Bacterial and Cellular Response to Yellow-Shaded Surface Modifications for Dental Implant Abutments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
2.3. Contact Angle and Surface Energy Evaluation
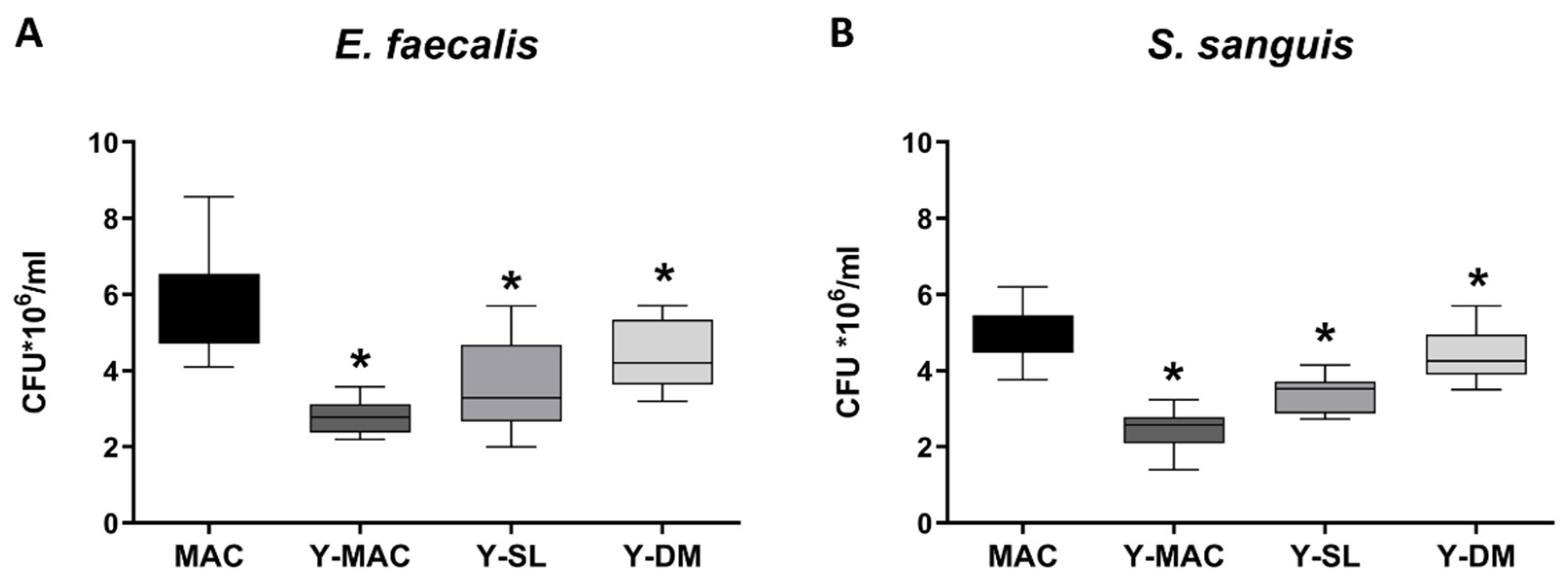
2.4. Bacterial Biofilm Evaluation
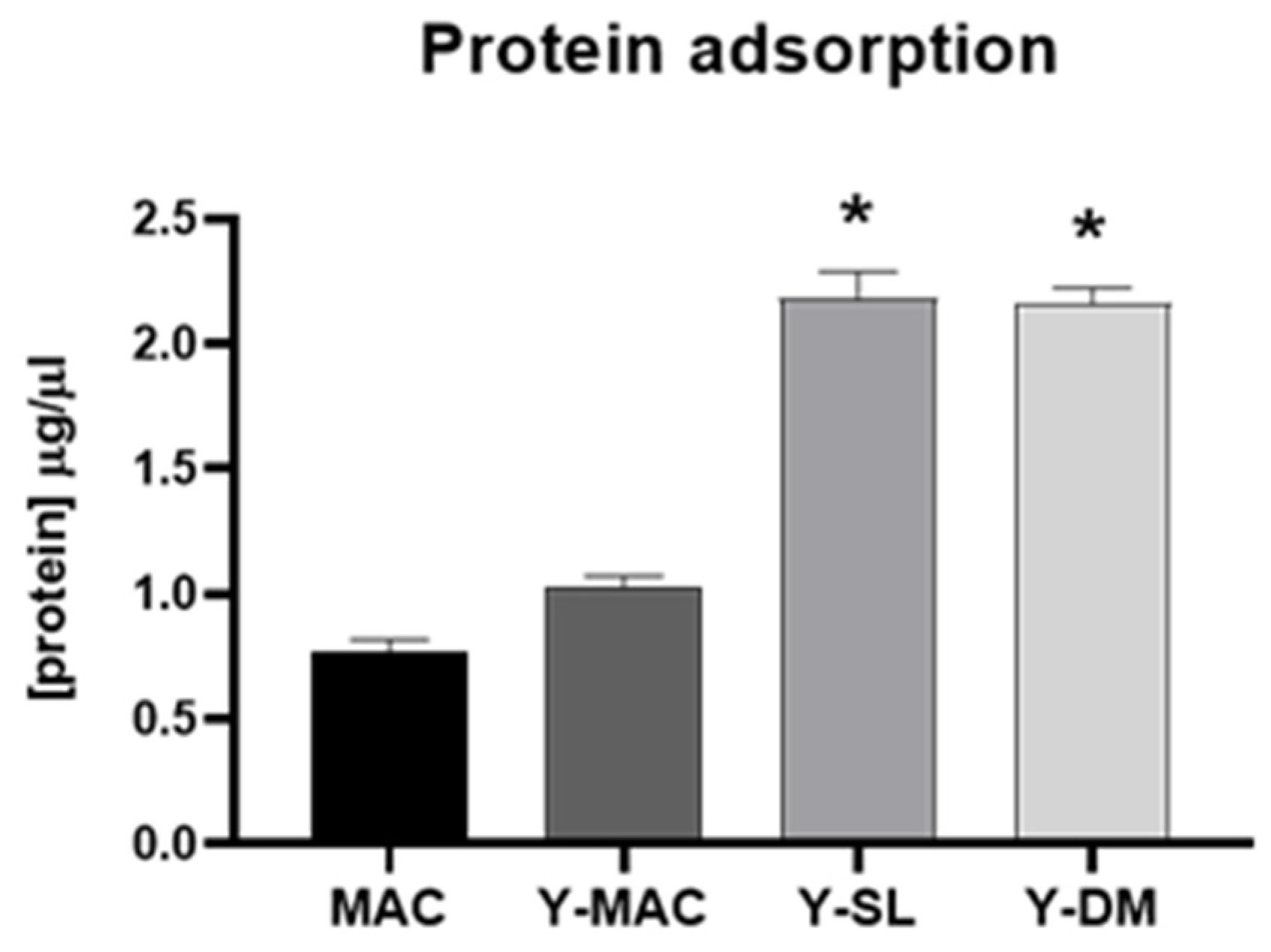
2.5. Protein Adsorption
2.6. Cell Culture
2.7. Cell Adhesion
2.8. Cell Proliferation
2.9. Statistics
3. Results
3.1. SEM
3.2. EDX
3.3. Wetting Properties
3.4. Evaluation of Bacterial Biofilm
3.5. Evaluation of Biological Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brånemark, P.I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkvist, S.; Rockler, B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 1983, 4, 25–28. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Berglundh, T.; Wennström, J.; Lindhe, J. The peri-implant hard and soft tissues at different implant systems. A comparative study in the dog. Clin. Oral Implant. Res. 1996, 7, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Chehroudi, B.; Gould, T.R.L.; Brunette, D.M. The role of connective tissue in inhibiting epithelial downgrowth on titanium-coated percutaneous implants. J. Biomed. Mater. Res. 1992, 26, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Geurs, N.C.; Vassilopoulos, P.J.; Reddy, M.S. Soft Tissue Considerations in Implant Site Development. Oral Maxillofac. Surg. Clin. 2010, 22, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Lindhe, J.; Lindhe, B.T. Dimension of the periimplant mucosa. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Ikeda, H.; Yamaza, T.; Yoshinari, M.; Ohsaki, Y.; Ayukawa, Y.; Kido, M.A.; Inoue, T.; Shimono, M.; Koyano, K.; Tanaka, T. Ultrastructural and Immunoelectron Microscopic Studies of the Peri-Implant Epithelium-Implant (Ti-6Al-4V) Interface of Rat Maxilla. J. Periodontol. 2000, 71, 961–973. [Google Scholar] [CrossRef]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.P.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17, 55–67. [Google Scholar] [CrossRef]
- Huh, J.B.; Rheu, G.B.; Kim, Y.S.; Jeong, C.M.; Lee, J.Y.; Shin, S.W. Influence of Implant transmucosal design on early peri-implant tissue responses in beagle dogs. Clin. Oral Implant. Res. 2014, 25, 962–968. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Linder, E.; Wennerberg, A.; Lindhe, J. The mucosal attachment to titanium implants with different surface characteristics: An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 448–455. [Google Scholar] [CrossRef]
