Red Blood Cells Oligosaccharides as Targets for Plasmodium Invasion
Abstract
1. Introduction
2. Sialic Acids
3. Antigens of Human ABO Blood Group System
4. Glycosaminoglycans (GAGs)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization WHO. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- World Health Organization WHO. Global Technical Strategy for Malaria 2016–2030; World Health Organization: Geneva, Switzerland, 2015.
- Crompton, P.D.; Moebius, J.; Portugal, S.; Waisberg, M.; Hart, G.; Garver, L.S.; Miller, L.H.; Barillas-Mury, C.; Pierce, S.K. Malaria Immunity in Man and Mosquito: Insights into Unsolved Mysteries of a Deadly Infectious Disease. Annu. Rev. Immunol. 2014, 32, 157–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Learn, G.H.; Rudicell, R.S.; Robertson, J.D.; Keele, B.F.; Ndjango, J.-B.N.; Sanz, C.M.; Morgan, D.B.; Locatelli, S.; et al. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature 2010, 467, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Prugnolle, F.; Durand, P.; Neel, C.; Ollomo, B.; Ayala, F.J.; Arnathau, C.; Etienne, L.; Mpoudi-Ngole, E.; Nkoghe, D.; Leroy, E.; et al. African great apes are natural hosts of multiple related malaria species, including Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2010, 107, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Dundas, K.; Shears, M.J.; Sinnis, P.; Wright, G.J. Important Extracellular Interactions between Plasmodium Sporozoites and Host Cells Required for Infection. Trends Parasitol. 2019, 35, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Ackerman, H.C.; Su, X.; Wellems, T.E. Malaria biology and disease pathogenesis: Insights for new treatments. Nat. Med. 2013, 19, 156–167. [Google Scholar] [CrossRef]
- Milner, D.A. Malaria Pathogenesis. Cold Spring Harb. Perspect. Med. 2018, 8, a025569. [Google Scholar] [CrossRef]
- Gaur, D.; Chitnis, C.E. Molecular interactions and signaling mechanisms during erythrocyte invasion by malaria parasites. Curr. Opin. Microbiol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Tham, W.-H.; Healer, J.; Cowman, A.F. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Salinas, N.D.; Tang, W.K.; Tolia, N.H. Blood-Stage Malaria Parasite Antigens: Structure, Function, and Vaccine Potential. J. Mol. Biol. 2019, 431, 4259–4280. [Google Scholar] [CrossRef]
- Patarroyo, M.A.; Molina-Franky, J.; Gómez, M.; Arévalo-Pinzón, G.; Patarroyo, M.E. Hotspots in Plasmodium and RBC Receptor-Ligand Interactions: Key Pieces for Inhibiting Malarial Parasite Invasion. Int. J. Mol. Sci. 2020, 21, 4729. [Google Scholar] [CrossRef]
- Kumar, H.; Tolia, N.H. Getting in: The structural biology of malaria invasion. PLoS Pathog. 2019, 15, e1007943. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, E.; Jodłowska, M.; Kaczmarek, R.; Zerka, A. Erythrocyte glycophorins as receptors for Plasmodium merozoites. Parasites Vectors 2019, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Blair, P.L.; Kaneko, O.; Peterson, D.S. An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 2001, 17, 297–299. [Google Scholar] [CrossRef]
- Sim, B.K.L. EBA-175: An Erythrocyte-binding ligand of Plasmodium falciparum. Parasitol. Today 1995, 11, 212–217. [Google Scholar] [CrossRef]
- Wanaguru, M.; Crosnier, C.; Johnson, S.; Rayner, J.C.; Wright, G.J. Biochemical Analysis of the Plasmodium falciparum Erythrocyte-binding Antigen-175 (EBA175)-Glycophorin-A Interaction. J. Biol. Chem. 2013, 288, 32106–32117. [Google Scholar] [CrossRef] [PubMed]
- Salinas, N.D.; Paing, M.M.; Tolia, N.H. Critical Glycosylated Residues in Exon Three of Erythrocyte Glycophorin A Engage Plasmodium falciparum EBA-175 and Define Receptor Specificity. mBio 2014, 5, e01606-14. [Google Scholar] [CrossRef]
- Thompson, J.K.; Triglia, T.; Reed, M.B.; Cowman, A.F. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol. Microbiol. 2001, 41, 47–58. [Google Scholar] [CrossRef]
- Narum, D.L.; Fuhrmann, S.R.; Luu, T.; Sim, B.K.L. A novel Plasmodium falciparum erythrocyte binding protein-2 (EBP2/BAEBL) involved in erythrocyte receptor binding. Mol. Biochem. Parasitol. 2002, 119, 159–168. [Google Scholar] [CrossRef]
- Lobo, C.-A.; Rodriguez, M.; Reid, M.; Lustigman, S. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 2003, 101, 4628–4631. [Google Scholar] [CrossRef]
- Maier, A.G.; Duraisingh, M.T.; Reeder, J.C.; Patel, S.S.; Kazura, J.W.; Zimmerman, P.A.; Cowman, A.F. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat. Med. 2003, 9, 87–92. [Google Scholar] [CrossRef]
- Jiang, L.; Duriseti, S.; Sun, P.; Miller, L.H. Molecular basis of binding of the Plasmodium falciparum receptor BAEBL to erythrocyte receptor glycophorin C. Mol. Biochem. Parasitol. 2009, 168, 49–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rydzak, J.; Kaczmarek, R.; Czerwinski, M.; Lukasiewicz, J.; Tyborowska, J.; Szewczyk, B.; Jaskiewicz, E. The Baculovirus-Expressed Binding Region of Plasmodium falciparum EBA-140 Ligand and Its Glycophorin C Binding Specificity. PLoS ONE 2015, 10, e0115437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukuda, M.; Lauffenburger, M.; Sasaki, H.; Rogers, M.E.; Dell, A. Structures of novel sialylated O-linked oligosaccharides isolated from human erythrocyte glycophorins. J. Biol. Chem. 1987, 262, 1952–1957. [Google Scholar] [CrossRef]
