Zinc in Human Health and Infectious Diseases
Abstract
:1. Introduction
2. Assessment of Zinc Status
3. Zinc Intake and Distribution
4. Regulation of Zinc Homeostasis
5. Zinc and Nutritional Immunity
| Disease | Zinc Salt/Formulation | Period | Population | Effect of Supplementation | References |
|---|---|---|---|---|---|
| Common cold | Zinc gluconate, 23.0 mg 6 times/d | 7 days | C:28 Z: 37 | Shortened duration of cold, more zinc-treated subjects are asymptomatic compared to control subjects | [108] |
| Zinc gluconate, 23.0 mg 6 times/d | 6 days | P: 28 Z: 29 | Reduced mean daily clinical score and nasal secretion weight and viral shedding | [114] | |
| Zinc gluconate, 23.0 mg 8 times/d | 5 days | P: 16 Z: 16 | No significant difference between nasal symptom scores, same median duration of viral shedding | [132] | |
| Zinc gluconate, 23.0 mg 6 times/d | 10 days | 75 | Shortens duration and reduces symptom severity | [109] | |
| Zinc gluconate, 13.3 mg 6 times/d | 8 days | P: 50 Z: 50 | Shortens duration and reduces symptom severity, especially cough, headache, nasal congestion/drainage | [133] | |
| Zinc gluconate, 4.5 mg 4–6 times/d | 10 days | P: 69 Z: 61 | No benefit was observed among the groups | [119] | |
| Zinc acetate, 10.0 mg 4 times/d | 6 days | P: 28 Z: 30 | No benefit was observed among the groups | [120] | |
| Zinc acetate, 9.0 mg 6 times/d | 14 days | P: 49 Z:52 | Overall symptom duration was significantly less | [110] | |
| Zinc acetate, 12.8 mg 6 times/d | 12 days | P: 23 Z: 25 | Reduced duration and severity of cold symptoms, especially cough | [111] | |
| Zinc gluconate, 13.5 mg Zinc acetate, 11.5 mg or 5.0 mg 6 times/d | 14 days | P: 67 ZG: 69 ZA5.0: 66 ZA11.5: 70 | Zinc gluconate treatment: reduced median duration of symptoms Zinc acetate lozenges: no effect on the duration or severity of symptoms | [112] | |
| Zinc sulfate, 15.0 mg/d | 7 month | P: 100 Z: 100 | Mean number of colds in the zinc group was significantly fewer | [134] | |
| Zinc gluconate, 15.0 mg/d | 7 month | P: 17 Z: 17 | More symptom-free episodes | [113] | |
| HIV/AIDS | Zinc sulfate, 45.5 mg/d | 1 month | P: 29 Z: 29 | Increase or stabilization in body weight, increase in plasma zinc levels, CD4+ T cells and plasma active zinc-bound thymulin; reduced or delayed frequency of opportunistic infections due to Pneumocystis jirovecii and C. albicans | [135] |
| Zinc gluconate, 45.0 mg 3 times/d | 15 days | P: 5 Z: 5 | Increased zinc concentrations in red blood cells, HLA-DR+ cells, stimulation of lymphocyte transformation, and phagocytosis of opsonized zymosan by neutrophils | [136] | |
| Zinc sulfate, 10.0 mg elemental zinc/d | 6 month | P: 41 Z: 44 | Decreased morbidity from diarrhea | [137] | |
| Zinc sulfate, 50.0 mg/d | 1 month | P: 34 Z: 31 | No improvements in immune responses to tuberculosis, CD4/CD8 ratio, lymphocyte subsets, and viral load | [138] | |
| Zinc sulfate, 25.0 mg/d | 6 month | P: 200 Z: 200 | No effect on birth outcomes by supplementation to pregnant HIV-positive women, no effect on T-lymphocyte counts | [139] | |
| Zinc sulfate, 25.0 mg/d | 6 month | P: 200 Z: 200 | Increased risk of wasting, no effect on viral load | [140] | |
| Zinc gluconate, 50.0 mg/d | 6 days | P: 45 Z: 44 | No improvements in antibody responses to a pneumococcal conjugate vaccine | [141] | |
| 12.0–15.0 mg zinc/d (women–men) | 18 month | P: 116 Z: 115 | Four-fold reduction in the likelihood of immunological failure, reduced rate of diarrhea | [142] | |
| Chelated zinc, 15.0 mg/d | 12 month | P: 17 Z: 13 | CD4+ cell count significantly increased | [143] | |
| Daily zinc intake (not specified) | 18 month | P: 128 Z: 126 | Nonsignificant decrease in Veterans Aging Cohort Study (VACS) index | [144] | |
| Zinc sulfate, 15.0 mg/d | 12 month | P: 40 Z:40 | No benefit was observed among the groups | [145] | |
| Zinc gluconate, 12.0–15.0 mg/d (women–men) | 18 month | P: 128 Z: 126 | No benefit was observed among the groups | [146] | |
| Zinc gluconate (high (Zhi) zinc): 90.0 mg elemental/d, Zinc gluconate (low (Zlow) zinc): 45.0 mg elemental/d | 16 weeks | Zhi: 27 Zlow: 25 | Increased serum zinc, decreased biomarkers associated with clinical comorbidities (decreased systemic inflammation (c reactive protein and TNF-α), monocyte activation, and enterocyte damage) | [147] | |
| Zinc sulfate, 20.0 mg/d | 24 weeks | P: 26 Z: 26 | Decrease in viral load, anthropometric indices, and morbidity profile in HIV-infected children started on antiretroviral therapy | [148] | |
| Chronic HepC | Polaprezinc, 75.0 mg 2 times/d | 24 weeks | P: 35 Z: 40 | Improved response to IFN-α therapy | [149] |
| Zinc sulfate, 300.0 mg/d Polaprezinc, 150.0 mg/d | 24 weeks | P: 10 ZS: 9 ZP: 15 | Normalized serum ALT levels, better eradication of HepC virus RNA | [150] | |
| Zinc gluconate, 78.0 mg/d 5 times/d | 6 month | P: 40 Z: 18 | Decreased incidences of gastrointestinal disturbances, body weight loss, and mild anemia | [151] | |
| Zinc gluconate, 30.0 mg/d | 1 year | C: 16 Z: 16 | No benefit was observed among the groups | [122] | |
| Polaprezinc, 75.0 mg 2 times/d | 48 weeks | P: 16 Z: 16 | No benefit was observed among the groups | [152] | |
| Polaprezinc, 75.0 mg 2 times/d | 24 weeks | P: 39 Z: 39 | No benefit was observed among the groups | [153] | |
| Polaprezinc, 75.0 mg 3 times/d | 6 month | P: 12 Z: 12 | Reduced serum AST, ALT, and ferritin | [154] | |
| Polaprezinc, 150.0 mg 2 times/d | 6 years | P: 30 Z: 32 | Reduced incidence of HCC (albumin-dependent) | [155] | |
| Polaprezinc, 150.0 mg/d | 48 weeks | P: 12 Z: 11 | Decrease in serum ALT levels and Th2 cells (%), decreased plasma thiobarbituric acid reactive substances, and prevented decrease in polyunsaturated fatty acids of erythrocyte membrane phospholipids | [156] | |
| Dengue fever | Zinc bisglycinate, 15.0 mg 3 times/d | 5 days | P: 25 Z: 25 | Lower mean time of defervescence and shorter time of hospitalization | [157] |
| COVID-19 | Zinc sulfate, 100.0 mg elemental/d | Until recovery or death | P: 46 Z: 196 | No benefit was observed among the groups | [123] |
| Zinc sulfate, 100.0 mg elemental zinc/d | 5 days | P: 521 Z: 411 | Increased frequency of being discharged home (OR 1.53, 95% CI 1.12–2.09) and reduction in mortality or transfer to hospice among patients who did not require ICU level of care | [158] | |
| Zinc sulfate, 100.0 mg elemental/d | 4 days | P: 2467 Z: 1006 | Increased rates of discharge home and 24% reduced risk of in-hospital mortality | [159] | |
| Zinc sulfate, 50.0 mg elemental/d | 5 days | P: 377 Z: 141 | Fewer hospitalizations | [160] | |
| Zinc acetate or gluconate, 2.0–2.5 mg/kg/day | 10 days | Case report Z: 28 | Short recovery time (~1,6 days) | [161] | |
| Zinc chloride, 0.24 mg/kg/d i.v. | 7 days | P: 18 Z: 15 | Increased zinc level but no effect on clinical outcome | [162] | |
| Zinc sulfate, 50.0 mg elemental/d 2 times/d | 15 days | P: 95 Z: 96 | Decreased duration of ventilation, decreased length of hospitalization, and reduced risk of in-hospital mortality | [163] | |
| Zinc bisglycinate, 15.0 mg/d | 6 weeks | P: 57 Z: 59 C: 56 | Significant decrease in SARS-CoV-2 infection | [164] |
| Disease | Zinc Salt/Formulation | Period | Population | Effect of Supplementation | References |
|---|---|---|---|---|---|
| Acute lower respiratory tract infection | Zinc bisglycinate, 30.0 mg elemental/d | 7 days | P: 32 Z: 32 | Shortened recovery time and duration of the hospital stay, and improved chest in-drawing, tachypnea, and fever | [157] |
| Zinc sulfate, 20.0 mg/d | 5 months | P: 124 Z: 134 | Reduced acute lower respiratory tract infection morbidity | [165] | |
| Zinc gluconate, 10.0 mg/d | 60 days | P: 48 Z: 48 | Reduced episodes of acute lower respiratory infections and severe acute lower respiratory infections, increased infection-free days | [166] | |
| Zinc oxide, 5.0 mg/d | 12 months | P: 167 Z: 162 | Decreased incidence of upper respiratory tract infections and diarrheal disease episodes | [167] | |
| Zinc gluconate, 10.0 mg/d | 6 months | P: 311 Z: 298 | Increased plasma zinc level and decreased episodes of infection | [168] | |
| Zinc acetate, 10.0 mg 2 times/d | 5 days | P: 74 Z: 76 | Increased recovery rates from illness and fever in boys | [169] | |
| Zinc sulfate, 15.0 mg/d | 6 months | P: 40 Z: 40 | Increased plasma retinol concentrations, earlier sputum conversion and resolution of X-ray lesion area | [170] | |
| Pneumonia | 10.0–20.0 mg zinc/d | 2 weeks | P: 280 Z: 280 | Acceleration in clinical resolution and shorter hospital stay | [171] |
| 10.0–20.0 mg zinc /d | 2 weeks | 610 | Marginal faster recovery time | [172] | |
| Zinc sulfate, 10.0 mg 2 times/d | until discharge | 299 | No benefit was observed among the groups | [173] | |
| Zinc sulfate, 12.5 mg elemental/d 2 times/d | until discharge | P: 47 Z: 47 | No benefit was observed among the groups | [174] | |
| Elemental zinc, 20.0 mg/d | until discharge | P: 84 Z: 80 | Reduced duration of severe pneumonia, duration of chest in-drawing respiratory rate hypoxia, and overall hospital duration | [115] | |
| Zinc sulfate, 10.0–20.0 mg/d | 7 days | P: 301 Z: 303 | Faster recovery from lower chest wall indrawing and sternal retraction | [175] | |