- Lee, D.J.; Ryu, J.S.; Shimono, M.; Lee, K.W.; Lee, J.M.; Jung, H.S. Differential Healing Patterns of Mucosal Seal on Zirconia and Titanium Implant. Front. Physiol. 2019, 10, 796. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Contaldo, M.; De Rosa, A.; Nucci, L.; Ballini, A.; Malacrinò, D.; La Noce, M.; Inchingolo, F.; Xhajanka, E.; Ferati, K.; Bexheti-Ferati, A.; et al. Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth. Materials 2021, 14, 3735. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Ueno, T.; Saruta, J.; Hirota, M.; Park, W.; Ogawa, T. Ultraviolet Treatment of Titanium to Enhance Adhesion and Retention of Oral Mucosa Connective Tissue and Fibroblasts. Int. J. Mol. Sci. 2021, 22, 12396. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Trujillo, E.G.; Pradies, G.; Petrillo, S.; Muzzi, M.; Carossa, S.; Mussano, F. Fibroblast Interaction with Different Abutment Surfaces: In Vitro Study. Int. J. Mol. Sci. 2020, 21, 1919. [Google Scholar] [CrossRef] [Green Version]
- Carossa, M.; Cavagnetto, D.; Mancini, F.; Mosca Balma, A.; Mussano, F. Plasma of Argon Treatment of the Implant Surface, Systematic Review of In Vitro Studies. Biomolecules 2022, 12, 1219. [Google Scholar] [CrossRef]
- Xing, R.; Salou, L.; Taxt-Lamolle, S.; Reseland, J.E.; Lyngstadaas, S.P.; Haugen, H.J. Surface hydride on titanium by cathodic polarization promotes human gingival fibroblast growth. J. Biomed. Mater. Res. Part A 2014, 102, 1389–1398. [Google Scholar] [CrossRef]
- Berry, C.C.; Campbell, G.; Spadiccino, A.; Robertson, M.; Curtis, A.S.G. The influence of microscale topography on fibroblast attachment and motility. Biomaterials 2004, 25, 5781–5788. [Google Scholar] [CrossRef]
- Roach, M.D.; Williamson, R.S.; Blakely, I.P.; Didier, L.M. Tuning anatase and rutile phase ratios and nanoscale surface features by anodization processing onto titanium substrate surfaces. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 58, 213–223. [Google Scholar] [CrossRef]
- Milleret, V.; Lienemann, P.S.; Gasser, A.; Bauer, S.; Ehrbar, M.; Wennerberg, A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin. Implant Dent. Relat. Res. 2019, 21 (Suppl. 1), 15–24. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef]