- Pisano, A.; Redmond, J.W.; Williams, K.L.; Gooley, A.A. Glycosylation sites identified by solid-phase Edman degradation: O-linked glycosylation motifs on human glycophorin A. Glycobiology 1993, 3, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Yoshima, H.; Furthmayr, H.; Kobata, A. Structures of the asparagine-linked sugar chains of glycophorin A. J. Biol. Chem. 1980, 255, 9713–9718. [Google Scholar] [CrossRef]
- Jaskiewicz, E.; Lisowska, E.; Lundblad, A. The role of carbohydrate in blood group N-related epitopes recognized by three new monoclonal antibodies. Glycoconiugate J. 1990, 7, 255–268. [Google Scholar] [CrossRef]
- Ashline, D.J.; Duk, M.; Lukasiewicz, J.; Reinhold, V.N.; Lisowska, E.; Jaskiewicz, E. The structures of glycophorin C N-glycans, a putative component of the GPC receptor site for Plasmodium falciparum EBA-140 ligand. Glycobiology 2015, 25, 570–581. [Google Scholar] [CrossRef][Green Version]
- Winzeler, E.A. Glycophorin alleles link to malaria protection. Science 2017, 356, 1122–1123. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Stefanoni, D.; Cendali, F.; Bertolone, L.; Gamboni, F.; Dzieciatkowska, M.; Rousakis, P.; Vergaki, A.; Soulakis, V.; et al. Beta thalassemia minor is a beneficial determinant of red blood cell storage lesion. Haematologica 2022, 107, 112–125. [Google Scholar] [CrossRef]
- Thein, S.L. The Molecular Basis of β-Thalassemia. Cold Spring Harb. Perspect. Med. 2014, 3, a011700. [Google Scholar]
- Glushakova, S.; Balaban, A.; McQueen, P.G.; Coutinho, R.; Miller, J.L.; Nossal, R.; Fairhurst, R.M.; Zimmerberg, J. Hemoglobinopathic Erythrocytes Affect the Intraerythrocytic Multiplication of Plasmodium falciparum in vitro. J. Infect. Dis. 2014, 210, 1100–1109. [Google Scholar] [CrossRef]
- Williams, T.N.; Weatherall, D.J.; Newbold, C.I. The membrane characteristics of Plasmodium falciparum-infected and -uninfected heterozygous α0thalassaemic erythrocytes. Br. J. Haematol. 2002, 118, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids: Fascinating sugars in higher animals and man. Zoology 2004, 107, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Burzyńska, P.; Sobala, Ł.F.; Mikołajczyk, K.; Jodłowska, M.; Jaśkiewicz, E. Sialic acids as receptors for pathogens. Biomolecules 2021, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R.; Kamerling, J.P. Exploration of the Sialic Acid World. Adv. Carbohydr. Chem. Biochem. 2018, 75, 1–213. [Google Scholar]
- Skarbek, K.; Milewska, M.J. Biosynthetic and synthetic access to amino sugars. Carbohydr. Res. 2016, 434, 44–71. [Google Scholar] [CrossRef]
- Shaw, L.; Schauer, R. The Biosynthesis of N-Glycoloylneuraminic Acid Occurs by Hydroxylation of the CMP-Glycoside of N-Acetylneuraminic Acid. Biol. Chem. Hoppe-Seyler 1988, 369, 477–486. [Google Scholar] [CrossRef]
- Hayakawa, T.; Aki, I.; Varki, A.; Satta, Y.; Takahata, N. Fixation of the Human-Specific CMP-N-Acetylneuraminic Acid Hydroxylase Pseudogene and Implications of Haplotype Diversity for Human Evolution. Genetics 2006, 172, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Peri, S.; Kulkarni, A.; Feyertag, F.; Berninsone, P.M.; Alvarez-Ponce, D. Phylogenetic Distribution of CMP-Neu5Ac Hydroxylase (CMAH), the Enzyme Synthetizing the Proinflammatory Human Xenoantigen Neu5Gc. Genome Biol. Evol. 2018, 10, 207–219. [Google Scholar] [CrossRef]
- Altman, M.O.; Gagneux, P. Absence of Neu5Gc and Presence of Anti-Neu5Gc Antibodies in Humans—An Evolutionary Perspective. Front. Immunol. 2019, 10, 789. [Google Scholar] [CrossRef]
- Chou, H.-H.; Takematsu, H.; Diaz, S.; Iber, J.; Nickerson, E.; Wright, K.L.; Muchmore, E.A.; Nelson, D.L.; Warren, S.T.; Varki, A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. USA 1998, 95, 11751–11756. [Google Scholar] [CrossRef]
- Chou, H.-H.; Hayakawa, T.; Diaz, S.; Krings, M.; Indriati, E.; Leakey, M.; Paabo, S.; Satta, Y.; Takahata, N.; Varki, A. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc. Natl. Acad. Sci. USA 2002, 99, 11736–11741. [Google Scholar] [CrossRef]
- Okerblom, J.; Varki, A. Biochemical, Cellular, Physiological, and Pathological Consequences of Human Loss of N-Glycolylneuraminic Acid. ChemBioChem 2017, 18, 1155–1171. [Google Scholar] [CrossRef]
- Paul, A.; Padler-Karavani, V. Evolution of sialic acids: Implications in xenotransplant biology. Xenotransplantation 2018, 25, e12424. [Google Scholar] [CrossRef]
- Mikolajczyk, K.; Kaczmarek, R.; Czerwinski, M. How glycosylation affects glycosylation: The role of N-glycans in glycosyltransferase activity. Glycobiology 2020, 30, 941–969. [Google Scholar] [CrossRef]
- Carbohydrate-Active enZYmes Database. Available online: https://www.cazy.org (accessed on 3 November 2022).
