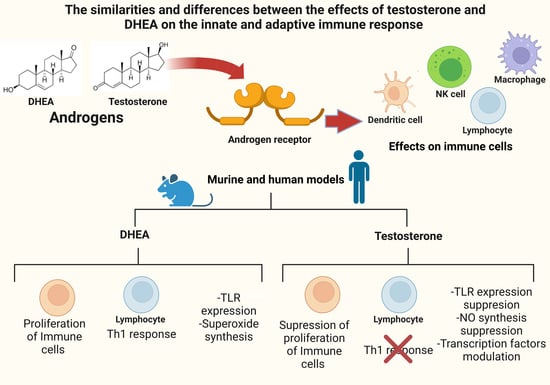
The Similarities and Differences between the Effects of Testosterone and DHEA on the Innate and Adaptive Immune Response
Abstract
:1. Introduction
2. Search Strategy and Screening Criteria
3. Synthesis of Androgens Testosterone, DHT, Androstenedione and DHEA and Its Main Properties
4. Mechanisms of Action of AR-Dependent Androgens (Canonical Pathway)
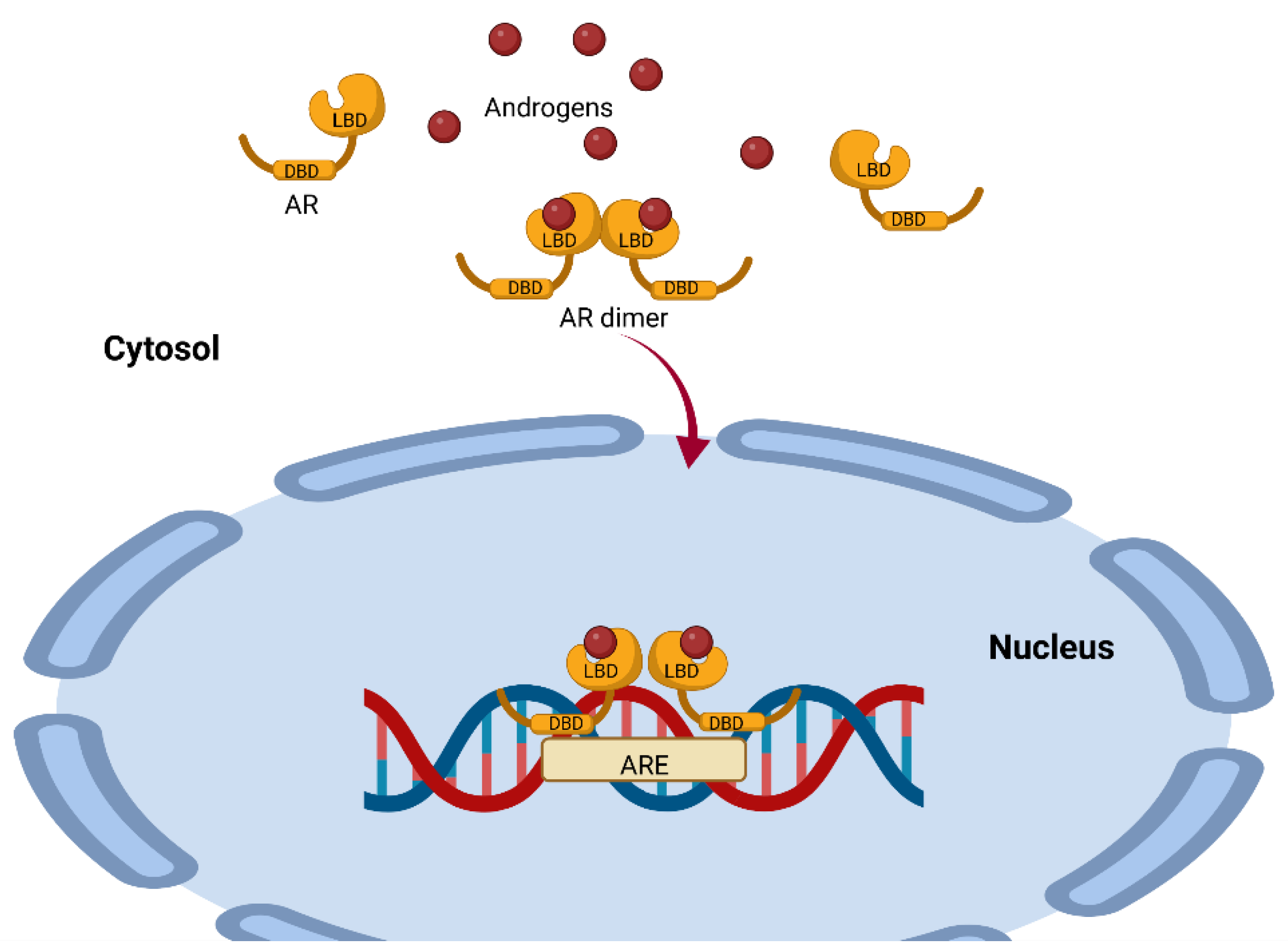
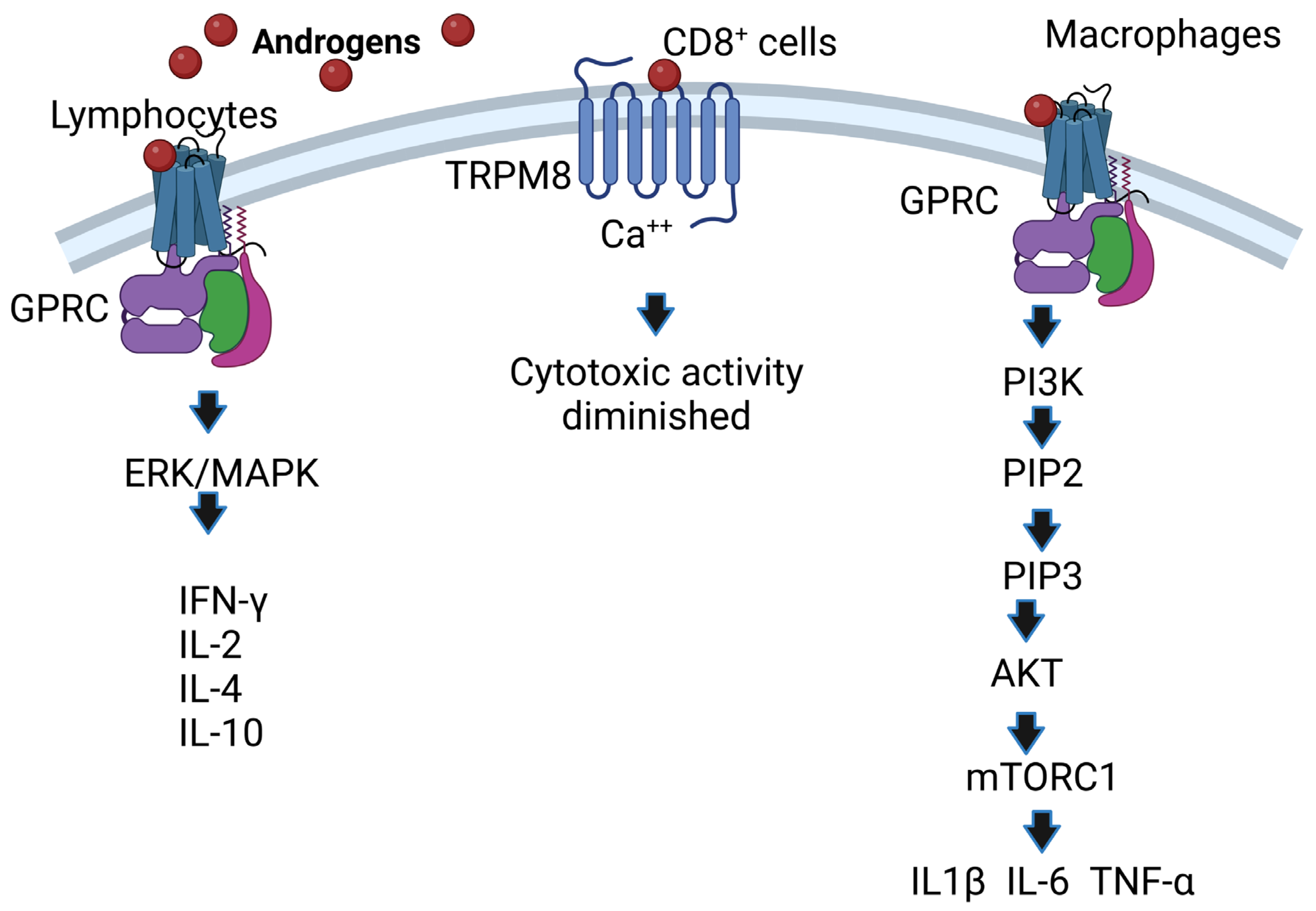
5. AR-Independent Androgen Mechanisms of Action (Noncanonical Pathway)
6. Effect of Androgens on Lymphoid Organs
7. Effect of Androgens on Toll-Like Receptors
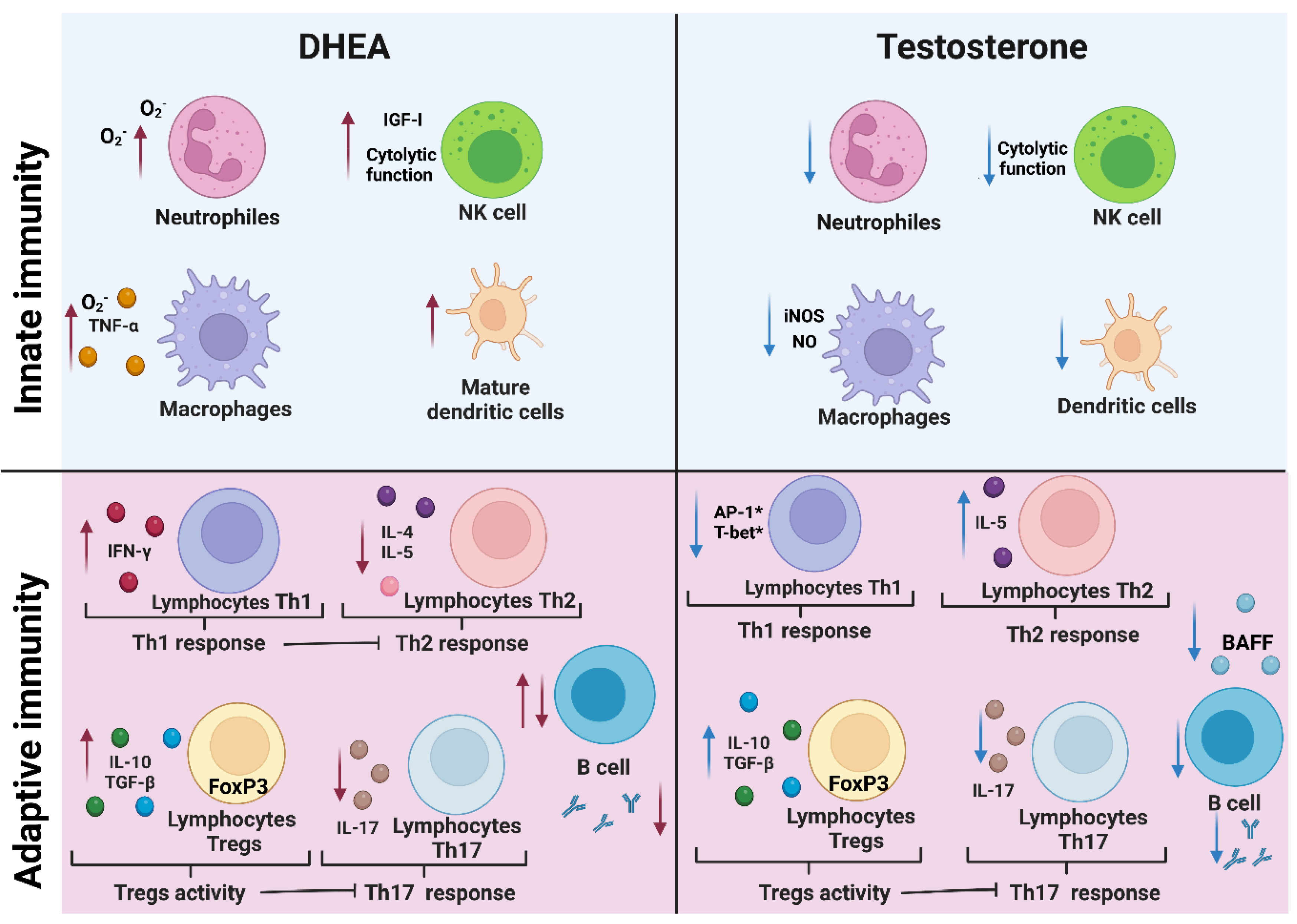
8. Androgens Affect Cells of the Innate Immune Response
8.1. Macrophages, Neutrophils, and NK Cells
8.2. Dendritic Cells
9. Effects of Androgens on Cells of the Adaptive Immune Response
9.1. Th1 and Th2 Lymphocytes
9.2. Th17 and Regulatory T Cells
9.3. Cytotoxic T-Lymphocytes
9.4. B Lymphocytes
10. Effect of Androgens on the Cytokines IFN-γ, TNF-α, IL-2, IL-10, TGF-B, IL-4, IL-5, IL-6, and IL-17
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benten, W.P.; Ulrich, P.; Kuhn-Velten, W.N.; Vohr, H.W.; Wunderlich, F. Testosterone-induced susceptibility to Plasmodium chabaudi malaria: Persistence after withdrawal of testosterone. J. Endocrinol. 1997, 153, 275–281. [Google Scholar] [CrossRef]
- Galindo-Sevilla, N.; Soto, N.; Mancilla, J.; Cerbulo, A.; Zambrano, E.; Chavira, R.; Huerto, J. Low serum levels of dehydroepiandrosterone and cortisol in human diffuse cutaneous leishmaniasis by Leishmania mexicana. Am. J. Trop. Med. Hyg. 2007, 76, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Filipin Mdel, V.; Caetano, L.C.; Brazao, V.; Santello, F.H.; Toldo, M.P.; do Prado, J.C., Jr. DHEA and testosterone therapies in Trypanosoma cruzi-infected rats are associated with thymic changes. Res. Vet. Sci. 2010, 89, 98–103. [Google Scholar] [CrossRef]
- Vom Steeg, L.G.; Klein, S.L. Sex Steroids Mediate Bidirectional Interactions between Hosts and Microbes. Horm. Behav. 2017, 88, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Lu, S.F.; McKenna, S.E.; Cologer-Clifford, A.; Nau, E.A.; Simon, N.G. Androgen receptor in mouse brain: Sex differences and similarities in autoregulation. Endocrinology 1998, 139, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Wong, K.A.; Baldwin, H.L.; Sorkin, J.D.; Johnson, M.L.; Bhasin, S.; Harman, S.M.; Blackman, M.R. Dehydroepiandrosterone secretion in healthy older men and women: Effects of testosterone and growth hormone administration in older men. J. Clin. Endocrinol. Metab. 2006, 91, 4445–4452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.L.; Bakhru, P.; Conley, B.; Nelson, J.S.; Free, M.; Martin, A.; Starmer, J.; Wilson, E.M.; Su, M.A. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat. Commun. 2016, 7, 11350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantopoulos, G.; Tchen, T.T. Cleavage of cholesterol side chain by adrenal cortex. I. Cofactor requirement and product of clevage. J. Biol. Chem. 1961, 236, 65–67. [Google Scholar] [CrossRef]
- Simpson, E.R.; Boyd, G.S. The cholesterol side-chain cleavage system of bovine adrenal cortex. Eur. J. Biochem. 1967, 2, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Adamkiewicz, M.; Zgliczynski, S.; Sfowinska-Srzednicka, J.; Jeske, W.; Rabijewski, M.; Pietrzyk, E.; Srzednicki, M.; Sadowski, Z. The relationship between plasma androgens (dehydroepiandrosterone sulfate and testosterone), insulin, coagulation and fibrinolytic factors in men with coronary arteriosclerosis. Aging Male 1998, 1, 270–279. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [PubMed] [Green Version]
- Fragkaki, A.G.; Angelis, Y.S.; Koupparis, M.; Tsantili-Kakoulidou, A.; Kokotos, G.; Georgakopoulos, C. Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Applied modifications in the steroidal structure. Steroids 2009, 74, 172–197. [Google Scholar] [CrossRef] [PubMed]
- Gubbels Bupp, M.R.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.X.; Lane, M.V.; Kemppainen, J.A.; French, F.S.; Wilson, E.M. Specificity of ligand-dependent androgen receptor stabilization: Receptor domain interactions influence ligand dissociation and receptor stability. Mol. Endocrinol. 1995, 9, 208–218. [Google Scholar] [PubMed] [Green Version]