- Köunönen, M.; Hormia, M.; Kivilahti, J.; Hautaniemi, J.; Thesleff, I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. J. Biomed. Mater. Res. 1992, 26, 1325–1341. [Google Scholar] [CrossRef]
- Okawachi, H.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Sakaguchi, M.; Yamane, K.; Koyano, K. Effect of Titanium Surface Calcium and Magnesium on Adhesive Activity of Epithelial-Like Cells and Fibroblasts. Biointerphases 2012, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Kim, J.G.; Kim, M.K.; Ansari, S.; Moshaverinia, A.; Choi, S.H.; Ryu, J.J. Effect of laser-dimpled titanium surfaces on attachment of epithelial-like cells and fibroblasts. J. Adv. Prosthodont. 2015, 7, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Pistilli, R.; Genova, T.; Canullo, L.; Faga, M.; Terlizzi, M.; Gribaudo, G.; Mussano, F. Effect of Bioactivation on Traditional Surfaces and Zirconium Nitride: Adhesion and Proliferation of Preosteoblastic Cells and Bacteria. Int. J. Oral Maxillofac. Implants 2018, 33, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Duraccio, D.; Strongone, V.; Malucelli, G.; Auriemma, F.; De Rosa, C.; Mussano, F.D.; Genova, T.; Faga, M.G. The role of alumina-zirconia loading on the mechanical and biological properties of UHMWPE for biomedical applications. Compos. Part B Eng. 2019, 164, 800–808. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Verga Falzacappa, E.; Scopece, P.; Munaron, L.; Rivolo, P.; Mandracci, P.; Benedetti, A.; Carossa, S.; Patelli, A. In vitro characterization of two different atmospheric plasma jet chemical functionalizations of titanium surfaces. Appl. Surf. Sci. 2017, 409, 314–324. [Google Scholar] [CrossRef] [Green Version]
- Mussano, F.; Genova, T.; Laurenti, M.; Zicola, E.; Munaron, L.; Rivolo, P.; Mandracci, P.; Carossa, S. Early Response of Fibroblasts and Epithelial Cells to Pink-Shaded Anodized Dental Implant Abutments: An In Vitro Study. Int. J. Oral Maxillofac. Implants 2018, 33, 571–579. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Serra, F.G.; Carossa, M.; Munaron, L.; Carossa, S. Nano-Pore Size of Alumina Affects Osteoblastic Response. Int. J. Mol. Sci. 2018, 19, 528. [Google Scholar] [CrossRef] [Green Version]