- Drula, E.; Garron, M.L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Friedrich, N.; Santos, J.M.; Liu, Y.; Palma, A.S.; Leon, E.; Saouros, S.; Kiso, M.; Blackman, M.J.; Matthews, S.; Feizi, T.; et al. Members of a Novel Protein Family Containing Microneme Adhesive Repeat Domains Act as Sialic Acid-binding Lectins during Host Cell Invasion by Apicomplexan Parasites. J. Biol. Chem. 2010, 285, 2064–2076. [Google Scholar] [CrossRef]
- Tolia, N.H.; Enemark, E.J.; Sim, B.K.L.; Joshua-Tor, L. Structural Basis for the EBA-175 Erythrocyte Invasion Pathway of the Malaria Parasite Plasmodium falciparum. Cell 2005, 122, 183–193. [Google Scholar] [CrossRef]
- Mayer, D.C.G.; Cofie, J.; Jiang, L.; Hartl, D.L.; Tracy, E.; Kabat, J.; Mendoza, L.H.; Miller, L.H. Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. Proc. Natl. Acad. Sci. USA 2009, 106, 5348–5352. [Google Scholar] [CrossRef]
- Lin, D.H.; Malpede, B.M.; Batchelor, J.D.; Tolia, N.H. Crystal and Solution Structures of Plasmodium falciparum Erythrocyte-binding Antigen 140 Reveal Determinants of Receptor Specificity during Erythrocyte Invasion. J. Biol. Chem. 2012, 287, 36830–36836. [Google Scholar] [CrossRef]
- Malpede, B.M.; Lin, D.H.; Tolia, N.H. Molecular Basis for Sialic Acid-dependent Receptor Recognition by the Plasmodium falciparum Invasion Protein Erythrocyte-binding Antigen-140/BAEBL. J. Biol. Chem. 2013, 288, 12406–12415. [Google Scholar] [CrossRef]
- Mayer, D.C.G.; Jiang, L.; Achur, R.N.; Kakizaki, I.; Gowda, D.C.; Miller, L.H. The glycophorin C N-linked glycan is a critical component of the ligand for the Plasmodium falciparum erythrocyte receptor BAEBL. Proc. Natl. Acad. Sci. USA 2006, 103, 2358–2362. [Google Scholar] [CrossRef]
- Yang, N.; Xing, M.; Ding, Y.; Wang, D.; Guo, X.; Sang, X.; Li, J.; Li, C.; Wang, Y.; Feng, Y.; et al. The Putative TCP-1 Chaperonin Is an Important Player Involved in Sialic Acid-Dependent Host Cell Invasion by Toxoplasma gondii. Front. Microbiol. 2020, 11, 258. [Google Scholar] [CrossRef]
- Zerka, A.; Olechwier, A.; Rydzak, J.; Jaskiewicz, E. Baculovirus-expressed Plasmodium reichenowi EBA-140 merozoite ligand is host specific. Parasitol. Int. 2016, 65, 708–714. [Google Scholar] [CrossRef]
- Zerka, A.; Kaczmarek, R.; Czerwinski, M.; Jaskiewicz, E. Plasmodium reichenowi EBA-140 merozoite ligand binds to glycophorin D on chimpanzee red blood cells, shedding new light on origins of Plasmodium falciparum. Parasites Vectors 2017, 10, 554. [Google Scholar] [CrossRef]
- Gilberger, T.-W.; Thompson, J.K.; Triglia, T.; Good, R.T.; Duraisingh, M.T.; Cowman, A.F. A Novel Erythrocyte Binding Antigen-175 Paralogue from Plasmodium falciparum Defines a New Trypsin-resistant Receptor on Human Erythrocytes. J. Biol. Chem. 2003, 278, 14480–14486. [Google Scholar] [CrossRef]