- Marchetti, P.M.; Barth, J.H. Clinical biochemistry of dihydrotestosterone. Ann. Clin. Biochem. 2013, 50 Pt 2, 95–107. [Google Scholar] [CrossRef]
- Dunn, J.F.; Nisula, B.C.; Rodbard, D. Transport of steroid hormones: Binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J. Clin. Endocrinol. Metab. 1981, 53, 58–68. [Google Scholar] [CrossRef]
- Morales, A.; Buvat, J.; Gooren, L.J.; Guay, A.T.; Kaufman, J.M.; Tan, H.M.; Torres, L.O. Endocrine aspects of sexual dysfunction in men. J. Sex. Med. 2004, 1, 69–81. [Google Scholar] [CrossRef]
- Miller, K.K.; Al-Rayyan, N.; Ivanova, M.M.; Mattingly, K.A.; Ripp, S.L.; Klinge, C.M.; Prough, R.A. DHEA metabolites activate estrogen receptors alpha and beta. Steroids 2013, 78, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Prough, R.A.; Clark, B.J.; Klinge, C.M. Novel mechanisms for DHEA action. J. Mol. Endocrinol. 2016, 56, R139–R155. [Google Scholar] [CrossRef]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.F.; Toft, D.O. Minireview: The intersection of steroid receptors with molecular chaperones: Observations and questions. Mol. Endocrinol. 2008, 22, 2229–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leon, J.T.; Iwai, A.; Feau, C.; Garcia, Y.; Balsiger, H.A.; Storer, C.L.; Suro, R.M.; Garza, K.M.; Lee, S.; Sang Kim, Y. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 11878–11883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riggs, D.L.; Roberts, P.J.; Chirillo, S.C.; Cheung-Flynn, J.; Prapapanich, V.; Ratajczak, T.; Gaber, R.; Picard, D.; Smith, D.F. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003, 22, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Saltzman, A.; Yeh, S.; Young, W.; Keller, E.; Lee, H.J.; Wang, C.; Mizokami, A. Androgen receptor: An overview. Crit. Rev. Eukaryot. Gene Expr. 1995, 5, 97–125. [Google Scholar] [CrossRef]
- Massie, C.E.; Adryan, B.; Barbosa-Morais, N.L.; Lynch, A.G.; Tran, M.G.; Neal, D.E.; Mills, I.G. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007, 8, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Dawood, S.; Holmes, M.D.; Collins, L.C.; Schnitt, S.J.; Cole, K.; Marotti, J.D.; Hankinson, S.E.; Colditz, G.A.; Tamimi, R.M. Androgen Receptor Expression and Breast Cancer Survival in Postmenopausal WomenAndrogen Receptor Expression and Breast Cancer Survival. Clin. Cancer Res. 2011, 17, 1867–1874. [Google Scholar] [CrossRef] [Green Version]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Lai, J.J.; Lai, K.P.; Zeng, W.; Chuang, K.H.; Altuwaijri, S.; Chang, C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: Lessons from conditional AR knockout mice. Am. J. Pathol. 2012, 181, 1504–1512. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Ma, W.; Wang, F.; Hua, S.; Liu, M. Identification of a prostate-specific G-protein coupled receptor in prostate cancer. Oncogene 2001, 20, 5903–5907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; Pang, Y.; Dong, J. Membrane androgen receptor characteristics of human ZIP9 (SLC39A) zinc transporter in prostate cancer cells: Androgen-specific activation and involvement of an inhibitory G protein in zinc and MAP kinase signaling. Mol. Cell. Endocrinol. 2017, 447, 23–34. [Google Scholar] [CrossRef]
- Nazareth, L.V.; Weigel, N.L. Activation of the human androgen receptor through a protein kinase A signaling pathway. J. Biol. Chem. 1996, 271, 19900–19907. [Google Scholar] [CrossRef] [Green Version]
- Estrada, M.; Espinosa, A.; Muller, M.; Jaimovich, E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology 2003, 144, 3586–3597. [Google Scholar] [CrossRef] [Green Version]
- Falkenstein, E.; Tillmann, H.C.; Christ, M.; Feuring, M.; Wehling, M. Multiple actions of steroid hormones—A focus on rapid, nongenomic effects. Pharmacol. Rev. 2000, 52, 513–556. [Google Scholar]
- Valverde, M.A.; Parker, M.G. Classical and novel steroid actions: A unified but complex view. Trends Biochem. Sci. 2002, 27, 172–173. [Google Scholar] [CrossRef]