| Zinc syrup, 20.0 mg elemental/d 2 times/d | until discharge | P: 225 Z: 225 | Faster resolution of respiratory signs | [176] | |
| Elemental zinc, 10.0 mg 2 times/d | 7 days | P: 53 Z: 64 | No benefit was observed among the groups | [177] | |
| Zinc gluconate, 10.0 mg/d–20.0 mg/d | 7 days | P: 176 Z: 176 | No benefit was observed among the groups | [178] | |
| Zinc syrup, 40.0 mg/d | until discharge | P: 150 Z: 150 | Shorter duration of relief of severe pneumonia signs and hospitalization time | [179] | |
| Zinc syrup, 10.0 mL/d | until discharge | P: 60 Z: 60 | Faster resolution of clinical symptoms | [180] | |
| Tuberculosis | Zinc sulfate, 220.0 mg/d | 18 months | Z: 8 | Reduced dose of clofazimine, withdrawal of steroids, toleration of dapsone, reduced incidence and severity of erythema nodosum leprosum, gradual decrease in the size of granuloma, and gradual increase in the number of lymphocytes | [181] |
| Zinc sulfate, 15.0 mg/d, +/−VitA 5000 IU/d | 6 months | P: 40 Z: 40 VitA: 40 Z+VitA: 40 | Marginal earlier sputum conversion No difference in clinical, nutritional, chest X-ray, or laboratory findings | [182] | |
| Zinc sulfate, 30.0 mg elemental every second day | 6 month | P: 37 Z: 37 | Elevated plasma zinc concentrations, elevated body weight, earlier sputum smear conversion, lower SGOT and SGPT concentrations after 2 months, decreased serum levels of total protein and albumin | [183] | |
| Zinc sulfate, 15.0 mg/d | 6 month | P: 40 Z: 40 | Increased plasma retinol concentrations, earlier sputum conversion and resolution of X-ray lesion area | [170] | |
| Shigellosis | Zinc acetate, 1.30 mg/kg 3 times/d | 1 month | P: 16 Z: 16 | Increased intestinal mucosal permeability and better nitrogen absorption, increased serum zinc and alkaline phosphatase activity | [184] |
| Zinc acetate, 20.0 mg/d | 2 weeks | P: 28 Z: 28 | Increased serum zinc level, lymphocyte proliferation in response to phytohemagglutinin and plasma invasion plasmid-encoded antigen-specific IgG titers | [185] | |
| Zinc acetate, 20.0 mg/d | 2 weeks | P: 28 Z: 28 | Increased serum zinc levels, serum shigellacidal antibody titers, CD20+ cells, and CD20+CD38+ cells | [186] | |
| Not specified, 20.0 mg/d | 2 weeks | P: 16 Z: 14 | Faster recovery from acute illness, increased mean body weight, and fewer episodes of diarrhea | [187] | |
| H. pylori | Polaprezinc, 150.0 mg 2 times/d | 7 days | P: 28 Z: 33 | Increased cure rate of H. pylori infection compared to single antibiotic treatment | [188] |
| Diarrhea | Zinc sulfate, 3.0–7.0 mg/kg/d elemental zinc/d | 4 month | P: 70 Z: 70 | Decreased incidence of diarrhea, number of diarrhea episodes per child, and frequency of stools per day | [189] |
| Diarrhea multiple different studies | Decreased duration, severity, and occurrence of diarrhea | [190] | |||
| Zinc acetate, zinc gluconate, zinc sulfate ranging from 5.0–40.0 mg/d | 5–15 days | P: 9353 Z: 9469 | Reductions in morbidity as a result of oral therapeutic zinc supplementation for acute diarrhea among children | [191] | |
| Zinc sulfate, 10.0 mg/d | 2 weeks | P: 536 Z: 538 | No benefit was observed among the groups | [121] | |
| 10.0–20.0 mg zinc/d | until discharge | P: 50 Z: 50 | Reduced frequency of diarrheal episodes | [192] | |
| Zinc gluconate syrup, 20.0 mg elemental/d, +/− daily Probiotics (Pr) | 7 days | P: 50 Pr: 50 Z: 46 | Reduced relative risk of diarrhea persistence, decreased duration and severity, reduced post-treatment complications | [193] | |
| Zinc tablet, 7.0 mg/d, therapeutic zinc (TZ), 20.0 mg/d, +/− micronutrient powder (MNP) | 9 month | P: 847 Z: 844 TZ: 848 MNP: 841 | No benefit was observed among the groups | [194] | |
| Zinc sulfate, 10.0–20.0 mg/d | 10 days | P: 50 Z: 53 | Reduced duration of diarrhea, fewer diarrheic episodes in the next 3 months | [195] |
| Disease | Zinc Salt/Formulation | Period | Population | Effect of Supplementation | References |
|---|---|---|---|---|---|
| Malaria | 10.0 mg zinc 6 times/week +/− VitA single dose, 200,000 IU | 6 months | P: 74 Z: 74 | Decreased malaria prevalence and fewer malaria episodes, longer time to first malaria episode, and 22% fewer fever episodes | [196] |
| Zinc gluconate, 10.0 mg 6 times/week | 46 weeks | P: 138 Z: 136 | Reduction in Plasmodium falciparum-mediated febrile episodes | [197] | |
| Zinc acetate or zinc gluconate, 70.0 mg 2 times/week | 15 month | P: 54 Z: 55 | Not statistically significant trend towards fewer malaria episodes; no effect on plasma and hair zinc, diarrhea, and respiratory illness | [198] | |
| Zinc sulfate, 12.5 mg 6 times/week | 6 months | P: 344 Z: 336 | Increased serum zinc levels and reduced prevalence of diarrhea | [199] | |
| Zinc sulfate, 20.0 mg or 40.0 mg/d | 4 days | P: 483 Z: 473 | Increased plasma zinc, no effect on fever, parasitemia, or hemoglobin concentration | [200] | |
| Zinc sulfate, 20.0 mg/d | 7 months | P: 189 Z: 191 | No significant effect on P. vivax incidence but significantly reduced diarrhea morbidity | [201] | |
| Zinc sulfate, 25.0 mg/d +/− VitA 2500 IU/d | until delivery | P: 362 VitA: 348 Z: 345 VitA+Z: 349 | 36% (95% CI = 9–56%) reduced risk of histopathology-positive placental infection | [202] | |
| 5.0 mg, 10.0 mg, or 15.0 mg zinc/d | 9 month | P: 785 C: 433 Z5: 429 Z10: 438 Z15: 436 | No benefit was observed among the groups | [203] | |
| Zinc gluconate, 10.0 mg/d +/− VitA, 200,000 IU/d at the beginning and end of the study | 6 month | C: 90 Z: 92 | Significantly fewer (27%) malaria diagnoses | [204] | |
| Zinc, 10.0 mg/d +/− Multi-nutrients (M) | P: 148 M: 148 Z: 145 M + Z: 146 | No benefit was observed among the groups | [205] | ||
| Leishmania infection | Zinc sulfate, 2.5 mg/kg, 5.0 mg/kg or 10.0 mg/kg 3 times/d | 45 days | P: 12 Z: 92 | Increased serum zinc levels and cure rate, decreased erythema and size of induration | [206] |
| Zinc, 45.0 mg/d | 20 days | P: 15 Z: 14 | Higher expression level of transferrin receptor | [207] | |
| Zinc sulfate, topical 2% | 3 month | P: 32 Z: 32 | No benefit was observed among the groups | [208] | |
| Zins syrup, total dose in 2 weeks of 2 mg/kg/d | 2 weeks | C: 26 Z: 26 | Accelerated reduction in splenomegaly | [209] | |
| Zinc sulfate, 10.0 mg/kg/d | 45 days | C: 50 Z: 50 | Zinc supplementation is as effective as systemic meglumine antimoniate treatment | [210] | |
| Zinc sulfate, itralesional injections of 2% zinc solution | 6 weeks | C: 35 Z: 31 | Higher efficacy after the second and fourth weeks | [211] | |
| C. albicans | Zinc syrup, 20.0 mg elemental/d | 2 weeks | P: 366 Z: 358 | Increased blood zinc concentration, reduced prevalence of candidemia and candiduria by 50%, lesser nosocomial urinary tract infection and bloodstream infection, shorter treatment with broad-spectrum antibiotics, shorter length of hospital stay | [212] |
6. Zinc and the Inflamed Immune System
7. Membrane Barrier Function and Zinc
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maret, W. The metals in the biological periodic system of the elements: Concepts and conjectures. Int. J. Mol. Sci. 2016, 17, 66. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.H.; Riddell, L.J.; Nowson, C.A.; Booth, A.O.; Szymlek-Gay, E.A. Iron and zinc nutrition in the economically-developed world: A review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef]
- Prasad, A.S.; Miale, A., Jr.; Farid, Z.; Sandstead, H.; Schulert, A. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypogonadism. J. Lab. Clin. Med. 1963, 61, 537–549. [Google Scholar]
- Jones, S.; Farr, G.; Nimmanon, T.; Ziliotto, S.; Gee, J.M.; Taylor, K.M. The importance of targeting signalling mechanisms of the SLC39A family of zinc transporters to inhibit endocrine resistant breast cancer. Explor. Target. Anti-Tumor Ther. 2022, 3, 224–239. [Google Scholar]
- Wan, Y.; Zhang, B. The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules 2022, 12, 900. [Google Scholar] [CrossRef]
- Bitirim, C.V. The role of zinc transporter proteins as predictive and prognostic biomarkers of hepatocellular cancer. PeerJ 2021, 9, e12314. [Google Scholar] [CrossRef]
- Fong, L.Y.; Farber, J.; Croce, C.M. Zinc intake, microRNA dysregulation, and esophageal cancer. Aging 2016, 8, 1161–1162. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, S.; Bergdahl, A. Zinc Homeostasis in Diabetes Mellitus and Vascular Complications. Biomedicines 2022, 10, 139. [Google Scholar] [CrossRef]
- Maret, W. Zinc in pancreatic islet biology, insulin sensitivity, and diabetes. Prev. Nutr. Food Sci. 2017, 22, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maret, W. Zinc and human disease. Met. Ions Life Sci. 2013, 13, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Rolles, B.; Slusarenko, A.J.; Rink, L. Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of Corona Virus Disease 19. Br. J. Nutr. 2022, 127, 214–232. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.C.; Caballero, B.H.; Cousins, R.J.; Tucker, K.L.; Ziegler, T.R. Modern Nutrition in Health and Disease, 11th ed.; Wolters Kluwer Health Adis (ESP): London, UK, 2012. [Google Scholar]
- National Institutes of Health. Zinc, Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 6 October 2022).