- Rayner, J.C.; Huber, C.S.; Barnwell, J.W. Conservation and divergence in erythrocyte invasion ligands: Plasmodium reichenowi EBL genes. Mol. Biochem. Parasitol. 2004, 138, 243–247. [Google Scholar] [CrossRef]
- Martin, M.J.; Rayner, J.C.; Gagneux, P.; Barnwell, J.W.; Varki, A. Evolution of human-chimpanzee differences in malaria susceptibility: Relationship to human genetic loss of N-glycolylneuraminic acid. Proc. Natl. Acad. Sci. USA 2005, 102, 12819–12824. [Google Scholar] [CrossRef]
- Varki, A.; Gagneux, P. Human-specific evolution of sialic acid targets: Explaining the malignant malaria mystery? Proc. Natl. Acad. Sci. USA 2009, 106, 14739–14740. [Google Scholar] [CrossRef]
- Wanaguru, M.; Liu, W.; Hahn, B.H.; Rayner, J.C.; Wright, G.J. RH5-Basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2013, 110, 20735–20740. [Google Scholar] [CrossRef]
- Dankwa, S.; Lim, C.; Bei, A.K.; Jiang, R.H.Y.; Abshire, J.R.; Patel, S.D.; Goldberg, J.M.; Moreno, Y.; Kono, M.; Niles, J.C.; et al. Ancient human sialic acid variant restricts an emerging zoonotic malaria parasite. Nat. Commun. 2016, 7, 11187. [Google Scholar] [CrossRef]
- Proto, W.R.; Siegel, S.V.; Dankwa, S.; Liu, W.; Kemp, A.; Marsden, S.; Zenonos, Z.A.; Unwin, S.; Sharp, P.M.; Wright, G.J.; et al. Adaptation of Plasmodium falciparum to humans involved the loss of an ape-specific erythrocyte invasion ligand. Nat. Commun. 2019, 10, 4512. [Google Scholar] [CrossRef]
- Rees, D.C.; William, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- Aminff, A.; Anderson, J.; Dabich, L.; Gathmann, W.D. Sialic acid content of erythrocytes in normal individuals and patients with certain hematologic disorders. Am. J. Hematol. 1980, 9, 381–389. [Google Scholar] [CrossRef]
- Onyemelukwe, G.C.; Esievo, K.A.N.; Kwanashie, C.N.; Kulkarni, A.G.; Obinechie, E.N. Erythrocyte sialic acid in human sickle-cell disease. J. Comp. Pathol. 1987, 97, 143–147. [Google Scholar] [CrossRef]
- Ashwood, H.E.; Ashwood, C.; Schmidt, A.P.; Gundry, R.L.; Hoffmeister, K.M.; Anani, W.Q. Characterization and statistical modeling of glycosylation changes in sickle cell disease. Blood Adv. 2021, 5, 1463–1473. [Google Scholar] [CrossRef]
- Cooling, L. Blood Groups in Infection and Host Susceptibility. Clin. Microbiol. Rev. 2015, 28, 801–870. [Google Scholar] [CrossRef]
- Cserti-Gazdewich, C.M.; Dhabangi, A.; Musoke, C.; Ssewanyana, I.; Ddungu, H.; Nakiboneka-Ssenabulya, D.; Nabukeera-Barungi, N.; Mpimbaza, A.; Dzik, W.H. Cytoadherence in paediatric malaria: ABO blood group, CD36, and ICAM1 expression and severe Plasmodium falciparum infection. Br. J. Haematol. 2012, 159, 223–236. [Google Scholar] [CrossRef]
- Arend, P. Position of human blood group O(H) and phenotype-determining enzymes in growth and infectious disease. Ann. N. Y. Acad. Sci. 2018, 1425, 5–18. [Google Scholar] [CrossRef]