- Shihan, M.; Bulldan, A.; Scheiner-Bobis, G. Non-classical testosterone signaling is mediated by a G-protein-coupled receptor interacting with Gnalpha11. Biochim. Biophys. Acta 2014, 1843, 1172–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Barritt, G.J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004, 64, 8365–8373. [Google Scholar] [CrossRef] [Green Version]
- Lan, X.; Zhao, J.; Song, C.; Yuan, Q.; Liu, X. TRPM8 facilitates proliferation and immune evasion of esophageal cancer cells. Biosci. Rep. 2019, 39, BSR20191878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, M.; Parrill, A.L.; Quarles, L.D. GPRC6A mediates the non-genomic effects of steroids. J. Biol. Chem. 2010, 285, 39953–39964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.Y.; Cho, C.L.; Huang, K.L.; Wang, J.C.; Hu, Y.C.; Lin, H.K.; Chang, C.; Huang, K.E. Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 2004, 19, 1181–1190. [Google Scholar] [CrossRef]
- Damasio, M.P.; Marchingo, J.M.; Spinelli, L.; Hukelmann, J.L.; Cantrell, D.A.; Howden, A.J.M. Extracellular signal-regulated kinase (ERK) pathway control of CD8+ T cell differentiation. Biochem. J. 2021, 478, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Y.; Chung, C.S.; Chaudry, I.H.; Ayala, A. MAPK p38 antagonism as a novel method of inhibiting lymphoid immune suppression in polymicrobial sepsis. Am. J. Physiol. Cell Physiol. 2001, 281, C662–C669. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chen, M.; Yan, B.; He, X.; Chen, X.; Li, D. Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells. PLoS ONE 2014, 9, e94496. [Google Scholar] [CrossRef]
- Carvalho, N.B.; de Lourdes Bastos, M.; Souza, A.S.; Netto, E.M.; Arruda, S.; Santos, S.B.; Carvalho, E.M. Impaired TNF, IL-1beta, and IL-17 production and increased susceptibility to Mycobacterium tuberculosis infection in HTLV-1 infected individuals. Tuberculosis 2018, 108, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Tsutsumi, H.; Sone, S.; Yoto, Y.; Oya, K.; Okamoto, Y.; Ogra, P.L.; Chiba, S. Characteristics of IL-6 and TNF-alpha production by respiratory syncytial virus-infected macrophages in the neonate. J. Med. Virol. 1996, 48, 199–203. [Google Scholar] [CrossRef]
- Olsen, N.J.; Kovacs, W.J. Effects of androgens on T and B lymphocyte development. Immunol. Res. 2001, 23, 281–288. [Google Scholar] [CrossRef]
- Greenstein, B.D.; de Bridges, E.F.; Fitzpatrick, F.T. Aromatase inhibitors regenerate the thymus in aging male rats. Int. J. Immunopharmacol. 1992, 14, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiao, S.; Tuckermann, J.; Okret, S.; Jondal, M. Thymus-derived glucocorticoids mediate androgen effects on thymocyte homeostasis. FASEB J. 2010, 24, 5043–5051. [Google Scholar] [PubMed]
- May, M.; Holmes, E.; Rogers, W.; Poth, M. Protection from glucocorticoid induced thymic involution by dehydroepiandrosterone. Life Sci. 1990, 46, 1627–1631. [Google Scholar] [CrossRef]
- Parker, C.R., Jr.; Conway-Myers, B.A. The effects of dehydroepiandrosterone (DHEA) on the thymus, spleen, and adrenals of prepubertal and adult female rats. Endocr. Res. 1998, 24, 113–126. [Google Scholar] [CrossRef]
- Angele, M.K.; Ayala, A.; Cioffi, W.G.; Bland, K.I.; Chaudry, I.H. Testosterone: The culprit for producing splenocyte immune depression after trauma hemorrhage. Am. J. Physiol. 1998, 274, C1530–C1536. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, I.; Giroux, N.; Olson, L.; Morrison, S.A.; Llanga, T.; Akinade, T.O.; Zhu, Y.; Zhong, Y.; Bose, S.; Arvai, S.; et al. DAMPs/PAMPs induce monocytic TLR activation and tolerance in COVID-19 patients; nucleic acid binding scavengers can counteract such TLR agonists. Biomaterials 2022, 283, 121393. [Google Scholar] [CrossRef]
- Matsuda, A.; Furukawa, K.; Suzuki, H.; Matsutani, T.; Tajiri, T.; Chaudry, I.H. Dehydroepiandrosterone modulates toll-like receptor expression on splenic macrophages of mice after severe polymicrobial sepsis. Shock 2005, 24, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Brauer, V.S.; Zambuzi, F.A.; Espíndola, M.S.; Cavalcanti Neto, M.P.; Prado, M.K.B.; Cardoso, P.M.; Soares, L.S.; Galvao-Lima, L.J.; Leopoldino, A.M.; Cardoso, C.R.d.B. The influence of dehydroepiandrosterone on effector functions of neutrophils. Braz. J. Pharm. Sci. 2021, 57, 419139. [Google Scholar] [CrossRef]
