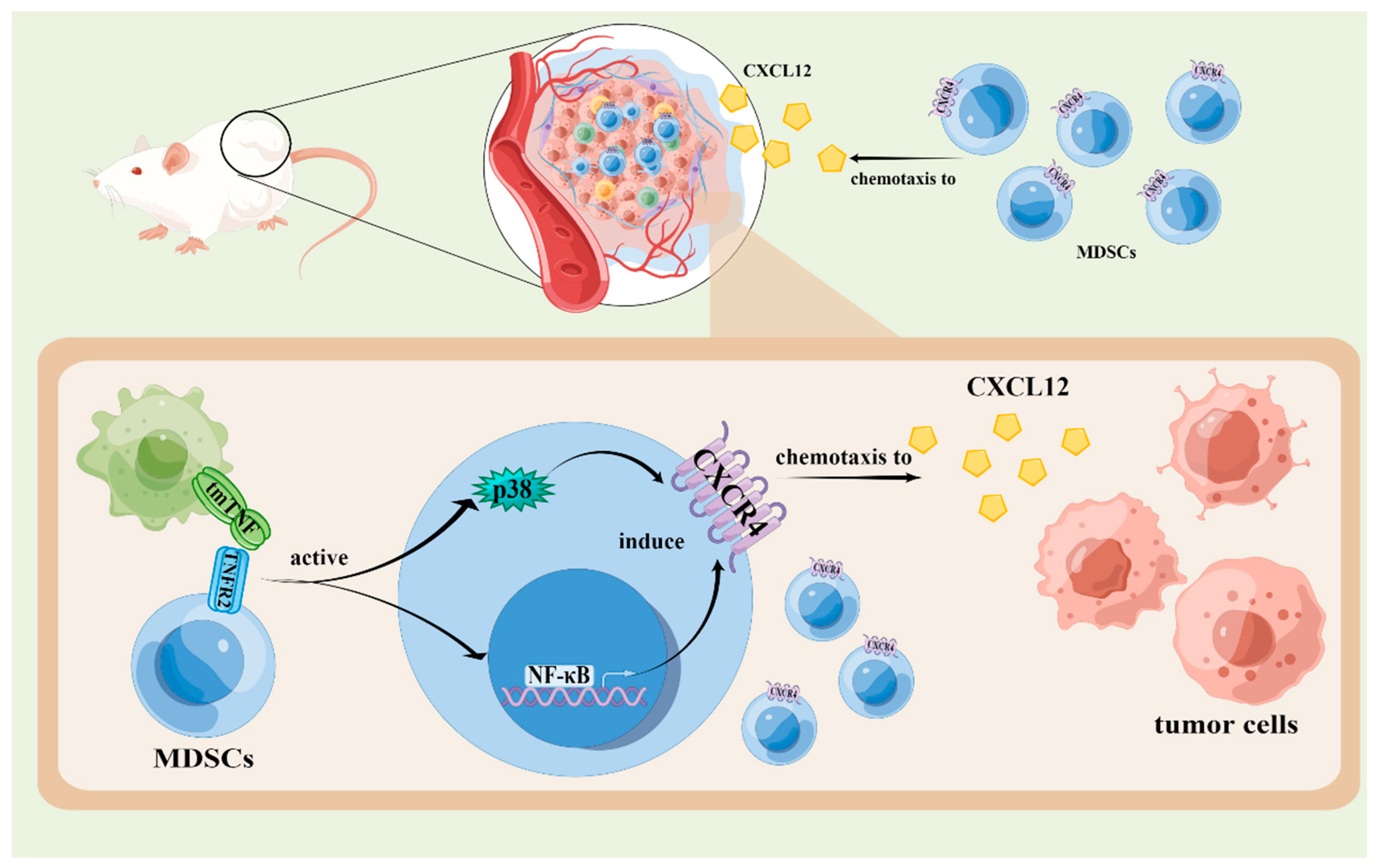
4.3. The Effects of TNF Signaling Pathways in the Chemotaxis of MDSCs
5. The Potential Therapeutic Effects of TNF Signaling Pathways in MDSCs
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Larbi, A. Immunity and Inflammation: From Jekyll to Hyde. Exp. Gerontol. 2018, 107, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- Antonatos, C.; Stavrou, E.F.; Evangelou, E.; Vasilopoulos, Y. Exploring pharmacogenetic variants for predicting response to anti-TNF therapy in autoimmune diseases: A meta-analysis. Pharmacogenomics 2021, 22, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M. Antibody therapeutics and immunoregulation in cancer and autoimmune disease. Semin. Cancer Biol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Shovman, O.; Tamar, S.; Amital, H.; Watad, A.; Shoenfeld, Y. Diverse patterns of anti-TNF-alpha-induced lupus: Case series and review of the literature. Clin. Rheumatol. 2018, 37, 563–568. [Google Scholar] [CrossRef]
- Shalaby, M.R.; Aggarwal, B.B.; Rinderknecht, E.; Svedersky, L.P.; Finkle, B.S.; Palladino, M.A., Jr. Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J. Immunol. Baltim. Md. 1950 1985, 135, 2069–2073. [Google Scholar]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Atretkhany, K.S.; Nosenko, M.A.; Gogoleva, V.S.; Zvartsev, R.V.; Qin, Z.; Nedospasov, S.A.; Drutskaya, M.S. TNF Neutralization Results in the Delay of Transplantable Tumor Growth and Reduced MDSC Accumulation. Front. Immunol. 2016, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Bazzoni, F.; Beutler, B. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 1996, 334, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Declercq, W.; Beyaert, R.; Fiers, W. Two tumour necrosis factor receptors: Structure and function. Trends Cell Biol. 1995, 5, 392–399. [Google Scholar] [CrossRef]
- Sobo-Vujanovic, A.; Vujanovic, L.; DeLeo, A.B.; Concha-Benavente, F.; Ferris, R.L.; Lin, Y.; Vujanovic, N.L. Inhibition of Soluble Tumor Necrosis Factor Prevents Chemically Induced Carcinogenesis in Mice. Cancer Immunol. Res. 2016, 4, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Ihnatko, R.; Kubes, M. TNF signaling: Early events and phosphorylation. Gen. Physiol. Biophys. 2007, 26, 159–167. [Google Scholar]
- Tartaglia, L.A.; Ayres, T.M.; Wong, G.H.; Goeddel, D.V. A novel domain within the 55 kd TNF receptor signals cell death. Cell 1993, 74, 845–853. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Ahmad, S.; Azid, N.A.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. The Key Role of TNF-TNFR2 Interactions in the Modulation of Allergic Inflammation: A Review. Front. Immunol. 2018, 9, 2572. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesi, R.J. MDSC: The Most Important Cell You Have Never Heard Of. Trends Pharmacol. Sci. 2019, 40, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Budhwar, S.; Verma, P.; Verma, R.; Rai, S.; Singh, K. The Yin and Yang of Myeloid Derived Suppressor Cells. Front. Immunol. 