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar]
- Sandstead, H.H.; William, A. Handbook on the Toxicology of Metals; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Elsevier Inc: Amsterdam, The Netherlands, 2007; pp. 925–947. [Google Scholar]
- Hall, A.G.; King, J.C.; McDonald, C.M. Comparison of Serum, Plasma, and Liver Zinc Measurements by AAS, ICP-OES, and ICP-MS in Diverse Laboratory Settings. Biol. Trace Elem. Res. 2022, 200, 2606–2613. [Google Scholar] [CrossRef]
- Maret, W. Optical methods for measuring zinc binding and release, zinc coordination environments in zinc finger proteins, and redox sensitivity and activity of zinc-bound thiols. In Methods in Enzymology; Sies, H., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2002; Volume 348, pp. 230–237. [Google Scholar]
- Carpenter, M.C.; Lo, M.N.; Palmer, A.E. Techniques for measuring cellular zinc. Arch. Biochem. Biophys. 2016, 611, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Rink, L. Zinc in Human Health; Ios Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Jackson, M. Physiology of zinc: General aspects. In Zinc in Human Biology; Springer: Berlin/Heidelberg, Germany, 1989; pp. 1–14. [Google Scholar]
- Maares, M.; Haase, H. A guide to human zinc absorption: General overview and recent advances of in vitro intestinal models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, C. Zinc: Physiology, deficiency, and parenteral nutrition. Nutr. Clin. Pract. 2015, 30, 371–382. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L. Metallothionein: Historical review and perspectives. Experientia Suppl. 1979, 34, 19–39. [Google Scholar] [PubMed]
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellularzinc homeostasis. Metallomics 2010, 2, 306–317. [Google Scholar] [CrossRef]
- Haase, H.; Beyersmann, D. Uptake and intracellular distribution of labile and total Zn(II) in C6 rat glioma cells investigated with fluorescent probes and atomic absorption. Biometals 1999, 12, 247–254. [Google Scholar] [CrossRef]
- Blaby-Haas, C.E.; Merchant, S.S. Lysosome-related organelles as mediators of metal homeostasis. J. Biol. Chem. 2014, 289, 28129–28136. [Google Scholar] [CrossRef] [Green Version]
- Krężel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.C.; Coles, J.A.; Deitmer, J.W. Homeostatic muffling. Nature 1991, 350, 564. [Google Scholar] [CrossRef]
- Haase, H.; Maret, W. The regulatory and signaling functions of zinc ions in human cellular physiology. In Cellular and Molecular Biology of Metals; CRC Press: Boca Raton, FL, USA, 2010; pp. 191–222. [Google Scholar]
- Rink, L. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.S.; King, J.C.; Lowe, N. A review of dietary zinc recommendations. Food Nutr. Bull. 2016, 37, 443–460. [Google Scholar] [CrossRef] [Green Version]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinhardt, H.J.; Adibi, S.A. Interaction between transport of zinc and other solutes in human intestine. Am. J. Physiol. 1984, 247, G176–G182. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc absorption in human small intestine. Am. J. Physiol. 1989, 256, G87–G91. [Google Scholar] [CrossRef] [PubMed]
- Ford, D. Intestinal and placental zinc transport pathways. Proc. Nutr. Soc. 2004, 63, 21–29. [Google Scholar] [CrossRef]
- Trame, S.; Wessels, I.; Haase, H.; Rink, L. A short 18 items food frequency questionnaire biochemically validated to estimate zinc status in humans. J. Trace Elem. Med. Biol. 2018, 49, 285–295. [Google Scholar] [CrossRef]
- Hunt, J.R.; Beiseigel, J.M.; Johnson, L.K. Adaptation in human zinc absorption as influenced by dietary zinc and bioavailability. Am. J. Clin. Nutr. 2008, 87, 1336–1345. [Google Scholar] [CrossRef] [Green Version]
- Lönnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378s–1383s. [Google Scholar] [CrossRef] [Green Version]
- Medicine, I.o. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006; p. 1344. [Google Scholar]
- Huth, P.J.; Fulgoni, V.L.; Keast, D.R.; Park, K.; Auestad, N. Major food sources of calories, added sugars, and saturated fat and their contribution to essential nutrient intakes in the U.S. diet: Data from the National Health and Nutrition Examination Survey (2003–2006). Nutr. J. 2013, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858s–885s. [Google Scholar] [CrossRef] [Green Version]
- Deutsche Gesellschaft für Ernährunf e. V. Referenzwerte für die Nährstoffzufuhr. Available online: https://www.dge.de/wissenschaft/referenzwerte/?L=0 (accessed on 6 October 2022).
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Agostoni, C.; Canani, R.; Fairweather-Tait, S.; Heinonen, M.; Korhonen, H.; La Vieille, S.; Marchelli, R.; Martin, A.; Naska, A.; Neuhäuser-Berthold, M. EFSA Panel on Dietetic Products, Nutrition and Allergies. Sci. Opin. Diet. Ref. Values Folate. EFSA J. 2014, 12, 3893. [Google Scholar]
- Brieger, A.; Rink, L. Zink und Immunfunktionen. Ernährung Med. 2010, 25, 156–160. [Google Scholar] [CrossRef]
- Ezzati, M.; Lopez, A.D.; Rodgers, A.A.; Murray, C.J.L. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Sandstead, H.H. Zinc deficiency. A public health problem? Am. J. Dis. Child. 1991, 145, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Rink, L. Multiple impacts of zinc on immune function. Metallomics 2014, 6, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Baarz, B.R.; Rink, L. Rebalancing the unbalanced aged immune system-a special focus on zinc. Ageing Res. Rev. 2021, 74, 101541. [Google Scholar] [CrossRef] [PubMed]
- Lestienne, I.; Icard-Vernière, C.; Mouquet, C.; Picq, C.; Trèche, S. Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chem. 2005, 89, 421–425. [Google Scholar] [CrossRef]
- Foster, M.; Samman, S. Vegetarian diets across the lifecycle: Impact on zinc intake and status. Adv. Food Nutr. Res. 2015, 74, 93–131. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, G.; Cianci, M.; Tucoulou, R.; Meyer-Klaucke, W.; Haase, H. The ligand environment of zinc stored in vesicles. Biochem. Biophys. Res. Commun. 2009, 380, 198–203. [Google Scholar] [CrossRef]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 2011, 3, 662–674. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.; Jacques, T.S. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism–dystonia. Nat. Commun. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, T.; Yoshigai, E.; Ohashi, T.; Fukada, T. Zinc transporters as potential therapeutic targets: An updated review. J. Pharmacol. Sci. 2022, 148, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013, 34, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1079–1086. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, D.E.; Stillman, M.J. The “magic numbers” of metallothionein. Metallomics 2011, 3, 444–463. [Google Scholar] [CrossRef]
- Thirumoorthy, N.; Shyam Sunder, A.; Manisenthil Kumar, K.; Senthil Kumar, M.; Ganesh, G.; Chatterjee, M. A review of metallothionein isoforms and their role in pathophysiology. World J. Surg. Oncol. 2011, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Vašák, M.; Meloni, G. Mammalian Metallothionein-3: New Functional and Structural Insights. Int. J. Mol. Sci. 2017, 18, 1117. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Wang, L.; Li, L.; Huang, Z.; Ye, L. Metallothionein 1: A New Spotlight on Inflammatory Diseases. Front. Immunol. 2021, 12, 739918. [Google Scholar] [CrossRef]
- Yin, X.; Knecht, D.A.; Lynes, M.A. Metallothionein mediates leukocyte chemotaxis. BMC Immunol. 2005, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, X.B.; Wei, H.W.; Wang, J.; Kong, Y.Q.; Wu, Y.Y.; Guo, J.L.; Li, T.F.; Li, J.K. Mammalian Metallothionein-2A and Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 1493. [Google Scholar] [CrossRef] [Green Version]
- Klaasen, C. Metallothionein iv; Birkhäuser: Basel, Switzerland, 2012. [Google Scholar]
- Min, K.S.; Kim, H.; Fujii, M.; Tetsuchikawahara, N.; Onosaka, S. Glucocorticoids suppress the inflammation-mediated tolerance to acute toxicity of cadmium in mice. Toxicol. Appl. Pharmacol. 2002, 178, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sobocinski, P.Z.; Canterbury, W.J., Jr.; Mapes, C.A.; Dinterman, R.E. Involvement of hepatic metallothioneins in hypozincemia associated with bacterial infection. Am. J. Physiol. 1978, 234, E399–E406. [Google Scholar] [CrossRef] [PubMed]
- Emeny, R.T.; Kasten-Jolly, J.; Mondal, T.; Lynes, M.A.; Lawrence, D.A. Metallothionein differentially affects the host response to Listeria infection both with and without an additional stress from cold-restraint. Cell Stress Chaperones 2015, 20, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclerc, E.; Heizmann, C.W. The importance of Ca2+/Zn2+ signaling S100 proteins and RAGE in translational medicine. Front. Biosci. (School Ed.) 2011, 3, 1232–1262. [Google Scholar] [CrossRef]
- Gilston, B.A.; Skaar, E.P.; Chazin, W.J. Binding of transition metals to S100 proteins. Sci. China Life Sci. 2016, 59, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Ali, S.A. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef]
- Heizmann, C.W.; Cox, J.A. New perspectives on S100 proteins: A multi-functional Ca(2+)-, Zn(2+)- and Cu(2+)-binding protein family. Biometals 1998, 11, 383–397. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Malavolta, M. Zinc dyshomeostasis, ageing and neurodegeneration: Implications of A2M and inflammatory gene polymorphisms. J. Alzheimer’s Dis. 2007, 12, 101–109. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499. [Google Scholar] [CrossRef] [PubMed]