- McQuaid, F.; Rowe, J.A. Rosetting revisited: A critical look at the evidence for host erythrocyte receptors in Plasmodium falciparum rosetting. Parasitology 2020, 147, 1–11. [Google Scholar] [CrossRef]
- Rowe, J.A.; Handel, I.G.; Thera, M.A.; Deans, A.-M.; Lyke, K.E.; Koné, A.; Diallo, D.A.; Raza, A.; Kai, O.; Marsh, K.; et al. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc. Natl. Acad. Sci. USA 2007, 104, 17471–17476. [Google Scholar] [CrossRef]
- Cserti-Gazdewich, C.M.; Mayr, W.R.; Dzik, W.H. Plasmodium falciparum malaria and the immunogenetics of ABO, HLA, and CD36 (platelet glycoprotein IV). Vox Sang. 2011, 100, 99–111. [Google Scholar] [CrossRef]
- Moll, K.; Palmkvist, M.; Ch’ng, J.; Kiwuwa, M.S.; Wahlgren, M. Evasion of Immunity to Plasmodium falciparum: Rosettes of Blood Group A Impair Recognition of PfEMP1. PLoS ONE 2015, 10, e0145120. [Google Scholar] [CrossRef]
- Hedberg, P.; Sirel, M.; Moll, K.; Kiwuwa, M.S.; Hoglund, P.; Ribacke, U.; Wahlgren, M. Red blood cell blood group A antigen level affects the ability of heparin and PfEMP1 antibodies to disrupt Plasmodium falciparum rosettes. Malar. J. 2021, 20, 441. [Google Scholar] [CrossRef]
- Cserti-Gazdewich, C.M. Plasmodium falciparum malaria and carbohydrate blood group evolution. ISBT Sci. Ser. 2010, 5, 256–266. [Google Scholar] [CrossRef]
- Vigan-Womas, I.; Guillotte, M.; Juillerat, A.; Hessel, A.; Raynal, B.; England, P.; Cohen, J.H.; Bertrand, O.; Peyrard, T.; Bentley, G.A.; et al. Structural Basis for the ABO Blood-Group Dependence of Plasmodium falciparum Rosetting. PLoS Pathog. 2012, 8, e1002781. [Google Scholar] [CrossRef]
- Svensson, L.; Rydberg, L.; De Mattos, L.C.; Henry, S.M. Blood group A 1 and A 2 revisited: An immunochemical analysis. Vox Sang. 2009, 96, 56–61. [Google Scholar] [CrossRef]
- Barragan, A.; Kremsner, P.G.; Wahlgren, M.; Carlson, J. Blood Group A Antigen Is a Coreceptor in Plasmodium falciparum Rosetting. Infect. Immun. 2000, 68, 2971–2975. [Google Scholar] [CrossRef]
- Rowe, J.A.; Claessens, A.; Corrigan, R.A.; Arman, M. Adhesion of Plasmodium falciparum -infected erythrocytes to human cells: Molecular mechanisms and therapeutic implications. Expert Rev. Mol. Med. 2009, 11, e16. [Google Scholar] [CrossRef]
- Resende, M.; Nielsen, M.A.; Dahlbäck, M.; Ditlev, S.B.; Andersen, P.; Sander, A.F.; Ndam, N.T.; Theander, T.G.; Salanti, A. Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA. Malar. J. 2008, 7, 104. [Google Scholar] [CrossRef]
- Niang, M.; Bei, A.K.; Madnani, K.G.; Pelly, S.; Dankwa, S.; Kanjee, U.; Gunalan, K.; Amaladoss, A.; Yeo, K.P.; Bob, N.S.; et al. STEVOR Is a Plasmodium falciparum Erythrocyte Binding Protein that Mediates Merozoite Invasion and Rosetting. Cell Host Microbe 2014, 16, 81–93. [Google Scholar] [CrossRef]
- Yam, X.Y.; Niang, M.; Madnani, K.G.; Preiser, P.R. Three Is a Crowd—New Insights into Rosetting in Plasmodium falciparum. Trends Parasitol. 2017, 33, 309–320. [Google Scholar] [CrossRef]