2018, 9, 2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Miao, K.; Yin, B.; Li, H.; Fan, J.; Zhu, Y.; Ba, H.; Zhang, Z.; Chen, F.; Wang, J.; et al. Cardioprotective Role of Myeloid-Derived Suppressor Cells in Heart Failure. Circulation 2018, 138, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, T.; Shao, S.; Shi, B.; Zhao, Y. Phenotype, development, and biological function of myeloid-derived suppressor cells. Oncoimmunology 2016, 5, e1004983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polz, J.; Remke, A.; Weber, S.; Schmidt, D.; Weber-Steffens, D.; Pietryga-Krieger, A.; Müller, N.; Ritter, U.; Mostböck, S.; Männel, D.N. Myeloid suppressor cells require membrane TNFR2 expression for suppressive activity. Immun. Inflamm. Dis. 2014, 2, 121–130. [Google Scholar] [CrossRef]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor Necrosis Factor alpha and Regulatory T Cells in Oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Brand, D.D.; Zheng, S.G. Role of TNF-TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Li, B.; Li, X.; Zhao, X.; Wan, L.; Lin, G.; Yu, M.; Wang, J.; Jiang, X.; Feng, W.; et al. Transmembrane TNF-α promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J. Immunol. Baltim. Md. 1950 2014, 192, 1320–1331. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Galan, L.; Vesin, D.; Uysal, H.; Blaser, G.; Benkhoucha, M.; Ryffel, B.; Quesniaux, V.F.J.; Garcia, I. Transmembrane Tumor Necrosis Factor Controls Myeloid-Derived Suppressor Cell Activity via TNF Receptor 2 and Protects from Excessive Inflammation during BCG-Induced Pleurisy. Front. Immunol. 2017, 8, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sade-Feldman, M.; Kanterman, J.; Ish-Shalom, E.; Elnekave, M.; Horwitz, E.; Baniyash, M. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity 2013, 38, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hodges, A.; Chen, S.H.; Pan, P.Y. Myeloid-derived suppressor cells as cellular immunotherapy in transplantation and autoimmune diseases. Cell. Immunol. 2021, 362, 104300. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Lin, Y.C.; Chang, L.Y.; Huang, S.K.; Huang, C.H.; Yang, C.K.; Huang, C.T.; Lin, C.Y. Therapeutic Role of Inducible Nitric Oxide Synthase Expressing Myeloid-Derived Suppressor Cells in Acetaminophen-Induced Murine Liver Failure. Front. Immunol. 2020, 11, 574839. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; Goncharov, A.G.; Seledtsova, G.V. Clinically feasible approaches to potentiating cancer cell-based immunotherapies. Hum. Vaccines Immunother. 2015, 11, 851–869. [Google Scholar] [CrossRef] [Green Version]
- Verschoor, C.P.; Johnstone, J.; Millar, J.; Dorrington, M.G.; Habibagahi, M.; Lelic, A.; Loeb, M.; Bramson, J.L.; Bowdish, D.M. Blood CD33(+)HLA-DR(−) myeloid-derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 2013, 93, 633–637. [Google Scholar] [CrossRef]
- Lechner, M.G.; Liebertz, D.J.; Epstein, A.L. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. Baltim. Md. 1950 2010, 185, 2273–2284. [Google Scholar] [CrossRef] [Green Version]