- Mussano, F.; Genova, T.; Rivolo, P.; Mandracci, P.; Munaron, L.; Faga, M.G.; Carossa, S. Role of surface finishing on the in vitro biological properties of a silicon nitride–titanium nitride (Si3N4–TiN) composite. J. Mater. Sci. 2017, 52, 467–477. [Google Scholar] [CrossRef]
- Genova, T.; Petrillo, S.; Zicola, E.; Roato, I.; Ferracini, R.; Tolosano, E.; Altruda, F.; Carossa, S.; Mussano, F.; Munaron, L. The crosstalk between osteodifferentiating stem cells and endothelial cells promotes angiogenesis and bone formation. Front. Physiol. 2019, 10, 1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinidis, I.; Kotsakis, G.; Gerdes, S.; Walter, M. Cross-sectional study on the prevalence and risk indicators of peri-implant diseases. Eur. J. Oral Implant. 2015, 8, 75–88. [Google Scholar]
- Koidou, V.P.; Argyris, P.P.; Skoe, E.P.; Mota Siqueira, J.; Chen, X.; Zhang, L.; Hinrichs, J.E.; Costalonga, M.; Aparicio, C. Peptide coatings enhance keratinocyte attachment towards improving the peri-implant mucosal seal. Biomater. Sci. 2018, 6, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Moon, H.J.; Kumar, P.T.S.; Han, P.; Ivanovski, S. Anodized anisotropic titanium surfaces for enhanced guidance of gingival fibroblasts. Mater. Sci. Eng. C 2020, 112, 110860. [Google Scholar] [CrossRef] [PubMed]
- Ghinassi, B.; Di Baldassarre, A.; D’addazio, G.; Traini, T.; Andrisani, M.; Di Vincenzo, G.; Gaggi, G.; Piattelli, M.; Caputi, S.; Sinjari, B. Gingival Response to Dental Implant: Comparison Study on the Effects of New Nanopored Laser-Treated vs. Traditional Healing Abutments. Int. J. Mol. Sci. 2020, 21, 6056. [Google Scholar] [CrossRef]
- Jin, C.; Ren, L.F.; Ding, H.Z.; Shi, G.S.; Lin, H.S.; Zhang, F. Enhanced attachment, proliferation, and differentiation of human gingival fibroblasts on titanium surface modified with biomolecules. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 2167–2177. [Google Scholar] [CrossRef]
- Kim, H.; Murakami, H.; Chehroudi, B.; Textor, M.; Brunette, D. Effects of surface topography on the connective tissue attachment to subcutaneous implants. Int. J. Oral Maxillofac. Implant. 2006, 21, 354–365. [Google Scholar]
- Li, J.; Zhang, K.; Chen, H.; Liu, T.; Yang, P.; Zhao, Y.; Huang, N. A novel coating of type IV collagen and hyaluronic acid on stent material-titanium for promoting smooth muscle cell contractile phenotype. Mater. Sci. Eng. C. Mater. Biol. Appl. 2014, 38, 235–243. [Google Scholar] [CrossRef]