- Goel, S.; Palmkvist, M.; Moll, K.; Joannin, N.; Lara, P.; Akhouri, R.R.; Moradi, N.; Öjemalm, K.; Westman, M.; Angeletti, D.; et al. RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nat. Med. 2015, 21, 314–317. [Google Scholar] [CrossRef]
- Chen, Q.; Heddini, A.; Barragan, A.; Fernandez, V.; Pearce, S.F.A.; Wahlgren, M. The Semiconserved Head Structure of Plasmodium falciparum Erythrocyte Membrane Protein 1 Mediates Binding to Multiple Independent Host Receptors. J. Exp. Med. 2000, 192, 1–10. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.; Stanley, P.; Bertozzi, C.; Hart, G.W.; Etzler, M.; Aebi, M.; Darvill, A.G.; et al. Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017. [Google Scholar]
- Drzeniek, Z.; Stöcker, G.; Siebertz, B.; Just, U.; Schroeder, T.; Ostertag, W.; Haubeck, H.-D. Heparan Sulfate Proteoglycan Expression Is Induced During Early Erythroid Differentiation of Multipotent Hematopoietic Stem Cells. Blood 1999, 93, 2884–2897. [Google Scholar] [CrossRef]
- Vogt, A.M.; Winter, G.; Wahlgren, M.; Spillman, D. Heparan sulphate identified on human erythrocytes: A Plasmodium falciparum receptor. Biochem. J. 2004, 381, 593–597. [Google Scholar] [CrossRef][Green Version]
- Molina-Franky, J.; Patarroyo, M.E.; Kalkum, M.; Patarroyo, M.A. The Cellular and Molecular Interaction between Erythrocytes and Plasmodium falciparum Merozoites. Front. Cell. Infect. Microbiol. 2022, 12, 816574. [Google Scholar] [CrossRef]
- Boyle, M.J.; Richards, J.S.; Gilson, P.R.; Chai, W.; Beeson, J.G. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood 2010, 115, 4559–4568. [Google Scholar] [CrossRef]
- Kobayashi, S.; Volden, P.; Timm, D.; Mao, K.; Xu, X.; Liang, Q. Transcription Factor GATA4 Inhibits Doxorubicin-induced Autophagy and Cardiomyocyte Death. J. Biol. Chem. 2010, 285, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Matuschewski, K. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO J. 2002, 21, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Akhouri, R.R.; Bhattacharyya, A.; Pattnaik, P.; Malhotra, P.; Sharma, A. Structural and functional dissection of the adhesive domains of Plasmodium falciparum thrombospondin-related anonymous protein (TRAP). Biochem. J. 2004, 379, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.M.; Barragan, A.; Chen, Q.; Kironde, F.; Spillmann, D.; Wahlgren, M. Heparan sulfate on endothelial cells mediates the binding ofPlasmodium falciparum–infected erythrocytes via the DBL1α domain of PfEMP1. Blood 2003, 101, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Barragan, A.; Fernandez, V.; Chen, Q.; von Euler, A.; Wahlgren, M.; Spillmann, D. The Duffy-binding-like domain 1 of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a heparan sulfate ligand that requires 12 mers for binding. Blood 2000, 95, 3594–3599. [Google Scholar] [CrossRef]