- Wang, X.; Pei, J.; Bao, P.; Liang, C.; Chu, M.; Guo, S.; Yan, P.; Guo, X. Identification of Yak’s TLR4 Alternative Spliceosomes and Bioinformatic Analysis of TLR4 Protein Structure and Function. Animals 2020, 11, 32. [Google Scholar] [CrossRef]
- Buoso, E.; Galasso, M.; Ronfani, M.; Serafini, M.M.; Lanni, C.; Corsini, E.; Racchi, M. Role of spliceosome proteins in the regulation of glucocorticoid receptor isoforms by cortisol and dehydroepiandrosterone. Pharmacol. Res. 2017, 120, 180–187. [Google Scholar] [CrossRef]
- Rettew, J.A.; Huet-Hudson, Y.M.; Marriott, I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol. Reprod. 2008, 78, 432–437. [Google Scholar] [CrossRef]
- Han, Q.; Yang, D.; Yin, C.; Zhang, J. Androgen Receptor (AR)-TLR4 Crosstalk Mediates Gender Disparities in Hepatocellular Carcinoma Incidence and Progression. J. Cancer 2020, 11, 1094–1103. [Google Scholar] [CrossRef] [Green Version]
- Al-Quraishy, S.; Dkhil, M.A.; Abdel-Baki, A.A.; Arauzo-Bravo, M.J.; Delic, D.; Wunderlich, F. Testosterone persistently dysregulates hepatic expression of Tlr6 and Tlr8 induced by Plasmodium chabaudi malaria. Parasitol. Res. 2014, 113, 3609–3620. [Google Scholar] [CrossRef]
- Ainola, M.; Porola, P.; Takakubo, Y.; Przybyla, B.; Kouri, V.P.; Tolvanen, T.A.; Hanninen, A.; Nordstrom, D.C. Activation of plasmacytoid dendritic cells by apoptotic particles-mechanism for the loss of immunological tolerance in Sjogren’s syndrome. Clin. Exp. Immunol. 2018, 191, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.K. Cellular and Molecular Immunology, 5th ed.; Saunders: Philadelphia, PA, USA, 2003; p. 562. [Google Scholar]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Friedl, R.; Brunner, M.; Moeslinger, T.; Spieckermann, P.G. Testosterone inhibits expression of inducible nitric oxide synthase in murine macrophages. Life Sci. 2000, 68, 417–429. [Google Scholar] [CrossRef]
- Benten, W.P.; Guo, Z.; Krucken, J.; Wunderlich, F. Rapid effects of androgens in macrophages. Steroids 2004, 69, 585–590. [Google Scholar] [CrossRef]
- Markman, J.L.; Porritt, R.A.; Wakita, D.; Lane, M.E.; Martinon, D.; Noval Rivas, M.; Luu, M.; Posadas, E.M.; Crother, T.R.; Arditi, M. Loss of testosterone impairs anti-tumor neutrophil function. Nat. Commun. 2020, 11, 1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliano-Juca, T.; Pencina, K.M.; Guo, W.; Li, Z.; Huang, G.; Basaria, S.; Bhasin, S. Differential effects of testosterone on circulating neutrophils, monocytes, and platelets in men: Findings from two trials. Andrology 2020, 8, 1324–1331. [Google Scholar] [CrossRef]
- Scalerandi, M.V.; Peinetti, N.; Leimgruber, C.; Cuello Rubio, M.M.; Nicola, J.P.; Menezes, G.B.; Maldonado, C.A.; Quintar, A.A. Inefficient N2-Like Neutrophils Are Promoted by Androgens During Infection. Front. Immunol. 2018, 9, 1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radford, D.J.; Wang, K.; McNelis, J.C.; Taylor, A.E.; Hechenberger, G.; Hofmann, J.; Chahal, H.; Arlt, W.; Lord, J.M. Dehydroepiandrosterone sulfate directly activates protein kinase C-beta to increase human neutrophil superoxide generation. Mol. Endocrinol. 2010, 24, 813–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, P.F.; Jacobson, M.S. Inhibition of macrophage superoxide generation by dehydroepiandrosterone. Am. J. Med. Sci. 1993, 306, 10–15. [Google Scholar] [CrossRef]
- Hamerman, J.A.; Ogasawara, K.; Lanier, L.L. NK cells in innate immunity. Curr. Opin. Immunol. 2005, 17, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Page, S.T.; Plymate, S.R.; Bremner, W.J.; Matsumoto, A.M.; Hess, D.L.; Lin, D.W.; Amory, J.K.; Nelson, P.S.; Wu, J.D. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: A physiological role for testosterone and/or its metabolites. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E856–E863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solerte, S.B.; Fioravanti, M.; Vignati, G.; Giustina, A.; Cravello, L.; Ferrari, E. Dehydroepiandrosterone sulfate enhances natural killer cell cytotoxicity in humans via locally generated immunoreactive insulin-like growth factor I. J. Clin. Endocrinol. Metab. 1999, 84, 3260–3267. