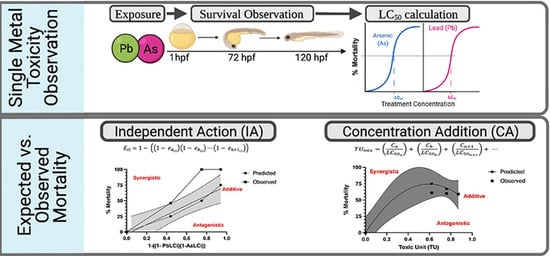
Joint Action Toxicity of Arsenic (As) and Lead (Pb) Mixtures in Developing Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
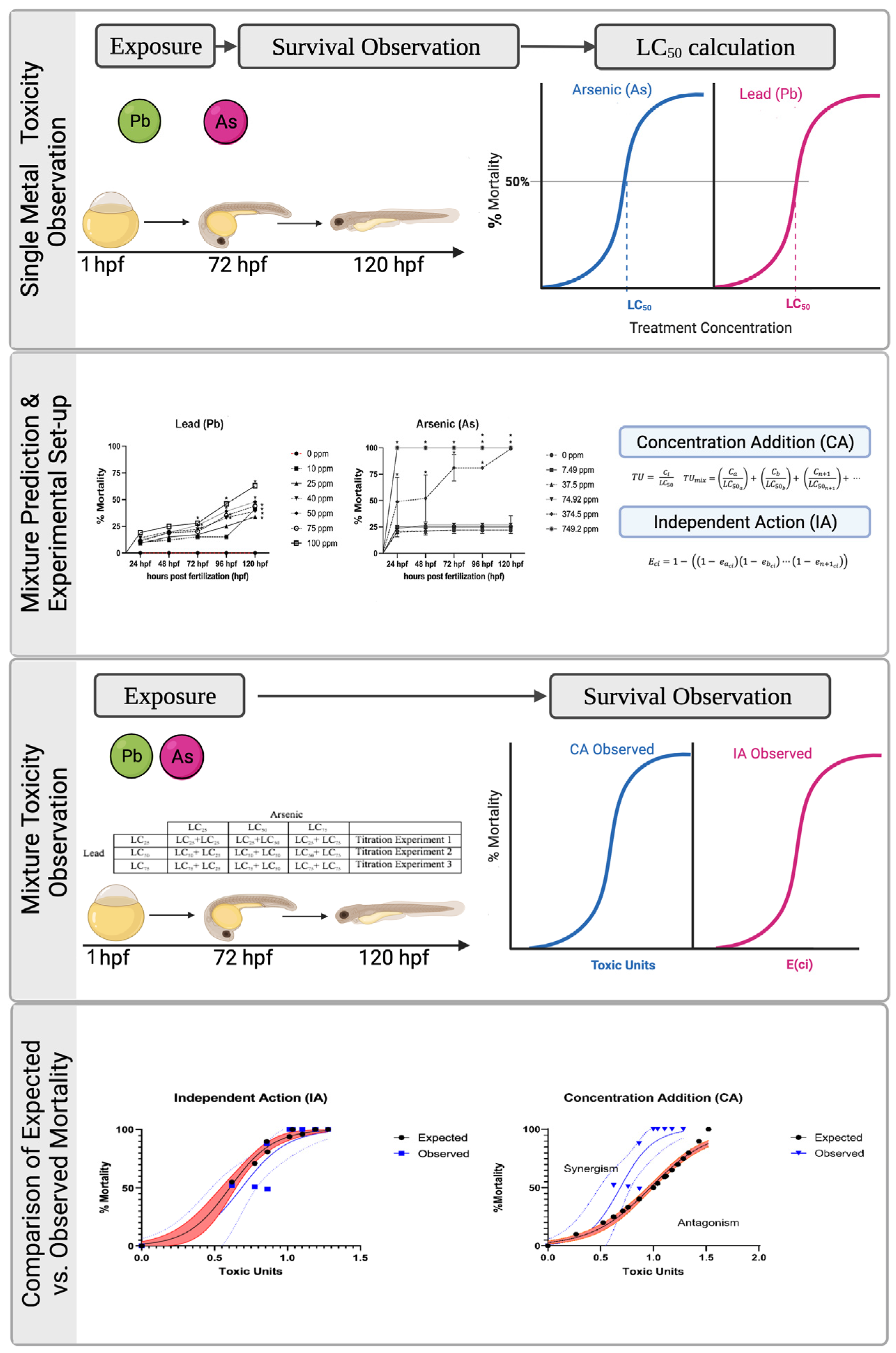
2.2. Arsenic and Lead Single Chemical Exposure
2.3. Single Arsenic and Lead LC Calculations
2.4. Binary Arsenic and Lead Mixture Exposure
2.5. Independent Action (IA) and Concentration Addition (CA) Model Calculations
2.6. Mixture Toxicity Analysis and Comparison of Measured with Predicted Toxicity
2.7. Type of Mixture Interaction Analysis
3. Results
3.1. Effects of Single Chemical Exposure on Mortality

| Chemical | Percent (%) Mortality | ||||
|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | |
| Lead (Pb) | 0 ppm | 39.0 ppm | 73.76 ppm | 99.94 ppm | 121.74 ppm |
| Arsenic (As) | 0 ppm | 40.18 ppm | 55.42 ppm | 66.64 ppm | 77.32 pm |
3.2. Effects of Metal Mixtures on Mortality
3.3. Mixture Toxicity Comparison of Predicted to Observed Toxicity
3.4. Type of Mixture Interaction Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamdi, M.; Sanchez, M.A.; Beene, L.C.; Liu, Q.; Landfear, S.M.; Rosen, B.P.; Liu, Z. Arsenic transport by zebrafish aquaglyceroporins. BMC Mol. Biol. 2009, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Styblo, M.; Del Razo, L.M.; Vega, L.; Germolec, D.R.; LeCluyse, E.; Hamilton, G.A.; Reed, W.; Wang, C.; Cullen, W.R.; Thomas, D.J. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000, 74, 289–299. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11005674 (accessed on 11 April 2018).
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, S.; Bhaumik, S.; Purkayastha, M.; Basu, S.; Chaudhuri, A.N.; Das Gupta, S. Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol. Lett. 2002, 136, 65–76. [Google Scholar] [CrossRef]
- De Castro, M.R.; Lima, J.V.; de Freitas, D.P.S.; Valente, R.D.S.; Dummer, N.S.; de Aguiar, R.B.; dos Santos, L.C.; Marins, L.F.; Geracitano, L.A.; Monserrat, J.M.; et al. Behavioral and neurotoxic effects of arsenic exposure in zebrafish (Danio rerio, Teleostei: Cyprinidae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 337–342. [Google Scholar] [CrossRef]
- Rudge, C.V.; Röllin, H.B.; Nogueira, C.M.; Thomassen, Y.; Rudge, M.C.; Odland, J. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J. Environ. Monit. 2009, 11, 1322–1330. [Google Scholar] [CrossRef] [Green Version]
- Mass, M.J.; Tennant, A.; Roop, B.C.; Cullen, W.R.; Styblo, M.; Thomas, D.J.; Kligerman, A.D. Methylated trivalent arsenic species are genotoxic. Chem. Res. Toxicol. 2001, 14, 355–361. [Google Scholar] [CrossRef]