- Zygiel, E.M.; Nolan, E.M. Transition Metal Sequestration by the Host-Defense Protein Calprotectin. Annu. Rev. Biochem. 2018, 87, 621–643. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, A.T.; Nolan, E.M. Avian MRP126 Restricts Microbial Growth through Ca(II)-Dependent Zn(II) Sequestration. Biochemistry 2020, 59, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Arcanjo, A.; Logullo, J.; Menezes, C.C.B.; de Souza Carvalho Giangiarulo, T.C.; Dos Reis, M.C.; de Castro, G.M.M.; da Silva Fontes, Y.; Todeschini, A.R.; Freire-de-Lima, L.; Decoté-Ricardo, D.; et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci. Rep. 2020, 10, 19630. [Google Scholar] [CrossRef]
- Liang, C.; Lian, N.; Li, M. The emerging role of neutrophil extracellular traps in fungal infection. Front. Cell. Infect. Microbiol. 2022, 12, 900895. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Caruso, J.A.; Deepe, G.S., Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 2013, 39, 697–710. [Google Scholar] [CrossRef] [Green Version]
- Kehl-Fie, T.E.; Skaar, E.P. Nutritional immunity beyond iron: A role for manganese and zinc. Curr. Opin. Chem. Biol. 2010, 14, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Botella, H.; Stadthagen, G.; Lugo-Villarino, G.; de Chastellier, C.; Neyrolles, O. Metallobiology of host-pathogen interactions: An intoxicating new insight. Trends Microbiol. 2012, 20, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Branch, A.H.; Stoudenmire, J.L.; Seib, K.L.; Cornelissen, C.N. Acclimation to Nutritional Immunity and Metal Intoxication Requires Zinc, Manganese, and Copper Homeostasis in the Pathogenic Neisseriae. Front. Cell. Infect. Microbiol. 2022, 12, 909888. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T. Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell-virus growth inhibition. Am. J. Biomed. Sci. Res. 2019, 2, 28–37. [Google Scholar] [CrossRef]
- Alamir, O.F.; Oladele, R.O.; Ibe, C. Nutritional immunity: Targeting fungal zinc homeostasis. Heliyon 2021, 7, e07805. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Chang, S.M.; Guthrie, G.J.; Maki, A.B.; Ryu, M.S.; Karabiyik, A.; Cousins, R.J. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia). PLoS ONE 2012, 7, e48679. [Google Scholar] [CrossRef] [Green Version]
- Gaetke, L.M.; McClain, C.J.; Talwalkar, R.T.; Shedlofsky, S.I. Effects of endotoxin on zinc metabolism in human volunteers. Am. J. Physiol. 1997, 272, E952–E956. [Google Scholar] [CrossRef]
- Lonergan, Z.R.; Skaar, E.P. Nutrient Zinc at the Host-Pathogen Interface. Trends Biochem. Sci. 2019, 44, 1041–1056. [Google Scholar] [CrossRef]
- Lappann, M.; Danhof, S.; Guenther, F.; Olivares-Florez, S.; Mordhorst, I.L.; Vogel, U. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol. Microbiol. 2013, 89, 433–449. [Google Scholar] [CrossRef]
- Mikhaylina, A.; Ksibe, A.Z.; Scanlan, D.J.; Blindauer, C.A. Bacterial zinc uptake regulator proteins and their regulons. Biochem. Soc. Trans. 2018, 46, 983–1001. [Google Scholar] [CrossRef] [Green Version]
- Maunders, E.A.; Ganio, K.; Hayes, A.J.; Neville, S.L.; Davies, M.R.; Strugnell, R.A.; McDevitt, C.A.; Tan, A. The Role of ZntA in Klebsiella pneumoniae Zinc Homeostasis. Microbiol. Spectr. 2022, 10, e0177321. [Google Scholar] [CrossRef]
- Price, S.L.; Vadyvaloo, V.; DeMarco, J.K.; Brady, A.; Gray, P.A.; Kehl-Fie, T.E.; Garneau-Tsodikova, S.; Perry, R.D.; Lawrenz, M.B. Yersiniabactin contributes to overcoming zinc restriction during Yersinia pestis infection of mammalian and insect hosts. Proc. Natl. Acad. Sci. USA 2021, 118, e2104073118. [Google Scholar] [CrossRef]
- Huang, K.; Wang, D.; Frederiksen, R.F.; Rensing, C.; Olsen, J.E.; Fresno, A.H. Investigation of the Role of Genes Encoding Zinc Exporters zntA, zitB, and fieF during Salmonella Typhimurium Infection. Front. Microbiol. 2017, 8, 2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overbeck, S.; Rink, L.; Haase, H. Modulating the immune response by oral zinc supplementation: A single approach for multiple diseases. Arch. Immunol. Et Ther. Exp. 2008, 56, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Eby, G.A.; Davis, D.R.; Halcomb, W.W. Reduction in duration of common colds by zinc gluconate lozenges in a double-blind study. Antimicrob. Agents Chemother. 1984, 25, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, J.C.; Conant Sloane, B.; Smith, D.S.; Turco, J.H.; Mercer, N.; Godfrey, N.J. Zinc gluconate and the common cold: A controlled clinical study. J. Int. Med. Res. 1992, 20, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Petrus, E.J.; Lawson, K.A.; Bucci, L.R.; Blum, K. Randomized, double-masked, placebo-controlled clinical study of the effectiveness of zinc acetate lozenges on common cold symptoms in allergy-tested subjects. Curr. Ther. Res. Clin. Exp. 1998, 59, 595–607. [Google Scholar] [CrossRef]
- Prasad, A.S.; Fitzgerald, J.T.; Bao, B.; Beck, F.W.; Chandrasekar, P.H. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2000, 133, 245–252. [Google Scholar] [CrossRef]
- Turner, R.B.; Cetnarowski, W.E. Effect of treatment with zinc gluconate or zinc acetate on experimental and natural colds. Clin. Infect. Dis. 2000, 31, 1202–1208. [Google Scholar] [CrossRef]
- Veverka, D.V.; Wilson, C.; Martinez, M.A.; Wenger, R.; Tamosuinas, A. Use of zinc supplements to reduce upper respiratory infections in United States Air Force Academy cadets. Complement. Ther. Clin. Pract. 2009, 15, 91–95. [Google Scholar] [CrossRef]
- Al-Nakib, W.; Higgins, P.G.; Barrow, I.; Batstone, G.; Tyrrell, D.A. Prophylaxis and treatment of rhinovirus colds with zinc gluconate lozenges. J. Antimicrob. Chemother. 1987, 20, 893–901. [Google Scholar] [CrossRef]
- Brooks, W.A.; Yunus, M.; Santosham, M.; Wahed, M.A.; Nahar, K.; Yeasmin, S.; Black, R.E. Zinc for severe pneumonia in very young children: Double-blind placebo-controlled trial. Lancet 2004, 363, 1683–1688. [Google Scholar] [CrossRef]
- Dhingra, U.; Kisenge, R.; Sudfeld, C.R.; Dhingra, P.; Somji, S.; Dutta, A.; Bakari, M.; Deb, S.; Devi, P.; Liu, E.; et al. Lower-Dose Zinc for Childhood Diarrhea—A Randomized, Multicenter Trial. N. Engl. J. Med. 2020, 383, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diarrhoeal Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 6 October 2022).
- Nowak, J.E.; Harmon, K.; Caldwell, C.C.; Wong, H.R. Prophylactic zinc supplementation reduces bacterial load and improves survival in a murine model of sepsis. Pediatr. Crit. Care Med. 2012, 13, e323–e329. [Google Scholar] [CrossRef] [PubMed]
- Weismann, K.; Jakobsen, J.P.; Weismann, J.E.; Hammer, U.M.; Nyholm, S.M.; Hansen, B.; Lomholt, K.E.; Schmidt, K. Zinc gluconate lozenges for common cold. A double-blind clinical trial. Dan. Med. Bull. 1990, 37, 279–281. [Google Scholar]
- Douglas, R.M.; Miles, H.B.; Moore, B.W.; Ryan, P.; Pinnock, C.B. Failure of effervescent zinc acetate lozenges to alter the course of upper respiratory tract infections in Australian adults. Antimicrob. Agents Chemother. 1987, 31, 1263–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.L.F.; Bhutta, Z.A.; Bhandari, N.; Teka, T.; Shahid, F.; Taneja, S.; Black, R.E.; Group, Z.S. Zinc during and in convalescence from diarrhea has no demonstrable effect on subsequent morbidity and anthropometric status among infants <6 mo of age. Am. J. Clin. Nutr. 2007, 85, 887–894. [Google Scholar]
- Abbasinazari, M.; Alavian, S.M.; Behnava, B.; Asgharinia, M.; Salimi, S.; Keshvari, M.; Mehrnoush, L.; Karim, P. Effect of zinc supplementation on viral response in patients with chronic hepatitis C and Beta thalassemia major, a pilot study. J. Clin. Diagn. Res. JCDR 2014, 8, HC16. [Google Scholar] [CrossRef]
- Natarajan, S.; Anbarasi, C.; Sathiyarajeswaran, P.; Manickam, P.; Geetha, S.; Kathiravan, R.; Prathiba, P.; Pitchiahkumar, M.; Parthiban, P.; Kanakavalli, K.; et al. Kabasura Kudineer (KSK), a poly-herbal Siddha medicine, reduced SARS-CoV-2 viral load in asymptomatic COVID-19 individuals as compared to vitamin C and zinc supplementation: Findings from a prospective, exploratory, open-labeled, comparative, randomized controlled trial, Tamil Nadu, India. Trials 2021, 22, 623. [Google Scholar] [CrossRef]
- Berendsen, A.A.M.; Kramer, C.S.; de Groot, L. The Newly Developed Elderly Nutrient-Rich Food Score Is a Useful Tool to Assess Nutrient Density in European Older Adults. Front. Nutr. 2019, 6, 119. [Google Scholar] [CrossRef] [Green Version]
- Mezzetti, A.; Pierdomenico, S.D.; Costantini, F.; Romano, F.; De Cesare, D.; Cuccurullo, F.; Imbastaro, T.; Riario-Sforza, G.; Di Giacomo, F.; Zuliani, G. Copper/zinc ratio and systemic oxidant load: Effect of aging and aging-related degenerative diseases. Free. Radic. Biol. Med. 1998, 25, 676–681. [Google Scholar] [CrossRef]
- Kawahara, M.; Tanaka, K.I.; Kato-Negishi, M. Crosstalk of copper and zinc in the pathogenesis of vascular dementia. J. Clin. Biochem. Nutr. 2022, 71, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Zhu, H. Serum Copper and Zinc Levels and Risk of Hepatocellular Carcinoma: A 1: 1 Matched Case-Control Study. Curr. Dev. Nutr. 2020, 4, 1397. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, C.; Yan, W.; Guo, S.; Liu, B. Five serum trace elements associated with risk of cardia and noncardia gastric cancer in a matched case–control study. Cancer Manag. Res. 2020, 12, 4441. [Google Scholar] [CrossRef] [PubMed]
- Emokpae, M.A.; Fatimehin, E.B.; Obazelu, P.A. Serum levels of copper, zinc and disease severity scores in sickle cell disease patients in Benin City, Nigeria. Afr. Health Sci. 2019, 19, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Pourfallah, F.; Javadian, S.; Zamani, Z.; Saghiri, R.; Sadeghi, S.; Zarea, B.; Faiaz, S.; Mirkhani, F.; Fatemi, N. Evaluation of serum levels of zinc, copper, iron, and zinc/copper ratio in cutaneous leishmaniasis. Iran. J. Arthropod-Borne Dis. 2009, 3, 7. [Google Scholar]