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Sun, R.; Fu, B.; Wang, F.; Guo, C.; Tian, Z.; Wei, H. IGF-1 promotes the development and cytotoxic activity of human NK cells. Nat. Commun. 2013, 4, 1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, A.; Chang, J.J.; Chan, E.S.; Pollard, R.B.; Sidhu, H.K.; Kulkarni, S.; Wen, T.F.; Lindsay, R.J.; Orellana, L.; Mildvan, D.; et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 2009, 15, 955–959. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.T.; Gray, A.; Higgins, S.A.; Hubby, B.; Kast, W.M. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate 2009, 69, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Corrales, J.J.; Almeida, M.; Burgo, R.; Mories, M.T.; Miralles, J.M.; Orfao, A. Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-presenting cells in aging type-2 diabetic men with partial androgen deficiency. J. Endocrinol. 2006, 189, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernykh, E.R.; Leplina, O.Y.; Tikhonova, M.A.; Seledtsova, N.V.; Tyrinova, T.V.; Khonina, N.A.; Ostanin, A.A.; Pasman, N.M. Elevated levels of dehydroepiandrosterone as a potential mechanism of dendritic cell impairment during pregnancy. BMC Immunol. 2015, 16, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canning, M.O.; Grotenhuis, K.; de Wit, H.J.; Drexhage, H.A. Opposing effects of dehydroepiandrosterone and dexamethasone on the generation of monocyte-derived dendritic cells. Eur. J. Endocrinol. 2000, 143, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Paharkova-Vatchkova, V.; Maldonado, R.; Kovats, S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J. Immunol. 2004, 172, 1426–1436. [Google Scholar] [CrossRef] [Green Version]
- Assani, K.; Tazi, M.F.; Amer, A.O.; Kopp, B.T. IFN-gamma stimulates autophagy-mediated clearance of Burkholderia cenocepacia in human cystic fibrosis macrophages. PLoS ONE 2014, 9, e96681. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.K.; Li, Y.; Oh, E.; Kim, J.; Yun, C.O. Oncolytic adenovirus expressing IL-23 and p35 elicits IFN-gamma- and TNF-alpha-co-producing T cell-mediated antitumor immunity. PLoS ONE 2013, 8, e67512. [Google Scholar]
- Hepworth, M.R.; Hardman, M.J.; Grencis, R.K. The role of sex hormones in the development of Th2 immunity in a gender-biased model of Trichuris muris infection. Eur. J. Immunol. 2010, 40, 406–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arredouani, S.; Kissick, H.; Dunn, L.; Sanda, M. PTP1B regulates lymphocytes responses androgen deprivation. J. ImmunoTherapy Cancer 2014, 2 (Suppl. S1), P2. [Google Scholar] [CrossRef]
- Yang, C.; Mai, H.; Peng, J.; Zhou, B.; Hou, J.; Jiang, D. STAT4: An immunoregulator contributing to diverse human diseases. Int. J. Biol. Sci. 2020, 16, 1575–1585. [Google Scholar] [CrossRef] [Green Version]
- Kissick, H.T.; Sanda, M.G.; Dunn, L.K.; Pellegrini, K.L.; On, S.T.; Noel, J.K.; Arredouani, M.S. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 9887–9892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liva, S.M.; Voskuhl, R.R. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 2001, 167, 2060–2067. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Benten, W.P.; Wang, L.; Hao, X.; Li, Q.; Zhang, H.; Guo, D.; Wang, Y.; Wunderlich, F.; Qiao, Z. Modulation of Leishmania donovani infection and cell viability by testosterone in bone marrow-derived macrophages: Signaling via surface binding sites. Steroids 2005, 70, 604–614. [Google Scholar] [CrossRef]
- Leplina, O.Y.; Tikhonova, M.; Sakchno, L.V.; Tyrinova, T.; Ostanin, A.A.; Chernykh, E.R. Effect of Dehydroepiandrosterone Sulfate on Maturation and Functional Properties of Interferon-±-Induced Dendritic Cells. Bull. Exp. Biol. Med. 2009, 148, 68–71. [Google Scholar] [CrossRef]
- Tabata, N.; Tagami, H.; Terui, T. Dehydroepiandrosterone may be one of the regulators of cytokine production in atopic dermatitis. Arch. Dermatol. Res. 1997, 289, 410–414. [Google Scholar] [CrossRef]
- Cai, C.W.; Blase, J.R.; Zhang, X.; Eickhoff, C.S.; Hoft, D.F. Th17 Cells Are More Protective Than Th1 Cells Against the Intracellular Parasite Trypanosoma cruzi. PLoS Pathog. 2016, 12, e1005902. [Google Scholar] [CrossRef] [Green Version]