- Petrick, J.S.; Jagadish, B.; Mash, A.E.A.; Aposhian, H.V. Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem. Res. Toxicol. 2001, 14, 651–656. [Google Scholar] [CrossRef]
- Hallauer, J.; Geng, X.; Yang, H.-C.; Shen, J.; Tsai, K.-J.; Liu, Z. The Effect of Chronic Arsenic Exposure in Zebrafish. Zebrafish 2016, 13, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-J.; Li, X.; Xu, Z.-Q.; Fang, K.-M.; Hong, H.-C.; Sun, H.-J.; Guan, D.-X.; Yu, X.-W. Environmentally relevant concentrations of arsenic induces apoptosis in the early life stage of zebrafish. Ecotoxicol. Environ. Saf. 2021, 227, 112883. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, J.; Carbrey, J.M.; Mukhopadhyay, R.; Agre, P.; Rosen, B.P. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. USA 2002, 99, 6053–6058. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ma, Y.; Li, D.; Gao, X.; Li, P.; Bai, N.; Luo, M.; Tan, X.; Lu, C.; Ma, X. Arsenic impairs embryo development via down-regulating Dvr1 expression in zebrafish. Toxicol. Lett. 2012, 212, 161–168. [Google Scholar] [CrossRef]
- Rocha-Amador, D.; Navarro, M.E.; Carrizales, L.; Morales, R.; Calderón, J. Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad. De Saude Publica 2007, 23 (Suppl. S4), S579–S587. [Google Scholar] [CrossRef]
- Jiang, C.B.; Kao, C.S.; Chien, L.C.; Chen, Y.J.; Liao, K.W. Associations among prenatal and postnatal arsenic, lead, and cadmium exposures and motor development in 3-year-old children: A longitudinal birth cohort study in Taiwan. Environ. Sci. Pollut. Res. 2022, 29, 43191–43200. [Google Scholar] [CrossRef]
- Wright, R.O.; Amarasiriwardena, C.; Woolf, A.D.; Jim, R.; Bellinger, D.C. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. NeuroToxicology 2006, 27, 210–216. [Google Scholar] [CrossRef]
- Rahbar, M.H.; Samms-Vaughan, M.; Lee, M.; Zhang, J.; Hessabi, M.; Bressler, J.; Bach, M.A.; Grove, M.L.; Shakespeare-Pellington, S.; Beecher, C.; et al. Interaction between a mixture of heavy metals (lead, mercury, arsenic, cadmium, manganese, aluminum) and GSTP1, GSTT1, and GSTM1 in relation to autism spectrum disorder. Res. Autism Spectr. Disord. 2020, 79, 101681. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip Toxicol 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Zhu, G.; Fan, G.; Feng, C.; Li, Y.; Chen, Y.; Zhou, F.; Du, G.; Jiao, H.; Liu, Z.; Xiao, X.; et al. The effect of lead exposure on brain iron homeostasis and the expression of DMT1/FP1 in the brain in developing and aged rats. Toxicol. Lett. 2013, 216, 108–123. [Google Scholar] [CrossRef]