- Tuncay, M.E.; Neselioglu, S.; Kalkan, E.A.; Inan, O.; Akkus, M.S.; Ates, I.; Erel, O. Modified Proline Metabolism and Prolidase Enzyme in COVID-19. Lab. Med. 2022, 53, 453–458. [Google Scholar] [CrossRef]
- Farr, B.M.; Conner, E.M.; Betts, R.F.; Oleske, J.; Minnefor, A.; Gwaltney, J.M., Jr. Two randomized controlled trials of zinc gluconate lozenge therapy of experimentally induced rhinovirus colds. Antimicrob Agents Chemother 1987, 31, 1183–1187. [Google Scholar] [CrossRef] [Green Version]
- Mossad, S.B.; Macknin, M.L.; Medendorp, S.V.; Mason, P. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann. Intern. Med. 1996, 125, 81–88. [Google Scholar] [CrossRef]
- Kurugöl, Z.; Akilli, M.; Bayram, N.; Koturoglu, G. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta Paediatr. 2006, 95, 1175–1181. [Google Scholar] [CrossRef]
- Kahmann, L.; Uciechowski, P.; Warmuth, S.; Plümäkers, B.; Gressner, A.M.; Malavolta, M.; Mocchegiani, E.; Rink, L. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 2008, 11, 227–237. [Google Scholar] [CrossRef]
- Zazzo, J.F.; Rouveix, B.; Rajagopalon, P.; Levacher, M.; Girard, P.M. Effect of zinc on the immune status of zinc-depleted AIDS related complex patients. Clin. Nutr. 1989, 8, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Bobat, R.; Coovadia, H.; Stephen, C.; Naidoo, K.L.; McKerrow, N.; Black, R.E.; Moss, W.J. Safety and efficacy of zinc supplementation for children with HIV-1 infection in South Africa: A randomised double-blind placebo-controlled trial. Lancet 2005, 366, 1862–1867. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Lewin, S.R.; Wightman, F.; Lee, M.; Ravindran, T.S.; Paton, N.I. A randomised controlled trial of oral zinc on the immune response to tuberculosis in HIV-infected patients. Int. J. Tuberc. Lung Dis. 2005, 9, 1378–1384. [Google Scholar] [PubMed]
- Fawzi, W.W.; Villamor, E.; Msamanga, G.I.; Antelman, G.; Aboud, S.; Urassa, W.; Hunter, D. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV-1-infected women in Tanzania. Am. J. Clin. Nutr. 2005, 81, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Villamor, E.; Aboud, S.; Koulinska, I.N.; Kupka, R.; Urassa, W.; Chaplin, B.; Msamanga, G.; Fawzi, W.W. Zinc supplementation to HIV-1-infected pregnant women: Effects on maternal anthropometry, viral load, and early mother-to-child transmission. Eur. J. Clin. Nutr. 2006, 60, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Deloria-Knoll, M.; Steinhoff, M.; Semba, R.D.; Nelson, K.; Vlahov, D.; Meinert, C.L. Effect of zinc and vitamin A supplementation on antibody responses to a pneumococcal conjugate vaccine in HIV-positive injection drug users: A randomized trial. Vaccine 2006, 24, 1670–1679. [Google Scholar] [CrossRef]
- Baum, M.K.; Lai, S.; Sales, S.; Page, J.B.; Campa, A. Randomized, controlled clinical trial of zinc supplementation to prevent immunological failure in HIV-infected adults. Clin. Infect. Dis. 2010, 50, 1653–1660. [Google Scholar] [CrossRef]
- Asdamongkol, N.; Phanachet, P.; Sungkanuparph, S. Low plasma zinc levels and immunological responses to zinc supplementation in HIV-infected patients with immunological discordance after antiretroviral therapy. Jpn. J. Infect. Dis. 2013, 66, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Lodi, S.; Freiberg, M.; Gnatienko, N.; Blokhina, E.; Yaroslavtseva, T.; Krupitsky, E.; Murray, E.; Samet, J.H.; Cheng, D.M. Per-protocol analysis of the ZINC trial for HIV disease among alcohol users. Trials 2021, 22, 226. [Google Scholar] [CrossRef]
- Silva, M.; Montes, C.G.; Canals, A.; Mackenna, M.J.; Wolff, M. Role and effects of zinc supplementation in HIV-infected patients with immunovirological discordance: A randomized, double blind, case control study. PLoS ONE 2021, 16, e0244823. [Google Scholar] [CrossRef]
- Freiberg, M.S.; Cheng, D.M.; Gnatienko, N.; Blokhina, E.; Coleman, S.M.; Doyle, M.F.; Yaroslavtseva, T.; Bridden, C.; So-Armah, K.; Tracy, R.; et al. Effect of Zinc Supplementation vs Placebo on Mortality Risk and HIV Disease Progression Among HIV-Positive Adults with Heavy Alcohol Use: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e204330. [Google Scholar] [CrossRef]
- Dirajlal-Fargo, S.; Yu, J.; Kulkarni, M.; Sattar, A.; Funderburg, N.; Barkoukis, H.; McComsey, G.A. Brief Report: Zinc Supplementation and Inflammation in Treated HIV. J. Acquir. Immune. Defic. Syndr. 2019, 82, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lodha, R.; Shah, N.; Mohari, N.; Mukherjee, A.; Vajpayee, M.; Singh, R.; Singla, M.; Saini, S.; Bhatnagar, S.; Kabra, S.K. Immunologic effect of zinc supplementation in HIV-infected children receiving highly active antiretroviral therapy: A randomized, double-blind, placebo-controlled trial. J. Acquir. Immune. Defic. Syndr. 2014, 66, 386–392. [Google Scholar] [CrossRef]
- Takagi, H.; Nagamine, T.; Abe, T.; Takayama, H.; Sato, K.; Otsuka, T.; Kakizaki, S.; Hashimoto, Y.; Matsumoto, T.; Kojima, A.; et al. Zinc supplementation enhances the response to interferon therapy in patients with chronic hepatitis C. J. Viral Hepat. 2001, 8, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Takagi, H.; Takayama, H.; Kojima, A.; Kakizaki, S.; Mori, M.; Nakajima, K. Preliminary study of combination therapy with interferon-alpha and zinc in chronic hepatitis C patients with genotype 1b. Biol. Trace Elem. Res. 2000, 75, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.S.; Guo, C.H.; Hsu, G.S.; Chiou, Y.L.; Yeh, M.S.; Yaun, S.R. The effect of zinc supplementation on the treatment of chronic hepatitis C patients with interferon and ribavirin. Clin. Biochem. 2005, 38, 614–620. [Google Scholar] [CrossRef]
- Kim, K.I.; Kim, S.R.; Sasase, N.; Akimoto, Y.; Shikata, M.; Ohtani, A.; Hirooka, T.; Tanaka, K. Blood cell, liver function, and response changes by PEG-interferon-alpha2b plus ribavirin with polaprezinc therapy in patients with chronic hepatitis C. Hepatol. Int. 2008, 2, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Takagi, H.; Sohara, N.; Kanda, D.; Kakizaki, S.; Sato, K.; Mori, M. Triple therapy of interferon and ribavirin with zinc supplementation for patients with chronic hepatitis C: A randomized controlled clinical trial. World J. Gastroenterol. WJG 2006, 12, 1265. [Google Scholar] [CrossRef] [Green Version]
- Himoto, T.; Hosomi, N.; Nakai, S.; Deguchi, A.; Kinekawa, F.; Matsuki, M.; Yachida, M.; Masaki, T.; Kurokochi, K.; Watanabe, S. Efficacy of zinc administration in patients with hepatitis C virus-related chronic liver disease. Scand. J. Gastroenterol. 2007, 42, 1078–1087. [Google Scholar] [CrossRef]
- Matsumura, H.; Nirei, K.; Nakamura, H.; Arakawa, Y.; Higuchi, T.; Hayashi, J.; Yamagami, H.; Matsuoka, S.; Ogawa, M.; Nakajima, N. Zinc supplementation therapy improves the outcome of patients with chronic hepatitis C. J. Clin. Biochem. Nutr. 2012, 51, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Murakami, Y.; Koyabu, T.; Kawashima, A.; Kakibuchi, N.; Kawakami, T.; Takaguchi, K.; Kita, K.; Okita, M. Zinc supplementation prevents the increase of transaminase in chronic hepatitis C patients during combination therapy with pegylated interferon α-2b and ribavirin. J. Nutr. Sci. Vitaminol. 2007, 53, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rerksuppaphol, S.; Rerksuppaphol, L. A randomized controlled trial of zinc supplementation as adjuvant therapy for dengue viral infection in Thai children. Int. J. Prev. Med. 2018, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, P.M.; Ahuja, T.; Petrilli, C.; Rajagopalan, H.; Jones, S.; Rahimian, J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020, 69, 1228. [Google Scholar] [CrossRef]
- Frontera, J.A.; Rahimian, J.O.; Yaghi, S.; Liu, M.; Lewis, A.; Mainali, S.; Huang, J.; Scher, E.; Wisniewski, T.; Troxel, A.B. Treatment with Zinc is Associated with Reduced In-Hospital Mortality among COVID-19 Patients: A Multi-Center Cohort Study. Res. Sq. 2020, rs-3–rs-94509. [Google Scholar] [CrossRef]
- Derwand, R.; Scholz, M.; Zelenko, V. COVID-19 outpatients: Early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: A retrospective case series study. Int. J. Antimicrob. Agents 2020, 56, 106214. [Google Scholar] [CrossRef] [PubMed]
- Finzi, E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int. J. Infect. Dis. 2020, 99, 307–309. [Google Scholar] [CrossRef]
- Patel, O.; Chinni, V.; El-Khoury, J.; Perera, M.; Neto, A.S.; McDonald, C.; See, E.; Jones, D.; Bolton, D.; Bellomo, R. A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients. J. Med. Virol. 2021, 93, 3261–3267. [Google Scholar] [CrossRef]
- Abd-Elsalam, S.; Soliman, S.; Esmail, E.S.; Khalaf, M.; Mostafa, E.F.; Medhat, M.A.; Ahmed, O.A.; El Ghafar, M.S.A.; Alboraie, M.; Hassany, S.M. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: A randomized, multicenter trial. Biol. Trace Elem. Res. 2021, 199, 3642–3646. [Google Scholar] [CrossRef]
- Stambouli, N.; Driss, A.; Gargouri, F.; Bahrini, K.; Arfaoui, B.; Abid, R.; Taamallah, K.; Hannachi, S.; Boughariou, S.; Rebai, A. COVID-19 prophylaxis with doxycycline and zinc in health care workers: A prospective, randomized, double-blind clinical trial. Int. J. Infect. Dis. 2022, 122, 553–558. [Google Scholar] [CrossRef]
- Malik, A.; Taneja, D.K.; Devasenapathy, N.; Rajeshwari, K. Zinc supplementation for prevention of acute respiratory infections in infants: A randomized controlled trial. Indian Pediatr. 2014, 51, 780–784. [Google Scholar] [CrossRef]