- Blanco, L.P.; Plegue, M.; Fung-Leung, W.P.; Holoshitz, J. Gender-biased regulation of human IL-17-producing cells in vitro by peptides corresponding to distinct HLA-DRB1 allele-coded sequences. J. Immune Based Ther. Vaccines Antimicrob. 2013, 2, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Massa, M.G.; David, C.; Jorg, S.; Berg, J.; Gisevius, B.; Hirschberg, S.; Linker, R.A.; Gold, R.; Haghikia, A. Testosterone Differentially Affects T Cells and Neurons in Murine and Human Models of Neuroinflammation and Neurodegeneration. Am. J. Pathol. 2017, 187, 1613–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggelakopoulou, M.; Kourepini, E.; Paschalidis, N.; Simoes, D.C.; Kalavrizioti, D.; Dimisianos, N.; Papathanasopoulos, P.; Mouzaki, A.; Panoutsakopoulou, V. ERbeta-Dependent Direct Suppression of Human and Murine Th17 Cells and Treatment of Established Central Nervous System Autoimmunity by a Neurosteroid. J. Immunol. 2016, 197, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Walecki, M.; Eisel, F.; Klug, J.; Baal, N.; Paradowska-Dogan, A.; Wahle, E.; Hackstein, H.; Meinhardt, A.; Fijak, M. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol. Biol. Cell 2015, 26, 2845–2857. [Google Scholar] [CrossRef]
- Afshan, G.; Afzal, N.; Qureshi, S. CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clin. Lab. 2012, 58, 567–571. [Google Scholar]
- Roden, A.C.; Moser, M.T.; Tri, S.D.; Mercader, M.; Kuntz, S.M.; Dong, H.; Hurwitz, A.A.; McKean, D.J.; Celis, E.; Leibovich, B.C.; et al. Augmentation of T cell levels and responses induced by androgen deprivation. J. Immunol. 2004, 173, 6098–6108. [Google Scholar] [CrossRef] [Green Version]
- Vom Steeg, L.G.; Dhakal, S.; Woldetsadik, Y.A.; Park, H.S.; Mulka, K.R.; Reilly, E.C.; Topham, D.J.; Klein, S.L. Androgen receptor signaling in the lungs mitigates inflammation and improves the outcome of influenza in mice. PLoS Pathog. 2020, 16, e1008506. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, M.; Dahlweid, F.M.; Giannoudis, P.V.; Wittwer, T.; Bottcher, F.; Breddin, M.; Pape, H.C. Dehydroepiandrosterone (DHEA) modulates the activity and the expression of lymphocyte subpopulations induced by cecal ligation and puncture. Shock 2002, 18, 445–449. [Google Scholar] [CrossRef]
- Rasmussen, K.R.; Healey, M.C.; Cheng, L.; Yang, S. Effects of dehydroepiandrosterone in immunosuppressed adult mice infected with Cryptosporidium parvum. J. Parasitol. 1995, 81, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Alari-Pahissa, E.; Ataya, M.; Moraitis, I.; Campos-Ruiz, M.; Altadill, M.; Muntasell, A.; Moles, A.; Lopez-Botet, M. NK cells eliminate Epstein-Barr virus bound to B cells through a specific antibody-mediated uptake. PLoS Pathog. 2021, 17, e1009868. [Google Scholar] [CrossRef] [PubMed]
- Weaver, R.; Reiling, L.; Feng, G.; Drew, D.R.; Mueller, I.; Siba, P.M.; Tsuboi, T.; Richards, J.S.; Fowkes, F.J.I.; Beeson, J.G. The association between naturally acquired IgG subclass specific antibodies to the PfRH5 invasion complex and protection from Plasmodium falciparum malaria. Sci. Rep. 2016, 6, 33094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viselli, S.M.; Reese, K.R.; Fan, J.; Kovacs, W.J.; Olsen, N.J. Androgens alter B cell development in normal male mice. Cell. Immunol. 1997, 182, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmson, A.S.; Lantero Rodriguez, M.; Stubelius, A.; Fogelstrand, P.; Johansson, I.; Buechler, M.B.; Lianoglou, S.; Kapoor, V.N.; Johansson, M.E.; Fagman, J.B.; et al. Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat. Commun. 2018, 9, 2067. [Google Scholar] [CrossRef]
- Kanda, N.; Tsuchida, T.; Tamaki, K. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin. Exp. Immunol. 1996, 106, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yu, L.; Zhao, J.; Ma, H. Effect of dehydroepiandrosterone on the immune function of mice in vivo and in vitro. Mol. Immunol. 2019, 112, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, Y.; Nakagawa, Y.; Ohzeki, T. Differential effect of DHEA on mitogen-induced proliferation of T and B lymphocytes. J. Steroid Biochem. Mol. Biol. 2006, 99, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinedi, E.; Suescun, M.O.; Hadid, R.; Daneva, T.; Gaillard, R.C. Effects of gonadectomy and sex hormone therapy on the endotoxin-stimulated hypothalamo-pituitary-adrenal axis: Evidence for a neuroendocrine-immunological sexual dimorphism. Endocrinology 1992, 131, 2430–2436. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Atkinson, E.J.; Dunstan, C.R.; O’Fallon, W.M. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J. Clin. Endocrinol. Metab. 2002, 87, 1550–1554. [Google Scholar] [CrossRef]