- Bauswein, M.; Singh, A.; Ralhan, A.; Neri, D.; Fuchs, K.; Blanz, K.D.; Schäfer, I.; Hector, A.; Handgretinger, R.; Hartl, D.; et al. Human T cells modulate myeloid-derived suppressor cells through a TNF-α-mediated mechanism. Immunol. Lett. 2018, 202, 31–37. [Google Scholar] [CrossRef]
- Wu, T.H.; Pabin, C.N.; Qin, Z.; Blankenstein, T.; Philip, M.; Dignam, J.; Schreiber, K.; Schreiber, H. Long-term suppression of tumor growth by TNF requires a Stat1- and IFN regulatory factor 1-dependent IFN-gamma pathway but not IL-12 or IL-18. J. Immunol. Baltim. Md. 1950 2004, 172, 3243–3251. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Rong, L.; Zhao, X.; Li, X.; Liu, X.; Deng, J.; Wu, H.; Xu, X.; Erben, U.; Wu, P.; et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J. Clin. Investig. 2012, 122, 4094–4104. [Google Scholar] [CrossRef] [Green Version]
- Ham, B.; Wang, N.; D’Costa, Z.; Fernandez, M.C.; Bourdeau, F.; Auguste, P.; Illemann, M.; Eefsen, R.L.; Høyer-Hansen, G.; Vainer, B.; et al. TNF Receptor-2 Facilitates an Immunosuppressive Microenvironment in the Liver to Promote the Colonization and Growth of Hepatic Metastases. Cancer Res. 2015, 75, 5235–5247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, G.; You, M.; Fan, H.; Ji, J.; Ding, L.; Li, P.; Hou, Y. 17β-estradiol contributes to the accumulation of myeloid-derived suppressor cells in blood by promoting TNF-α secretion. Acta Biochim. Biophys. Sin. 2015, 47, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Milette, S.; Hashimoto, M.; Perrino, S.; Qi, S.; Chen, M.; Ham, B.; Wang, N.; Istomine, R.; Lowy, A.M.; Piccirillo, C.A.; et al. Sexual dimorphism and the role of estrogen in the immune microenvironment of liver metastases. Nat. Commun. 2019, 10, 5745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Liang, H.; Burnette, B.; Weicheslbaum, R.R.; Fu, Y.X. Radiation and anti-PD-L1 antibody combinatorial therapy induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression. Oncoimmunology 2014, 3, e28499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte, R.N.; Dotan, S.; Elkabets, M.; White, M.R.; Reich, E.; Carmi, Y.; Song, X.; Dvozkin, T.; Krelin, Y.; Voronov, E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006, 25, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Beldi, G.; Khosravi, M.; Abdelgawad, M.E.; Salomon, B.L.; Uzan, G.; Haouas, H.; Naserian, S. TNFα/TNFR2 signaling pathway: An active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res. Ther. 2020, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Richter, G.; Schüler, T.; Ibe, S.; Cao, X.; Blankenstein, T. B cells inhibit induction of T cell-dependent tumor immunity. Nat. Med. 1998, 4, 627–630. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Xu, X.; Meng, Q.; Erben, U.; Wang, P.; Glauben, R.; Kühl, A.A.; Wu, H.; Ma, C.W.; Hu, M.; Wang, Y.; et al. Myeloid-derived suppressor cells promote B-cell production of IgA in a TNFR2-dependent manner. Cell. Mol. Immunol. 2017, 14, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Sun, H.W.; Yang, Y.Y.; Chen, H.T.; Yu, X.J.; Wu, W.C.; Xu, Y.T.; Jin, L.L.; Wu, X.J.; Xu, J.; et al. Reprogramming immunosuppressive myeloid cells by activated T cells promotes the response to anti-PD-1 therapy in colorectal cancer. Signal Transduct. Target. Ther. 2021, 6, 4. [Google Scholar] [CrossRef]