- Shah, U.H.; Abu-Shaheen, A.K.; Malik, M.A.; Alam, S.; Riaz, M.; Al-Tannir, M.A. The efficacy of zinc supplementation in young children with acute lower respiratory infections: A randomized double-blind controlled trial. Clin. Nutr. 2013, 32, 193–199. [Google Scholar] [CrossRef]
- Martinez-Estevez, N.; Alvarez-Guevara, A.; Rodriguez-Martinez, C. Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: A 12-month randomised controlled trial. Allergol. Et Immunopathol. 2016, 44, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Sazawal, S.; Black, R.E.; Jalla, S.; Mazumdar, S.; Sinha, A.; Bhan, M.K. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: A double-blind, controlled trial. Pediatrics 1998, 102, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mahalanabis, D.; Lahiri, M.; Paul, D.; Gupta, S.; Gupta, A.; Wahed, M.A.; Khaled, M.A. Randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am. J. Clin. Nutr. 2004, 79, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Karyadi, E.; West, C.E.; Schultink, W.; Nelwan, R.H.; Gross, R.; Amin, Z.; Dolmans, W.M.; Schlebusch, H.; van der Meer, J.W. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: Effects on clinical response and nutritional status. Am. J. Clin. Nutr. 2002, 75, 720–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baruah, A.; Saikia, H. Effect of Zinc Supplementation in Children with Severe Pneumonia: A Randomised Controlled Study. J. Clin. Diagn. Res. 2018, 12, 8–11. [Google Scholar] [CrossRef]
- Basnet, S.; Shrestha, P.S.; Sharma, A.; Mathisen, M.; Prasai, R.; Bhandari, N.; Adhikari, R.K.; Sommerfelt, H.; Valentiner-Branth, P.; Strand, T.A. A randomized controlled trial of zinc as adjuvant therapy for severe pneumonia in young children. Pediatrics 2012, 129, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Bose, A.; Coles, C.L.; John, H.; Moses, P.; Raghupathy, P.; Kirubakaran, C.; Black, R.E.; Brooks, W.A.; Santosham, M. Efficacy of zinc in the treatment of severe pneumonia in hospitalized children <2 y old. Am. J. Clin. Nutr. 2006, 83, 1089–1096. [Google Scholar]
- Fataki, M.R.; Kisenge, R.R.; Sudfeld, C.R.; Aboud, S.; Okuma, J.; Mehta, S.; Spiegelman, D.; Fawzi, W.W. Effect of zinc supplementation on duration of hospitalization in Tanzanian children presenting with acute pneumonia. J. Trop. Pediatr. 2014, 60, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Howie, S.; Bottomley, C.; Chimah, O.; Ideh, R.; Ebruke, B.; Okomo, U.; Onyeama, C.; Donkor, S.; Rodrigues, O.; Tapgun, M. Zinc as an adjunct therapy in the management of severe pneumonia among Gambian children: Randomized controlled trial. J. Glob. Health 2018, 8, 010418. [Google Scholar] [CrossRef]
- Sempértegui, F.; Estrella, B.; Rodríguez, O.; Gómez, D.; Cabezas, M.; Salgado, G.; Sabin, L.L.; Hamer, D.H. Zinc as an adjunct to the treatment of severe pneumonia in Ecuadorian children: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 497–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, G.S.; Dutta, A.K.; Shah, D.; Mishra, O.P. Role of zinc in severe pneumonia: A randomized double bind placebo controlled study. Ital. J. Pediatr. 2012, 38, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, M.G.; Ndeezi, G.; Mboijana, C.K.; Kiguli, S.; Bimenya, G.S.; Nankabirwa, V.; Tumwine, J.K. Zinc adjunct therapy reduces case fatality in severe childhood pneumonia: A randomized double blind placebo-controlled trial. BMC Med. 2012, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Shehzad, N.; Anwar, M.I.; Muqaddas, T. Zinc supplementation for the treatment of severe pneumonia in hospitalized children: A randomized controlled trial. Sudan. J. Paediatr. 2015, 15, 37. [Google Scholar] [PubMed]
- Qasemzadeh, M.J.; Fathi, M.; Tashvighi, M.; Gharehbeglou, M.; Yadollah-Damavandi, S.; Parsa, Y.; Rahimi, E. The effect of adjuvant zinc therapy on recovery from pneumonia in hospitalized children: A double-blind randomized controlled trial. Scientifica 2014, 2014, 694193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, N.; Bumb, R.; Mangal, H. Oral zinc in recurrent Erythema Nodosum Leprosum reaction. Lepr. India 1983, 55, 547–552. [Google Scholar]
- Pakasi, T.A.; Karyadi, E.; Suratih, N.M.; Salean, M.; Darmawidjaja, N.; Bor, H.; van der Velden, K.; Dolmans, W.M.; van der Meer, J.W. Zinc and vitamin A supplementation fails to reduce sputum conversion time in severely malnourished pulmonary tuberculosis patients in Indonesia. Nutr. J. 2010, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Zolfaghari, B.; Ghanbari, M.; Musavi, H.; Bavandpour Baghshahi, P.; Taghikhani, M.; Pourfallah, F. Investigation of Zinc Supplement Impact on the Serum Biochemical Parameters in Pulmonary Tuberculosis: A Double Blinded Placebo Control Trial. Rep. Biochem. Mol. Biol. 2021, 10, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.N.; Sarker, S.A.; Wahed, M.A.; Khatun, M.; Rahaman, M.M. Enteric protein loss and intestinal permeability changes in children during acute shigellosis and after recovery: Effect of zinc supplementation. Gut 1994, 35, 1707–1711. [Google Scholar] [CrossRef] [Green Version]
- Raqib, R.; Roy, S.K.; Rahman, M.J.; Azim, T.; Ameer, S.S.; Chisti, J.; Andersson, J. Effect of zinc supplementation on immune and inflammatory responses in pediatric patients with shigellosis. Am. J. Clin. Nutr. 2004, 79, 444–450. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.J.; Sarker, P.; Roy, S.K.; Ahmad, S.M.; Chisti, J.; Azim, T.; Mathan, M.; Sack, D.; Andersson, J.; Raqib, R. Effects of zinc supplementation as adjunct therapy on the systemic immune responses in shigellosis. Am. J. Clin. Nutr. 2005, 81, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.K.; Raqib, R.; Khatun, W.; Azim, T.; Chowdhury, R.; Fuchs, G.J.; Sack, D.A. Zinc supplementation in the management of shigellosis in malnourished children in Bangladesh. Eur. J. Clin. Nutr. 2008, 62, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Kashimura, H.; Suzuki, K.; Hassan, M.; Ikezawa, K.; Sawahata, T.; Watanabe, T.; Nakahara, A.; Mutoh, H.; Tanaka, N. Polaprezinc, a mucosal protective agent, in combination with lansoprazole, amoxycillin and clarithromycin increases the cure rate of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1999, 13, 483–487. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, Y.S.; Shouman, A.E.; Hakim, S.A.; El Gendy, Y.G.A.; Wahdan, M.M.M. Effect of Zinc Supplementation in Children Less than 5 Years on Diarrhea Attacks: A Randomized Controlled Trial. Glob. Pediatr. Health 2022, 9, 2333794X221099266. [Google Scholar] [CrossRef] [PubMed]
- Hoque, K.M.; Binder, H.J. Zinc in the treatment of acute diarrhea: Current status and assessment. Gastroenterology 2006, 130, 2201–2205. [Google Scholar] [CrossRef]
- Lamberti, L.M.; Fischer Walker, C.L.; Chan, K.Y.; Jian, W.-Y.; Black, R.E. Oral zinc supplementation for the treatment of acute diarrhea in children: A systematic review and meta-analysis. Nutrients 2013, 5, 4715–4740. [Google Scholar] [CrossRef]
- Laghari, G.S.; Hussain, Z.; Shahzad, H. Effect of zinc supplementation on the frequency and consistency of stool in children with acute diarrhea. Cureus 2019, 11, e4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadipour, S.; Mohsenzadeh, A.; Alimadadi, H.; Salehnia, M.; Fallahi, A. Treating viral diarrhea in children by probiotic and zinc supplements. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 162–170. [Google Scholar] [CrossRef]
- Barffour, M.A.; Hinnouho, G.M.; Wessells, K.R.; Kounnavong, S.; Ratsavong, K.; Sitthideth, D.; Bounheuang, B.; Sengnam, K.; Chanhthavong, B.; Arnold, C.D.; et al. Effects of therapeutic zinc supplementation for diarrhea and two preventive zinc supplementation regimens on the incidence and duration of diarrhea and acute respiratory tract infections in rural Laotian children: A randomized controlled trial. J. Glob. Health 2020, 10, 010424. [Google Scholar] [CrossRef] [PubMed]
- Negruț, N. Zinc therapy for acute diarrhea in children under three years of age. BMC Infect. Dis. 2014, 14, P80. [Google Scholar] [CrossRef] [Green Version]
- Zeba, A.N.; Sorgho, H.; Rouamba, N.; Zongo, I.; Rouamba, J.; Guiguemdé, R.T.; Hamer, D.H.; Mokhtar, N.; Ouedraogo, J.B. Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: A randomized double blind trial. Nutr. J. 2008, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, A.H.; Genton, B.; Baisor, M.; Paino, J.; Tamja, S.; Adiguma, T.; Wu, L.; Rare, L.; Bannon, D.; Tielsch, J.M.; et al. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: A randomized trial in preschool children in Papua New Guinea. Am. J. Trop. Med. Hyg. 2000, 62, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, C.J.; Evans, P.H.; Dardenne, M.; Prentice, A.; Lunn, P.G.; Northrop-Clewes, C.A.; Hoare, S.; Cole, T.J.; Horan, S.J.; Longman, S.C.; et al. A trial of zinc supplementation in young rural Gambian children. Br. J. Nutr. 1993, 69, 243–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, O.; Becher, H.; van Zweeden, A.B.; Ye, Y.; Diallo, D.A.; Konate, A.T.; Gbangou, A.; Kouyate, B.; Garenne, M. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: Randomised double blind placebo controlled trial. BMJ 2001, 322, 1567. [Google Scholar] [CrossRef] [Green Version]
- Zinc Against Plasmodium Study Group. Effect of zinc on the treatment of Plasmodium falciparum malaria in children: A randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Richard, S.A.; Zavaleta, N.; Caulfield, L.E.; Black, R.E.; Witzig, R.S.; Shankar, A.H. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 75, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Darling, A.M.; Mugusi, F.M.; Etheredge, A.J.; Gunaratna, N.S.; Abioye, A.I.; Aboud, S.; Duggan, C.; Mongi, R.; Spiegelman, D.; Roberts, D.; et al. Vitamin A and Zinc Supplementation among Pregnant Women to Prevent Placental Malaria: A Randomized, Double-Blind, Placebo-Controlled Trial in Tanzania. Am. J. Trop. Med. Hyg. 2017, 96, 826–834. [Google Scholar] [CrossRef] [Green Version]