- Malkin, C.J.; Pugh, P.J.; Jones, R.D.; Kapoor, D.; Channer, K.S.; Jones, T.H. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metab. 2004, 89, 3313–3318. [Google Scholar] [CrossRef] [Green Version]
- Daynes, R.A.; Dudley, D.J.; Araneo, B.A. Regulation of murine lymphokine production in vivo. II. Dehydroepiandrosterone is a natural enhancer of interleukin 2 synthesis by helper T cells. Eur. J. Immunol. 1990, 20, 793–802. [Google Scholar] [CrossRef]
- Santos, C.D.; Toldo, M.P.; Santello, F.H.; Filipin Mdel, V.; Brazao, V.; do Prado Junior, J.C. Dehydroepiandrosterone increases resistance to experimental infection by Trypanosoma cruzi. Vet. Parasitol. 2008, 153, 238–243. [Google Scholar] [CrossRef]
- Perez, A.R.; Bertoya, A.A.; Revelli, S.; Garcia, F. A high corticosterone/DHEA-s ratio in young rats infected with Trypanosoma cruzi is associated with increased susceptibility. Mem. Inst. Oswaldo Cruz 2011, 106, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Karitskaia, I.A.; Aksenov, N.D.; Vinogradova, T.A.; Marakhova, I.I. Il-2-regulated expression of Na+,K(+)-ATPase in activated human lymphocytes. Tsitologiia 2005, 47, 28–37. [Google Scholar] [PubMed]
- Padgett, D.A.; Loria, R.M. Endocrine regulation of murine macrophage function: Effects of dehydroepiandrosterone, androstenediol, and androstenetriol. J. Neuroimmunol. 1998, 84, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Araneo, B.A.; Dowell, T.; Diegel, M.; Daynes, R.A. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood 1991, 78, 688–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Campbell, H.D.; Young, I.G. Sex hormones and dexamethasone modulate interleukin-5 gene expression in T lymphocytes. J. Steroid Biochem Mol. Biol. 1993, 44, 203–210. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Wysocka, M.; Hieny, S.; Scharton-Kersten, T.; Cheever, A.; Kuhn, R.; Muller, W.; Trinchieri, G.; Sher, A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 1996, 157, 798–805. [Google Scholar] [PubMed]
- Kulkarni, A.B.; Huh, C.G.; Becker, D.; Geiser, A.; Lyght, M.; Flanders, K.C.; Roberts, A.B.; Sporn, M.B.; Ward, J.M.; Karlsson, S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 1993, 90, 770–774. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.F.; Chang, H.L.; Tseng, J. Dehydroepiandrosterone induces the transforming growth factor-beta production by murine macrophages. Int. J. Tissue React. 1997, 19, 141–148. [Google Scholar] [PubMed]
- Cypowyj, S.; Picard, C.; Marodi, L.; Casanova, J.L.; Puel, A. Immunity to infection in IL-17-deficient mice and humans. Eur. J. Immunol. 2012, 42, 2246–2254. [Google Scholar] [CrossRef] [Green Version]
- Babaloo, Z.; Aliparasti, M.R.; Babaiea, F.; Almasi, S.; Baradaran, B.; Farhoudi, M. The role of Th17 cells in patients with relapsing-remitting multiple sclerosis: Interleukin-17A and interleukin-17F serum levels. Immunol. Lett. 2015, 164, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Unutmaz, D. RORC2: The master of human Th17 cell programming. Eur. J. Immunol. 2009, 39, 1452–1455. [Google Scholar] [CrossRef]
- Kalimi, M.; Shafagoj, Y.; Loria, R.; Padgett, D.; Regelson, W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol. Cell. Biochem. 1994, 131, 99–104. [Google Scholar] [CrossRef]