- Smithskamp, H.; Wolthuis, F.H. New Concepts in Treatment of Malignant Tertian Malaria with Cerebral Involvement. Br. Med. J. 1971, 1, 714–716. [Google Scholar] [CrossRef][Green Version]
- Leitgeb, A.M.; Blomqvist, K.; Cho-Ngwa, F.; Samje, M.; Nde, P.; Titanji, V.; Wahlgreen, M. Low Anticoagulant Heparin Disrupts Plasmodium falciparum Rosettes in Fresh Clinical Isolates. Am. J. Trop. Med. Hyg. 2011, 84, 390–396. [Google Scholar] [CrossRef]
- Boyle, M.J.; Skidmore, M.; Dickerman, B.; Cooper, L.; Devlin, A.; Yates, E.; Horrocks, P.; Freeman, C.; Chai, W.; Beeson, J.G. Identification of Heparin Modifications and Polysaccharide Inhibitors of Plasmodium falciparum Merozoite Invasion That Have Potential for Novel Drug Development. Antimicrob. Agents Chemother. 2017, 61, e00709-17. [Google Scholar] [CrossRef]
- Skidmore, M.A.; Mustaffa, K.M.F.; Cooper, L.C.; Guimond, S.E.; Yates, E.A.; Craig, A.G. A semi-synthetic glycosaminoglycan analogue inhibits and reverses Plasmodium falciparum cytoadherence. PLoS ONE 2017, 12, e0186276. [Google Scholar] [CrossRef]
- Fried, M.; Duffy, P.E. Adherence of Plasmodium falciparum to Chondroitin Sulfate A in the Human Placenta. Science 1996, 272, 1502–1504. [Google Scholar] [CrossRef]
- Tran, E.E.; Cheeks, M.L.; Kakuru, A.; Muhindo, M.K.; Natureeba, P.; Nakalembe, M.; Ategeka, J.; Nayebare, P.; Kamya, M.; Havlir, D.; et al. The impact of gravidity, symptomatology and timing of infection on placental malaria. Malar. J. 2020, 19, 227. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Gangnard, S.; Round, A.; Dechavanne, S.; Juillerat, A.; Raynal, B.; Faure, G.; Baron, B.; Ramboarina, S.; Singh, S.K.; et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc. Natl. Acad. Sci. USA 2010, 107, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Hviid, L.; Lopez-Perez, M.; Larsen, M.D.; Vidarsson, G. No sweet deal: The antibody-mediated immune response to malaria. Trends Parasitol. 2022, 38, 428–434. [Google Scholar] [CrossRef]
- Bastos, M.F.; Albrecht, L.; Kozlowski, E.O.; Lopes, S.C.P.; Blanco, Y.C.; Carlos, B.C.; Castiñeiras, C.; Vicente, C.P.; Werneck, C.C.; Wunderlich, G.; et al. Fucosylated Chondroitin Sulfate Inhibits Plasmodium falciparum Cytoadhesion and Merozoite Invasion. Antimicrob. Agents Chemother. 2014, 58, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Vilanova, E.; Mourao, P.A.S.; Fernandex-Busquets, X. Marine organism sulfated polysaccharides exhibiting significant antimalarial activity and inhibition of red blood cell invasion by Plasmodium. Sci. Rep. 2016, 6, 24368. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.L.; Dans, M.G.; Balbin, J.M.; de Koning-Ward, T.F.; Gilson, P.R.; Beeson, J.G.; Boyle, M.J.; Wilson, D.W. Targeting malaria parasite invasion of red blood cells as an antimalarial strategy. FEMS Microbiol. Rev. 2019, 43, 223–238. [Google Scholar] [CrossRef]