- Hamilton, D.W.; Brunette, D.M. “Gap guidance” of fibroblasts and epithelial cells by discontinuous edged surfaces. Exp. Cell Res. 2005, 309, 429–437. [Google Scholar] [CrossRef]
- Guida, L.; Oliva, A.; Basile, M.A.; Giordano, M.; Nastri, L.; Annunziata, M. Human gingival fibroblast functions are stimulated by oxidized nano-structured titanium surfaces. J. Dent. 2013, 41, 900–907. [Google Scholar] [CrossRef]
- Ritz, U.; Nusselt, T.; Sewing, A.; Ziebart, T.; Kaufmann, K.; Baranowski, A.; Rommens, P.M.; Hofmann, A. The effect of different collagen modifications for titanium and titanium nitrite surfaces on functions of gingival fibroblasts. Clin. Oral Investig. 2017, 21, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Laurenti, M.; Gaglioti, D.; Scarpellino, G.; Rivolo, P.; Faga, M.G.; Fiorio, P.A.; Munaron, L.; Mandracci, P.; et al. Beta1-integrin and TRPV4 are involved in osteoblast adhesion to different titanium surface topographies. Appl. Surf. Sci. 2020, 507, 145112. [Google Scholar] [CrossRef]
- Krzywicka, M.; Szymańska, J.; Tofil, S.; Malm, A.; Grzegorczyk, A. Surface Properties of Ti6Al7Nb Alloy: Surface Free Energy and Bacteria Adhesion. J. Funct. Biomater. 2022, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coatings 2020, 142, 105557. [Google Scholar] [CrossRef]
- Ijaola, A.O.; Bamidele, E.A.; Akisin, C.J.; Bello, I.T.; Oyatobo, A.T.; Abdulkareem, A.; Farayibi, P.K.; Asmatulu, E. Wettability Transition for Laser Textured Surfaces: A Comprehensive Review. Surf. Interfaces 2020, 21, 100802. [Google Scholar] [CrossRef]
- Jain, A.; Kumari, N.; Jagadevan, S.; Bajpai, V. Surface properties and bacterial behavior of micro conical dimple textured Ti6Al4V surface through micro-milling. Surf. Interfaces 2020, 21, 100714. [Google Scholar] [CrossRef]
- Hsu, L.C.; Fang, J.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Effect of micro- and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.J.; Lim, B.S.; Lee, S.J. Surface characteristics of orthodontic adhesives and effects on streptococcal adhesion. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 489–495. [Google Scholar] [CrossRef]
- Flanagan, D. Enterococcus faecalis and Dental Implants. J. Oral Implantol. 2017, 43, 8–11. [Google Scholar] [CrossRef]