- Pinto, A.; Malacrida, B.; Oieni, J.; Serafini, M.M.; Davin, A.; Galbiati, V.; Corsini, E.; Racchi, M. DHEA modulates the effect of cortisol on RACK1 expression via interference with the splicing of the glucocorticoid receptor. Br. J. Pharmacol. 2015, 172, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Qiu, W.B.; Mei, Y.F.; Wang, D.M.; Li, Y.G.; Tan, X.R. Testosterone alleviates tumor necrosis factor-alpha-mediated tissue factor pathway inhibitor downregulation via suppression of nuclear factor-kappa B in endothelial cells. Asian J. Androl. 2009, 11, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norata, G.D.; Tibolla, G.; Seccomandi, P.M.; Poletti, A.; Catapano, A.L. Dihydrotestosterone decreases tumor necrosis factor-alpha and lipopolysaccharide-induced inflammatory response in human endothelial cells. J. Clin. Endocrinol. Metab. 2006, 91, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, B.M.; Mountain, D.J.; Brock, T.C.; Chapman, J.R.; Kirkpatrick, S.S.; Freeman, M.B.; Klein, F.A.; Grandas, O.H. Low testosterone elevates interleukin family cytokines in a rodent model: A possible mechanism for the potentiation of vascular disease in androgen-deficient males. J. Surg. Res. 2014, 190, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, L.; Wang, Y.; Liu, L.; Yang, F.; Sun, Y.; Jiao, X.; Bao, L.; Chen, P.; Liang, Q.; et al. Dihydrotestosterone regulates oxidative stress and immunosuppressive cytokines in a female BALB/c mouse model of Graves’ disease. Autoimmunity 2019, 52, 117–125. [Google Scholar] [CrossRef]
- Choi, I.S.; Cui, Y.; Koh, Y.A.; Lee, H.C.; Cho, Y.B.; Won, Y.H. Effects of dehydroepiandrosterone on Th2 cytokine production in peripheral blood mononuclear cells from asthmatics. Korean J. Intern. Med. 2008, 23, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Vignozzi, L.; Cellai, I.; Santi, R.; Lombardelli, L.; Morelli, A.; Comeglio, P.; Filippi, S.; Logiodice, F.; Carini, M.; Nesi, G.; et al. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J. Endocrinol. 2012, 214, 31–43. [Google Scholar] [CrossRef] [PubMed]

| Type | Cell | Testosterone | REF | DHEA | REF |
|---|---|---|---|---|---|
| Innate | Macrophages | ↓ the secretion of NO | [64] | ↑ synthesis of O2− | [70] |
| NK cells | ↓ proliferation | [72] | ↑ cytotoxic activity | [73] | |
| Neutrophis | ↓ bactericidal activity | [68] | ↑ synthesis of O2− | [69] | |
| Dendritic cells | ↓ maturation | [76] | ↑ maduration | [78] | |
| Adaptive | Th1 lymphocytes | ↓ the expression of T-bet | [84] | ↑ activation | [89] |
| Th2 lymphocytes | is favored by the suppression of IL-12 | [87] | ↓ activation | [90] | |
| Regulatory T lymphocytes | ↑ the expression of Foxp3 | [95] | ↑ the expression of Foxp3 | [95] | |
| Th17 lymphocytes | ↓ proliferation | [93] | ↓ proliferation | [94] | |
| B cells | ↓ proliferation and antibody secretion | [103] | modulates their proliferation | [106,107] |
| Cytokine | Function | Testosterone (Reference) | DHT (Reference) | DHEA (Reference) |
|---|---|---|---|---|
| IFN-γ | Lymphocyte and macrophage activation. | does not change [116] | ↑ [116] | ↑ [112] |
| TNF-α | Proinflamatory response, macrophage activation | Inhibits its effects [126] | Inhibits its effects [127] | ↓ [115] |
| IL-2 | Lymphocyte activation | ↓ [128] | ↑ [129] | ↑ [106] |
| IL-10 | Antiinflamatory response, immunological tolerance | ↑ [95] | ↑ [87] | ↑ [68] |
| TGF-β | Antiinflamatory response | ↑ [120] | ↑ [129] | ↑ [120] |
| IL-4 | Th2 response | - [116] | ↓ [116] | ↓ [90] |
| IL-5 | Antibody secretion | ↑ [117] | ↓ [116] | ↓ [130] |
| IL-6 | B cell differentiation | ↓ [128] | ↓ [127] | ↓ [115] |
| IL-17 | Chronic proinflammatory response | ↓ [123] | ↓ [131] | ↓ [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buendía-González, F.O.; Legorreta-Herrera, M. The Similarities and Differences between the Effects of Testosterone and DHEA on the Innate and Adaptive Immune Response. Biomolecules 2022, 12, 1768. https://doi.org/10.3390/biom12121768
Buendía-González FO, Legorreta-Herrera M. The Similarities and Differences between the Effects of Testosterone and DHEA on the Innate and Adaptive Immune Response. Biomolecules. 2022; 12(12):1768. https://doi.org/10.3390/biom12121768
Chicago/Turabian StyleBuendía-González, Fidel Orlando, and Martha Legorreta-Herrera. 2022. "The Similarities and Differences between the Effects of Testosterone and DHEA on the Innate and Adaptive Immune Response" Biomolecules 12, no. 12: 1768. https://doi.org/10.3390/biom12121768
APA StyleBuendía-González, F. O., & Legorreta-Herrera, M. (2022). The Similarities and Differences between the Effects of Testosterone and DHEA on the Innate and Adaptive Immune Response. Biomolecules, 12(12), 1768. https://doi.org/10.3390/biom12121768