- Xu, J.; Chakrabarti, A.K.; Tan, J.L.; Ge, L.; Gambotto, A.; Vujanovic, N.L. Essential role of the TNF-TNFR2 cognate interaction in mouse dendritic cell-natural killer cell crosstalk. Blood 2007, 109, 3333–3341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ba, H.; Li, B.; Li, X.; Li, C.; Feng, A.; Zhu, Y.; Wang, J.; Li, Z.; Yin, B. Transmembrane tumor necrosis factor-α promotes the recruitment of MDSCs to tumor tissue by upregulating CXCR4 expression via TNFR2. Int. Immunopharmacol. 2017, 44, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Vredevoogd, D.W.; Kuilman, T.; Ligtenberg, M.A.; Boshuizen, J.; Stecker, K.E.; de Bruijn, B.; Krijgsman, O.; Huang, X.; Kenski, J.C.N.; Lacroix, R.; et al. Augmenting Immunotherapy Impact by Lowering Tumor TNF Cytotoxicity Threshold. Cell 2020, 180, 404–405. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.D.; Lu, C.; Payne, D.; Paschall, A.V.; Klement, J.D.; Redd, P.S.; Ibrahim, M.L.; Yang, D.; Han, Q.; Liu, Z.; et al. Autocrine IL6-Mediated Activation of the STAT3-DNMT Axis Silences the TNFalpha-RIP1 Necroptosis Pathway to Sustain Survival and Accumulation of Myeloid-Derived Suppressor Cells. Cancer Res. 2020, 80, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Steed, P.M.; Tansey, M.G.; Zalevsky, J.; Zhukovsky, E.A.; Desjarlais, J.R.; Szymkowski, D.E.; Abbott, C.; Carmichael, D.; Chan, C.; Cherry, L.; et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science 2003, 301, 1895–1898. [Google Scholar] [CrossRef]
- Zhao, A.; Li, F.; Wei, C.; Zhou, Z.; Luo, X.; Wu, H.; Ning, C.; Liu, W.; Li, D.; Lin, D.; et al. TNFalpha antagonist in combination with PD-1 blocker to prevent or retard malignant transformation of B[a]P-induced chronic lung inflammation. Carcinogenesis 2022, 43, 445–456. [Google Scholar] [CrossRef]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef]
- Scaffidi, C.; Kirchhoff, S.; Krammer, P.H.; Peter, M.E. Apoptosis signaling in lymphocytes. Curr. Opin. Immunol. 1999, 11, 277–285. [Google Scholar] [CrossRef]
- Pereira, R.; Lago, P.; Faria, R.; Torres, T. Safety of Anti-TNF Therapies in Immune-Mediated Inflammatory Diseases: Focus on Infections and Malignancy. Drug Dev. Res. 2015, 76, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]

| Disease Models | Pharmacological or Biological Agents | Mechanisms | Reference |
|---|---|---|---|
| Melanoma | Decitabine (DCA): A DNA methyltransferase inhibitor. | DAC activates the intrinsic TNF-α-RIP1signaling pathway (an apoptosis pathway) in myeloid-derived suppressor cells (MDSCs), inhibiting the accumulation of MDSCs and enhancing the sensitivity of the tumor to immune checkpoint blockade (ICB) therapy. | [53,54] |