- Somé, J.W.; Abbeddou, S.; Yakes Jimenez, E.; Hess, S.Y.; Ouédraogo, Z.P.; Guissou, R.M.; Vosti, S.A.; Ouédraogo, J.B.; Brown, K.H. Effect of zinc added to a daily small-quantity lipid-based nutrient supplement on diarrhoea, malaria, fever and respiratory infections in young children in rural Burkina Faso: A cluster-randomised trial. BMJ Open 2015, 5, e007828. [Google Scholar] [CrossRef]
- Owusu-Agyei, S.; Newton, S.; Mahama, E.; Febir, L.G.; Ali, M.; Adjei, K.; Tchum, K.; Alhassan, L.; Moleah, T.; Tanumihardjo, S.A. Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutr. J. 2013, 12, 131. [Google Scholar] [CrossRef] [Green Version]
- Veenemans, J.; Milligan, P.; Prentice, A.M.; Schouten, L.R.; Inja, N.; van der Heijden, A.C.; de Boer, L.C.; Jansen, E.J.; Koopmans, A.E.; Enthoven, W.T.; et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: A randomised trial. PLoS Med. 2011, 8, e1001125. [Google Scholar] [CrossRef] [Green Version]
- Sharquie, K.E.; Najim, R.A.; Farjou, I.B.; Al-Timimi, D.J. Oral zinc sulphate in the treatment of acute cutaneous leishmaniasis. Clin. Exp. Dermatol. 2001, 26, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Rivero, M.; Rojas, E.; Verduguez-Orellana, A.; Pardo, H.; Torrico, M.C.; Cloetens, L.; Akesson, B.; Sejas, E. Nutritional status in patients with cutaneous leishmaniasis and a study of the effects of zinc supplementation together with antimony treatment. Food Nutr. Res. 2014, 58, 23353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farajzadeh, S.; Ahmadi, R.; Mohammadi, S.; Pardakhty, A.; Khalili, M.; Aflatoonian, M. Evaluation of the efficacy of intralesional Glucantime plus niosomal zinc sulphate in comparison with intralesional Glucantime plus cryotherapy in the treatment of acute cutaneous leishmaniasis, a randomized clinical trial. J. Parasit. Dis. 2018, 42, 616–620. [Google Scholar] [CrossRef]
- Carbone, D.C.B.; Zanoni, L.Z.G.; Cônsolo, F.Z.; Sanches, S.C.; Reis, V.Q.D.; Muller, K.T.C.; Carvalho, C.M.E.; Silva, M.C. Potential role of zinc in the visceromegaly regression and recovery of hematological parameters during treatment of visceral leishmaniasis in children from an endemic area. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e50. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Karimi, G.; Tafaghodi, M.; Raftari, S.; Nahidi, Y. Comparison of intralesional two percent zinc sulfate and glucantime injection in treatment of acute cutaneous leishmaniasis. Indian J. Dermatol. 2012, 57, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Iraji, F.; Vali, A.; Asilian, A.; Shahtalebi, M.A.; Momeni, A.Z. Comparison of intralesionally injected zinc sulfate with meglumine antimoniate in the treatment of acute cutaneous leishmaniasis. Dermatology 2004, 209, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhu, L.; Zhu, T.; Jian, Y.; Ding, Y.; Zhou, M.; Feng, X. Zinc supplementation reduces Candida infections in pediatric intensive care unit: A randomized placebo-controlled clinical trial. J. Clin. Biochem. Nutr. 2019, 64, 170–173. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C. Nonresolving inflammation redux. Immunity 2022, 55, 592–605. [Google Scholar] [CrossRef]
- Gudernatsch, V.; Stefańczyk, S.A.; Mirakaj, V. Novel Resolution Mediators of Severe Systemic Inflammation. ImmunoTargets Ther. 2020, 9, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Hasan, R.; Rink, L.; Haase, H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 2013, 19, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Rink, L.; Haase, H. Chelation of Free Zn2+ Impairs Chemotaxis, Phagocytosis, Oxidative Burst, Degranulation, and Cytokine Production by Neutrophil Granulocytes. Biol. Trace Elem. Res. 2016, 171, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Businco, L.; Menghi, A.M.; Rossi, P.; D’Amelio, R.; Galli, E. Zinc-dependent chemotactic defect in an infant with acrodermatitis. Arch. Dis. Child. 1980, 55, 966–968. [Google Scholar] [CrossRef]
- Bellomo, E.; Birla Singh, K.; Massarotti, A.; Hogstrand, C.; Maret, W. The metal face of protein tyrosine phosphatase 1B. Coord. Chem. Rev. 2016, 327–328, 70–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustelin, T.; Vang, T.; Bottini, N. Protein tyrosine phosphatases and the immune response. Nat. Rev. Immunol. 2005, 5, 43–57. [Google Scholar] [CrossRef]
- Hasegawa, H.; Suzuki, K.; Suzuki, K.; Nakaji, S.; Sugawara, K. Effects of zinc on the reactive oxygen species generating capacity of human neutrophils and on the serum opsonic activity in vitro. Luminescence 2000, 15, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Sheline, C.T.; Behrens, M.M.; Choi, D.W. Zinc-induced cortical neuronal death: Contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J. Neurosci. 2000, 20, 3139–3146. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. MedComm 2022, 3, e162. [Google Scholar] [CrossRef]
- Wessels, I.; Jansen, J.; Rink, L.; Uciechowski, P. Immunosenescence of polymorphonuclear neutrophils. ScientificWorldJournal 2010, 10, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Haase, H.; Engelhardt, G.; Rink, L.; Uciechowski, P. Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms. J. Nutr. Biochem. 2013, 24, 289–297. [Google Scholar] [CrossRef]
- Wessels, I.; Cousins, R.J. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G768–G778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besecker, B.Y.; Exline, M.C.; Hollyfield, J.; Phillips, G.; Disilvestro, R.A.; Wewers, M.D.; Knoell, D.L. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am. J. Clin. Nutr. 2011, 93, 1356–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Samman, S. Zinc and regulation of inflammatory cytokines: Implications for cardiometabolic disease. Nutrients 2012, 4, 676–694. [Google Scholar] [CrossRef] [Green Version]
- Haase, H.; Ober-Blöbaum, J.L.; Engelhardt, G.; Hebel, S.; Heit, A.; Heine, H.; Rink, L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J. Immunol. 2008, 181, 6491–6502. [Google Scholar] [CrossRef]
- Cho, J.; Tsichlis, P.N. Phosphorylation at Thr-290 regulates Tpl2 binding to NF-kappaB1/p105 and Tpl2 activation and degradation by lipopolysaccharide. Proc. Natl. Acad. Sci. USA 2005, 102, 2350–2355. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Petris, M.J.; Peck, S.C. Separation of zinc-dependent and zinc-independent events during early LPS-stimulated TLR4 signaling in macrophage cells. FEBS Lett. 2014, 588, 2928–2935. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Sarkar, F.H. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition 2011, 27, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Kucuk, O.; Sarkar, F.H. Antioxidant effect of zinc in humans. Free. Radic. Biol. Med. 2004, 37, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.L.; Turer, E.E.; Lee, E.G.; Ahmad, R.C.; Wheeler, M.T.; Tsui, C.; Hurley, P.; Chien, M.; Chai, S.; Hitotsumatsu, O.; et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 2004, 5, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- von Bülow, V.; Dubben, S.; Engelhardt, G.; Hebel, S.; Plümäkers, B.; Heine, H.; Rink, L.; Haase, H. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J. Immunol. 2007, 179, 4180–4186. [Google Scholar] [CrossRef] [Green Version]
- Brieger, A.; Rink, L.; Haase, H. Differential regulation of TLR-dependent MyD88 and TRIF signaling pathways by free zinc ions. J. Immunol. 2013, 191, 1808–1817. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.P.; Rinaldi, N.A.; Ho, E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol. Nutr. Food Res. 2015, 59, 991–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Yang, Z.; Wang, J.; Yu, J.; Guo, J.; Liu, S.; Qian, C.; Song, L.; Wu, Y.; Cheng, J. Zinc Chloride Transiently Maintains Mouse Embryonic Stem Cell Pluripotency by Activating Stat3 Signaling. PLoS ONE 2016, 11, e0148994. [Google Scholar] [CrossRef] [Green Version]
- Fraker, P.; King, L. Changes in regulation of lymphopoiesis and myelopoiesis in the zinc-deficient mouse. Nutr. Rev. 1998, 56, S65–S69. [Google Scholar] [CrossRef]
- Supasai, S.; Aimo, L.; Adamo, A.M.; Mackenzie, G.G.; Oteiza, P.I. Zinc deficiency affects the STAT1/3 signaling pathways in part through redox-mediated mechanisms. Redox Biol. 2017, 11, 469–481. [Google Scholar] [CrossRef]
- Gruber, K.; Maywald, M.; Rosenkranz, E.; Haase, H.; Plumakers, B.; Rink, L. Zinc deficiency adversely influences interleukin-4 and interleukin-6 signaling. J. Biol. Regul. Homeost. Agents 2013, 27, 661–671. [Google Scholar]
- Lue, C.; Kiyono, H.; McGhee, J.R.; Fujihashi, K.; Kishimoto, T.; Hirano, T.; Mestecky, J. Recombinant human interleukin 6 (rhIL-6) promotes the terminal differentiation of in vivo-activated human B cells into antibody-secreting cells. Cell. Immunol. 1991, 132, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, C.; Fukada, T.; Kanamoto, M.; Ohashi, W.; Hojyo, S.; Atsumi, T.; Ueda, N.; Azuma, I.; Hirota, H.; Murakami, M.; et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int. Immunol. 2010, 22, 375–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, C.E.; Kim, C.; Weyand, C.M.; Goronzy, J.J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 2020, 145, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.W.; Prasad, A.S.; Kaplan, J.; Fitzgerald, J.T.; Brewer, G.J. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 1997, 272, E1002–E1007. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, B.; Choi, Y.H.; Hwang, Y.; Kim, D.H.; Cho, S.; Hong, S.J.; Lee, W.W. Inhibition of interleukin-1β-mediated interleukin-1 receptor-associated kinase 4 phosphorylation by zinc leads to repression of memory T helper type 17 response in humans. Immunology 2015, 146, 645–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, M.M.; Subramanian Vignesh, K.; Landero Figueroa, J.A.; Caruso, J.A.; Deepe, G.S., Jr. Zinc Induces Dendritic Cell Tolerogenic Phenotype and Skews Regulatory T Cell-Th17 Balance. J. Immunol. 2016, 197, 1864–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faber, C.; Gabriel, P.; Ibs, K.H.; Rink, L. Zinc in pharmacological doses suppresses allogeneic reaction without affecting the antigenic response. Bone Marrow Transpl. 2004, 33, 1241–1246. [Google Scholar] [CrossRef]