- Bharara, R.; Singh, S.; Pattnaik, P.; Chitnis, C.E.; Sharma, A. Structural analogs of sialic acid interfere with the binding of erythrocyte binding antigen-175 to glycophorin A, an interaction crucial for erythrocyte invasion by Plasmodium falciparum. Mol. Biochem. Parasitol. 2004, 138, 123–129. [Google Scholar] [CrossRef]
- White, N.J.; Pukrittayakamee, S.; Hien, T.T.; Faiz, M.A.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet 2014, 383, 723–735. [Google Scholar] [CrossRef]
- Arya, A.; Kojom Foko, L.P.; Chaudhry, S.; Sharma, A.; Singh, V. Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions—India and sub-Saharan Africa. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 43–56. [Google Scholar] [CrossRef]
- Leitgeb, A.M.; Charunwatthana, P.; Rueangveerayut, R.; Uthaisin, C.; Silamut, K.; Chotivanich, K.; Sila, P.; Moll, K.; Lee, S.J.; Lindgren, M.; et al. Inhibition of merozoite invasion and transient de-sequestration by sevuparin in humans with Plasmodium falciparum malaria. PLoS ONE 2017, 12, e0188754. [Google Scholar] [CrossRef]
- Mordmuller, B.; Sulyok, M.; Egger-Adam, D.; Resende, M.; De Jongh, W.A.; Jensen, M.H.; Smedegaard, H.H.; Ditlev, S.B.; Soegaard, M.; Poulsen, L.; et al. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate to prevent pregnancy-associated malaria. Clin. Infect. Dis. 2019, 69, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Feijó, D.F.; Morrot, A.; Freire-de-Lima, C.G. Decoding the Role of Glycans in Malaria. Front. Microbiol. 2017, 8, 1071. [Google Scholar] [CrossRef] [PubMed]
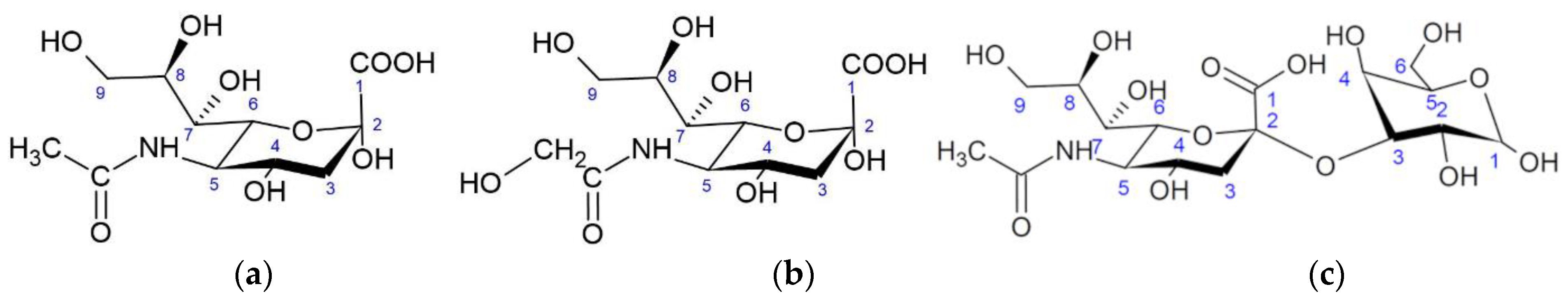
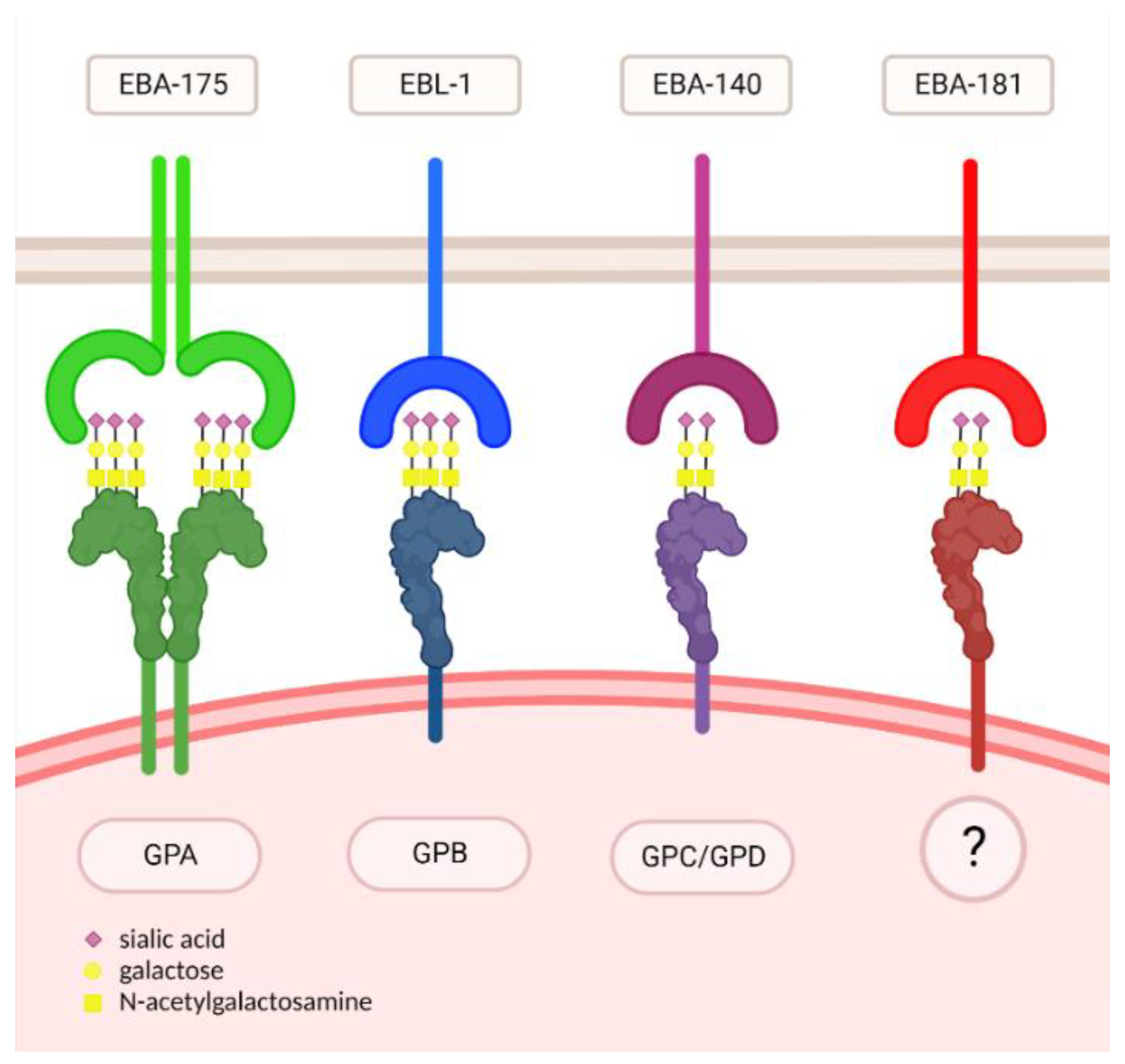
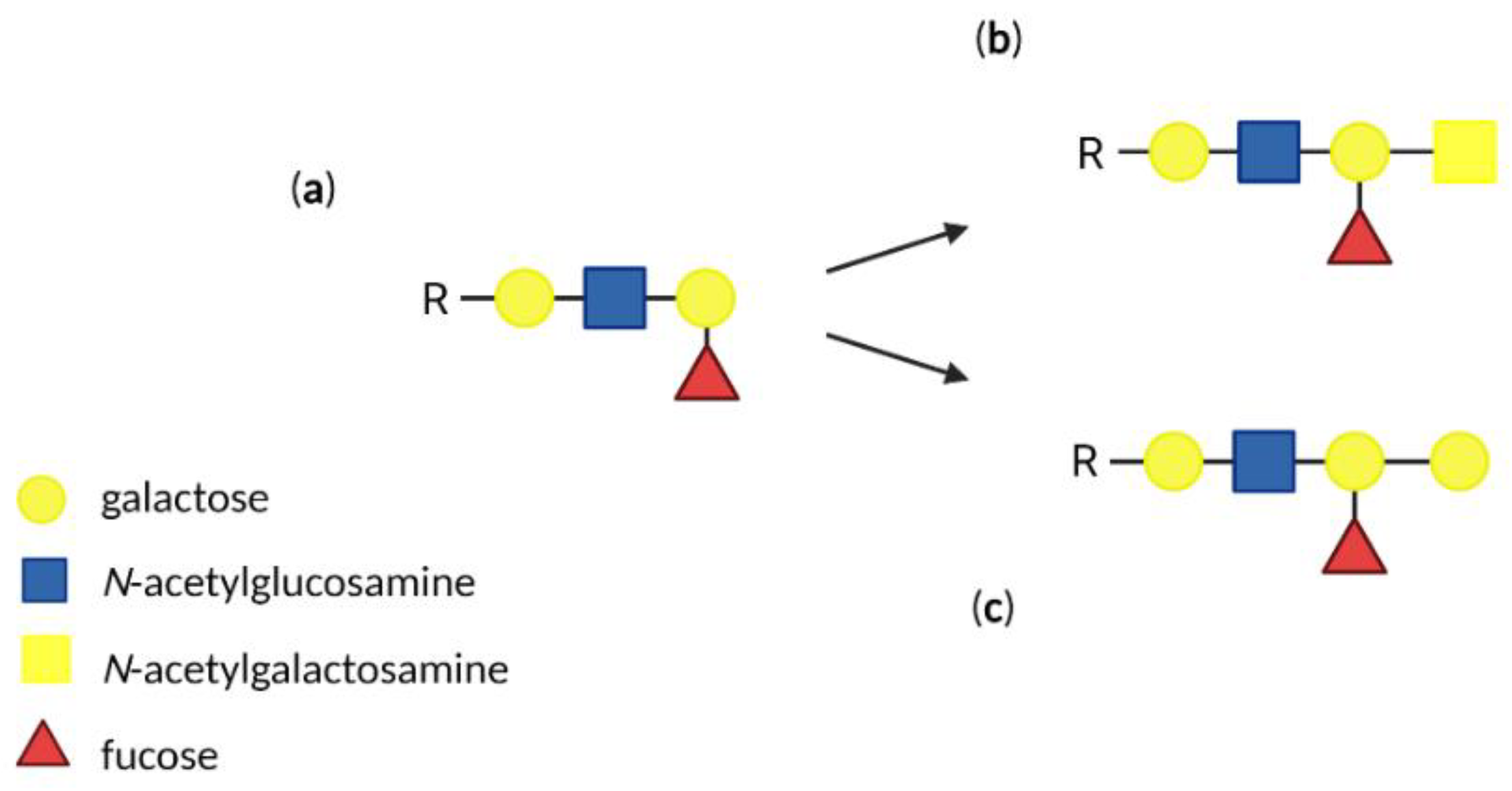
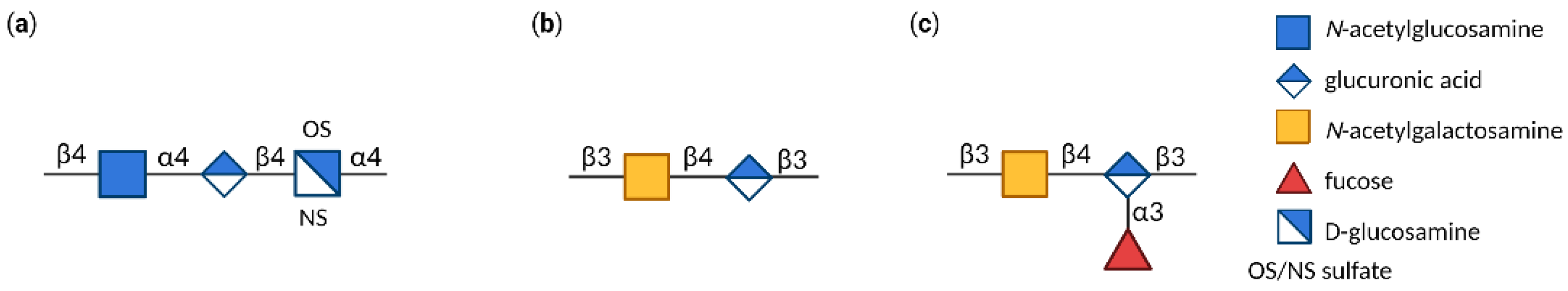
| Ligand | Receptor | Oligosaccharide |
|---|---|---|
| EBA-175 | GPA | Neu5Ac(α2,3)-Gal- |
| EBL-1 | GPB | Neu5Ac(α2,3)-Gal- |
| EBA-140 | GPC/GPD | Neu5Gc(α2,3)-Gal- |
| EBA-181 | ? | Neu5Gc(α2,3)-Gal- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burzyńska, P.; Jodłowska, M.; Zerka, A.; Czujkowski, J.; Jaśkiewicz, E. Red Blood Cells Oligosaccharides as Targets for Plasmodium Invasion. Biomolecules 2022, 12, 1669. https://doi.org/10.3390/biom12111669
Burzyńska P, Jodłowska M, Zerka A, Czujkowski J, Jaśkiewicz E. Red Blood Cells Oligosaccharides as Targets for Plasmodium Invasion. Biomolecules. 2022; 12(11):1669. https://doi.org/10.3390/biom12111669
Chicago/Turabian StyleBurzyńska, Patrycja, Marlena Jodłowska, Agata Zerka, Jan Czujkowski, and Ewa Jaśkiewicz. 2022. "Red Blood Cells Oligosaccharides as Targets for Plasmodium Invasion" Biomolecules 12, no. 11: 1669. https://doi.org/10.3390/biom12111669
APA StyleBurzyńska, P., Jodłowska, M., Zerka, A., Czujkowski, J., & Jaśkiewicz, E. (2022). Red Blood Cells Oligosaccharides as Targets for Plasmodium Invasion. Biomolecules, 12(11), 1669. https://doi.org/10.3390/biom12111669