| 3-methylcholanthrene (MCA)-induced tumor | XPro1595: A dominant-negative tumor necrosis factor (DN-TNF) and specific antagonist of sTNF. It inactivates endogenous sTNF by forming hetero trimers. | Treatment of XPro1595 can decrease the phosphorylation of STAT3 in MDSCs, inhibiting the recruitment and immunosuppressive function of MDSCs in MCA-induced tumor, delaying the development of the tumor. | [13,29,55] |
| Fibrosarcoma | Infliximab: A kind of purified recombinational DNA-derived chimeric monoclonal anti-TNF that specifically binds to TNF and blocks its interaction with cell surface receptors. | MDSCs treated with infliximab produce less NO and cannot inhibit the proliferation of T cells. By downregulating the recruitment and immunosuppressive function of MDSCs, infliximab suppresses the growth of tumors. | [9] |
| Fibrosarcoma | Etanercept: An engineered recombinant protein that comprises of TNFR2 and the fragment crystallizable (Fc) portion of human IgG1. It can specifically bind to TNF and block its interaction with cell surface receptors, downregulating the recruitment and immunosuppressive function of MDSCs | Etanercept inhibits the expression of IL-10 and TGF-β in MDSCs, impairing their immunosuppressive function. Moreover, the expression of ADAM17 in MDSCs decreases after the treatment of etanercept. This gene encodes TACE that cuts CD26L in the surface of T cells, preventing naive T cells from migrating to the inflammation site and inhibiting their activation. Therefore, etanercept suppresses tumor growth by downregulating the recruitment and immunosuppressive function of MDSCs. | [9] |
| Benzo(a)pyrene-induced chronic pneumonia | By downregulating the recruitment and immunosuppressive function of MDSCs, etanercept prevents or delays the malignant transformation of chronic inflammation induced by benzo(a)pyrene. | [56] | |
| Inflammation induced by bacille Calmette-Guerin (BCG) | Etanercept promotes the differentiation of MDSCs to mature dendritic cells and macrophages and inhibits the immunosuppressive function of MDSCs by decreasing the generation of NO and ROS, which reconstructs the activity of NK cells and T cells and promotes the recovery of immune function in organisms. | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Yu, C.; Jiao, L.; Miao, K.; Ni, L.; Rao, X.; Zhou, L.; Zhao, C. The Function and Therapeutic Implications of TNF Signaling in MDSCs. Biomolecules 2022, 12, 1627. https://doi.org/10.3390/biom12111627
Yu K, Yu C, Jiao L, Miao K, Ni L, Rao X, Zhou L, Zhao C. The Function and Therapeutic Implications of TNF Signaling in MDSCs. Biomolecules. 2022; 12(11):1627. https://doi.org/10.3390/biom12111627
Chicago/Turabian StyleYu, Kun, Chengxin Yu, Liping Jiao, Kun Miao, Li Ni, Xiaoquan Rao, Ling Zhou, and Chunxia Zhao. 2022. "The Function and Therapeutic Implications of TNF Signaling in MDSCs" Biomolecules 12, no. 11: 1627. https://doi.org/10.3390/biom12111627
APA StyleYu, K., Yu, C., Jiao, L., Miao, K., Ni, L., Rao, X., Zhou, L., & Zhao, C. (2022). The Function and Therapeutic Implications of TNF Signaling in MDSCs. Biomolecules, 12(11), 1627. https://doi.org/10.3390/biom12111627