- Schubert, C.; Guttek, K.; Grüngreiff, K.; Thielitz, A.; Bühling, F.; Reinhold, A.; Brocke, S.; Reinhold, D. Oral zinc aspartate treats experimental autoimmune encephalomyelitis. Biometals 2014, 27, 1249–1262. [Google Scholar] [CrossRef]
- Maywald, M.; Meurer, S.K.; Weiskirchen, R.; Rink, L. Zinc supplementation augments TGF-β1-dependent regulatory T cell induction. Mol. Nutr. Food Res. 2017, 61, 201600493. [Google Scholar] [CrossRef]
- Rosenkranz, E.; Metz, C.H.; Maywald, M.; Hilgers, R.D.; Weßels, I.; Senff, T.; Haase, H.; Jäger, M.; Ott, M.; Aspinall, R.; et al. Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol. Nutr. Food Res. 2016, 60, 661–671. [Google Scholar] [CrossRef]
- Maywald, M.; Rink, L. Zinc supplementation induces CD4(+)CD25(+)Foxp3(+) antigen-specific regulatory T cells and suppresses IFN-γ production by upregulation of Foxp3 and KLF-10 and downregulation of IRF-1. Eur. J. Nutr. 2017, 56, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Fragale, A.; Gabriele, L.; Stellacci, E.; Borghi, P.; Perrotti, E.; Ilari, R.; Lanciotti, A.; Remoli, A.L.; Venditti, M.; Belardelli, F.; et al. IFN regulatory factor-1 negatively regulates CD4+ CD25+ regulatory T cell differentiation by repressing Foxp3 expression. J. Immunol. 2008, 181, 1673–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Wara, A.K.; Icli, B.; Sun, X.; Packard, R.R.; Esen, F.; Stapleton, C.J.; Subramaniam, M.; Kretschmer, K.; Apostolou, I.; et al. Kruppel-like factor KLF10 targets transforming growth factor-beta1 to regulate CD4(+)CD25(-) T cells and T regulatory cells. J. Biol. Chem. 2009, 284, 24914–24924. [Google Scholar] [CrossRef] [Green Version]
- Cakman, I.; Rohwer, J.; Schütz, R.M.; Kirchner, H.; Rink, L. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech. Ageing Dev. 1996, 87, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000, 182 (Suppl. S1), S62–S68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Godmere, M. Zinc modulates mRNA levels of cytokines. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1095–E1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, Y.J.; Hodes, R.J. T-cell development is regulated by the coordinated function of proximal and distal Lck promoters active at different developmental stages. Eur. J. Immunol. 2016, 46, 2401–2408. [Google Scholar] [CrossRef] [Green Version]
- Kaltenberg, J.; Plum, L.M.; Ober-Blöbaum, J.L.; Hönscheid, A.; Rink, L.; Haase, H. Zinc signals promote IL-2-dependent proliferation of T cells. Eur. J. Immunol. 2010, 40, 1496–1503. [Google Scholar] [CrossRef]
- Plum, L.M.; Brieger, A.; Engelhardt, G.; Hebel, S.; Nessel, A.; Arlt, M.; Kaltenberg, J.; Schwaneberg, U.; Huber, M.; Rink, L.; et al. PTEN-inhibition by zinc ions augments interleukin-2-mediated Akt phosphorylation. Metallomics 2014, 6, 1277–1287. [Google Scholar] [CrossRef]
- Pinna, K.; Kelley, D.S.; Taylor, P.C.; King, J.C. Immune functions are maintained in healthy men with low zinc intake. J. Nutr. 2002, 132, 2033–2036. [Google Scholar] [CrossRef] [Green Version]
- Kloubert, V.; Wessels, I.; Wolf, J.; Blaabjerg, K.; Janssens, V.; Hapala, J.; Wagner, W.; Rink, L. Zinc deficiency leads to reduced interleukin-2 production by active gene silencing due to enhanced CREMα expression in T cells. Clin. Nutr. 2021, 40, 3263–3278. [Google Scholar] [CrossRef] [PubMed]
- Baarz, B.R.; Laurentius, T.; Wolf, J.; Wessels, I.; Bollheimer, L.C.; Rink, L. Short-term zinc supplementation of zinc-deficient seniors counteracts CREMα—Mediated IL-2 suppression. Immun. Ageing 2022, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Southon, S.; Gee, J.M.; Johnson, I.T. Hexose transport and mucosal morphology in the small intestine of the zinc-deficient rat. Br. J. Nutr. 1984, 52, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Southon, S.; Gee, J.M.; Bayliss, C.E.; Wyatt, G.M.; Horn, N.; Johnson, I.T. Intestinal microflora, morphology and enzyme activity in zinc-deficient and Zn-supplemented rats. Br. J. Nutr. 1986, 55, 603–611. [Google Scholar] [CrossRef]
- Elmes, M.E.; Jones, J.G. Ultrastructural changes in the small intestine of zinc deficient rats. J. Pathol. 1980, 130, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Southon, S.; Livesey, G.; Gee, J.M.; Johnson, I.T. Intestinal cellular proliferation and protein synthesis in zinc-deficient rats. Br. J. Nutr. 1985, 53, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Finamore, A.; Massimi, M.; Conti Devirgiliis, L.; Mengheri, E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J. Nutr. 2008, 138, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Grandjean, C.J.; Antonson, D.L.; Vanderhoof, J.A. Effects of short-term isolated zinc deficiency on intestinal growth and activities of several brush border enzymes in weaning rats. Pediatr. Res. 1985, 19, 1333–1336. [Google Scholar] [CrossRef]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Koza, J.; Straubing, S.; Schwerdtle, T.; Haase, H. In Vitro Studies on Zinc Binding and Buffering by Intestinal Mucins. Int. J. Mol. Sci. 2018, 19, 2662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447s–463s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, A.; Sada, K.K.; Ketheeswaran, S.; Dubey, A.K.; Bhat, M.S. Role of Zinc in Mucosal Health and Disease: A Review of Physiological, Biochemical, and Molecular Processes. Cureus 2020, 12, e8197. [Google Scholar] [CrossRef]
- Canani, R.B.; Cirillo, P.; Buccigrossi, V.; Ruotolo, S.; Passariello, A.; De Luca, P.; Porcaro, F.; De Marco, G.; Guarino, A. Zinc inhibits cholera toxin-induced, but not Escherichia coli heat-stable enterotoxin-induced, ion secretion in human enterocytes. J. Infect. Dis. 2005, 191, 1072–1077. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Knoell, D.L. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L1132–L1141. [Google Scholar] [CrossRef] [Green Version]
- Roscioli, E.; Jersmann, H.P.; Lester, S.; Badiei, A.; Fon, A.; Zalewski, P.; Hodge, S. Zinc deficiency as a codeterminant for airway epithelial barrier dysfunction in an ex vivo model of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3503–3510. [Google Scholar] [CrossRef] [Green Version]
- Lansdown, A.B.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Agren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef]
- Meydani, S.N.; Barnett, J.B.; Dallal, G.E.; Fine, B.C.; Jacques, P.F.; Leka, L.S.; Hamer, D.H. Serum zinc and pneumonia in nursing home elderly. Am. J. Clin. Nutr. 2007, 86, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Lassi, Z.S.; Kurji, J.; Oliveira, C.S.; Moin, A.; Bhutta, Z.A. Zinc supplementation for the promotion of growth and prevention of infections in infants less than six months of age. Cochrane Database Syst. Rev. 2020, 4, Cd010205. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Ñamendys-Silva, S.A. Respiratory support for patients with COVID-19 infection. Lancet Respir. Med. 2020, 8, e18. [Google Scholar] [CrossRef]
- Mantovani, A.; Morrone, M.C.; Patrono, C.; Santoro, M.G.; Schiaffino, S.; Remuzzi, G.; Bussolati, G. Long Covid: Where we stand and challenges ahead. Cell Death Differ. 2022, 29, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, B.A.; Zhang, S.; Tamashiro, E.; Bhargave, G.; Palmer, J.N.; Cohen, N.A. Zinc increases ciliary beat frequency in a calcium-dependent manner. Am. J. Rhinol. Allergy 2010, 24, 6–10. [Google Scholar] [CrossRef]
- Darma, A.; Ranuh, I.G.M.R.G.; Merbawani, W.; Setyoningrum, R.A.; Hidajat, B.; Hidayati, S.N.; Endaryanto, A.; Sudarmo, S.M. Zinc supplementation effect on the bronchial cilia length, the number of cilia, and the number of intact bronchial cell in zinc deficiency rats. Indones. Biomed. J. 2020, 12, 78–84. [Google Scholar] [CrossRef]
- Lin, F.-c.; Young, H.A. Interferons: Success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Suara, R.O.; Crowe, J.E., Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 2004, 48, 783–790. [Google Scholar] [CrossRef]
- Che, Z.; Sun, J. Investigation on relationship between whole blood zinc and Fe elements with children pneumonia caused by respiratory syncytial virus. Int. J. Lab. Med. 2016, 2401–2402, 2405. [Google Scholar]
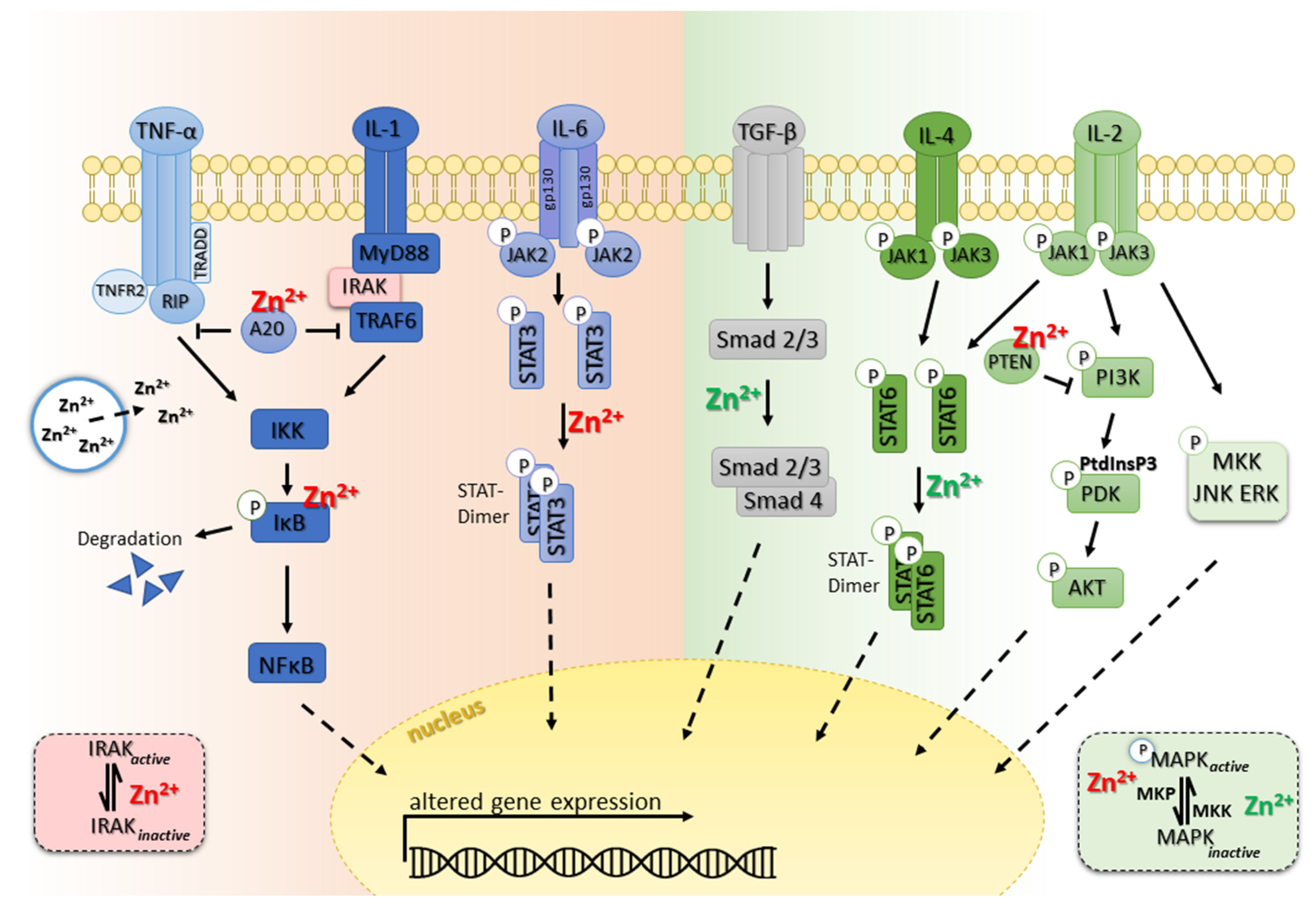
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maywald, M.; Rink, L. Zinc in Human Health and Infectious Diseases. Biomolecules 2022, 12, 1748. https://doi.org/10.3390/biom12121748
Maywald M, Rink L. Zinc in Human Health and Infectious Diseases. Biomolecules. 2022; 12(12):1748. https://doi.org/10.3390/biom12121748
Chicago/Turabian StyleMaywald, Martina, and Lothar Rink. 2022. "Zinc in Human Health and Infectious Diseases" Biomolecules 12, no. 12: 1748. https://doi.org/10.3390/biom12121748
APA StyleMaywald, M., & Rink, L. (2022). Zinc in Human Health and Infectious Diseases. Biomolecules, 12(12), 1748. https://doi.org/10.3390/biom12121748