- Kumar, P.S.; Mason, M.R.; Brooker, M.R.; O’Brien, K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J. Clin. Periodontol. 2012, 39, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Daubert, D.; Pozhitkov, A.; McLean, J.; Kotsakis, G. Titanium as a modifier of the peri-implant microbiome structure. Clin. Implant Dent. Relat. Res. 2018, 20, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Moritz, Y.; Saringer, C.; Tkadletz, M.; Stark, A.; Schell, N.; Letofsky-Papst, I.; Czettl, C.; Pohler, M.; Schalk, N. Oxidation behavior of arc evaporated TiSiN coatings investigated by in-situ synchrotron X-ray diffraction and HR-STEM. Surf. Coat. Technol. 2020, 404, 126632. [Google Scholar] [CrossRef]
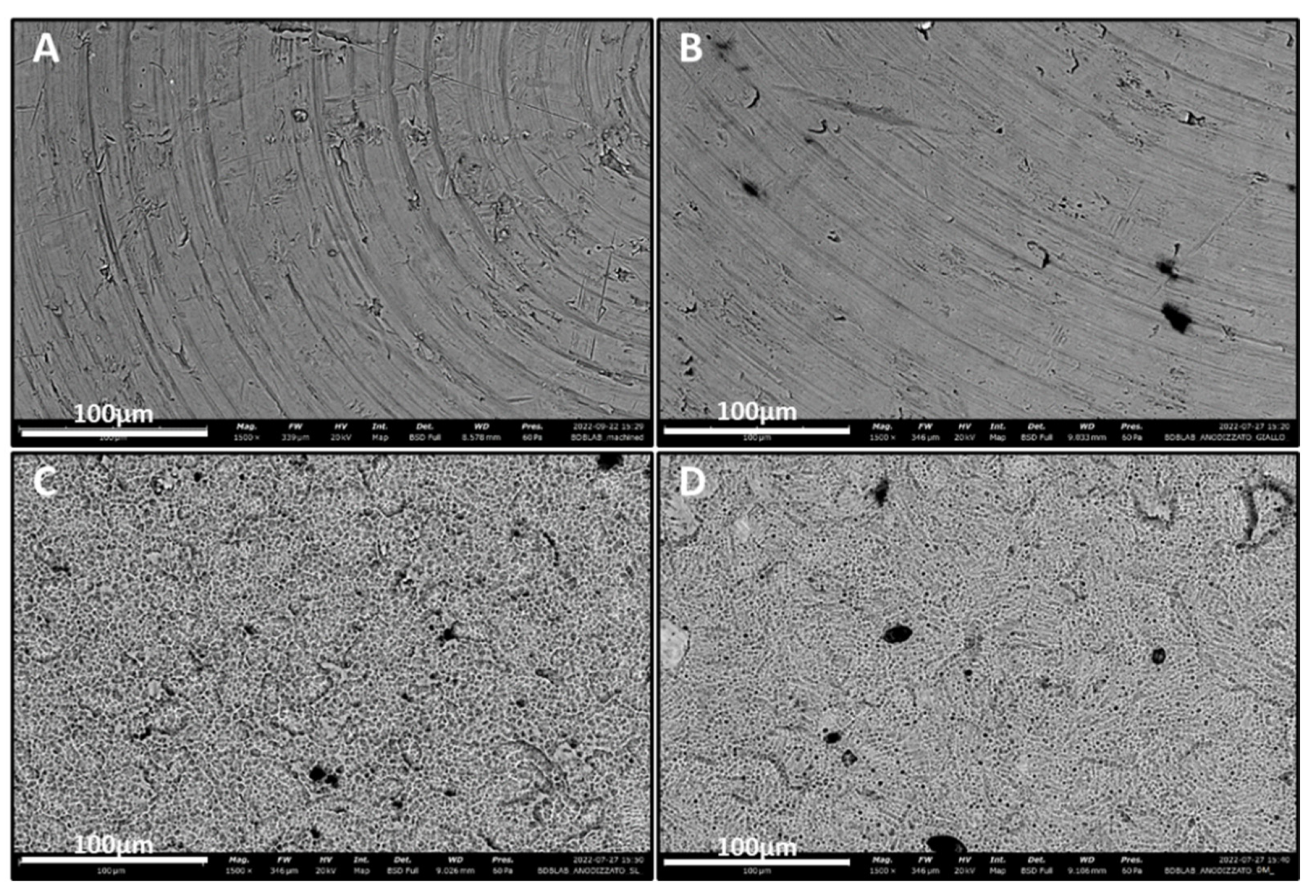


| Element | Atomic Conc. | Weight Conc. | ||
|---|---|---|---|---|
| Atomic Number | Symbol | Name | ||
| MAC | ||||
| 8 | O | Oxygen | 7.670 | 2.700 |
| 22 | Ti | Titanium | 92.330 | 97.300 |
| Y-MAC | ||||
| 8 | O | Oxygen | 57.012 | 30.700 |
| 22 | Ti | Titanium | 42.988 | 69.300 |
| Y-SL | ||||
| 8 | O | Oxygen | 50.478 | 25.400 |
| 22 | Ti | Titanium | 49.522 | 74.600 |
| Y-DM | ||||
| 8 | O | Oxygen | 49.412 | 24.600 |
| 22 | Ti | Titanium | 50.588 | 75.400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genova, T.; Chinigò, G.; Munaron, L.; Rivolo, P.; Luganini, A.; Gribaudo, G.; Cavagnetto, D.; Mandracci, P.; Mussano, F. Bacterial and Cellular Response to Yellow-Shaded Surface Modifications for Dental Implant Abutments. Biomolecules 2022, 12, 1718. https://doi.org/10.3390/biom12111718
Genova T, Chinigò G, Munaron L, Rivolo P, Luganini A, Gribaudo G, Cavagnetto D, Mandracci P, Mussano F. Bacterial and Cellular Response to Yellow-Shaded Surface Modifications for Dental Implant Abutments. Biomolecules. 2022; 12(11):1718. https://doi.org/10.3390/biom12111718
Chicago/Turabian StyleGenova, Tullio, Giorgia Chinigò, Luca Munaron, Paola Rivolo, Anna Luganini, Giorgio Gribaudo, Davide Cavagnetto, Pietro Mandracci, and Federico Mussano. 2022. "Bacterial and Cellular Response to Yellow-Shaded Surface Modifications for Dental Implant Abutments" Biomolecules 12, no. 11: 1718. https://doi.org/10.3390/biom12111718
APA StyleGenova, T., Chinigò, G., Munaron, L., Rivolo, P., Luganini, A., Gribaudo, G., Cavagnetto, D., Mandracci, P., & Mussano, F. (2022). Bacterial and Cellular Response to Yellow-Shaded Surface Modifications for Dental Implant Abutments. Biomolecules, 12(11), 1718. https://doi.org/10.3390/biom12111718