- Bihaqi, S.W.; Zawia, N.H. Enhanced taupathy and AD-like pathology in aged primate brains decades after infantile exposure to lead (Pb). Neurotoxicology 2013, 39, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Zheng, G.; Shen, X.-F.; Liu, X.-Q.; Luo, W.-J.; Chen, J.-Y. Reduction of brain barrier tight junctional proteins by lead exposure: Role of activation of nonreceptor tyrosine kinase Src via chaperon GRP78. Toxicol. Sci. 2014, 138, 393–402. [Google Scholar] [CrossRef]
- Senut, M.-C.; Sen, A.; Cingolani, P.; Shaik, A.; Land, S.J.; Ruden, D.M. Lead exposure disrupts global DNA methylation in human embryonic stem cells and alters their neuronal differentiation. Toxicol. Sci. 2014, 139, 142–161. [Google Scholar] [CrossRef] [Green Version]
- Molina, R.M.; Phattanarudee, S.; Kim, J.; Thompson, K.; Wessling-Resnick, M.; Maher, T.J.; Brain, J.D. Ingestion of Mn and Pb by rats during and after pregnancy alters iron metabolism and behavior in offspring. NeuroToxicology 2011, 32, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Xie, J.; Zhang, S.; Yin, G.; Gao, Y.; Zhang, Y.; Bo, D.; Li, Z.; Liu, S.; Feng, C.; et al. Lead, cadmium, arsenic, and mercury combined exposure disrupted synaptic homeostasis through activating the Snk-SPAR pathway. Ecotoxicol. Environ. Saf. 2018, 163, 674–684. [Google Scholar] [CrossRef]
- Rai, A.; Maurya, S.K.; Sharma, R.; Ali, S. Down-regulated GFAPα: A major player in heavy metal induced astrocyte damage. Toxicol. Mech. Methods 2013, 23, 99–107. [Google Scholar] [CrossRef]
- Abbas, S.; Khan, K.; Khan, M.P.; Nagar, G.K.; Tewari, D.; Maurya, S.K.; Dubey, J.; Ansari, N.G.; Bandyopadhyay, S.; Chattopadhyay, N. Developmental exposure to As, Cd, and Pb mixture diminishes skeletal growth and causes osteopenia at maturity via osteoblast and chondrocyte malfunctioning in female rats. Toxicol. Sci. 2013, 134, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, R.; Mishra, J.; Tripathi, S.; Khare, P.; Bandyopadhyay, S. Arsenic, Cadmium, and Lead Like Troglitazone Trigger PPARγ-Dependent Poly (ADP-Ribose) Polymerase Expression and Subsequent Apoptosis in Rat Brain Astrocytes. Mol. Neurobiol. 2018, 55, 2125–2149. [Google Scholar] [CrossRef]
- Muthusamy, S.; Peng, C.; Ng, J.C. The binary, ternary and quaternary mixture toxicity of benzo[a]pyrene, arsenic, cadmium and lead in HepG2 cells. Toxicol. Res. 2016, 5, 703. [Google Scholar] [CrossRef] [Green Version]
- Rai, N.K.; Ashok, A.; Rai, A.; Tripathi, S.; Nagar, G.K.; Mitra, K.; Bandyopadhyay, S. Exposure to As, Cd and Pb-mixture impairs myelin and axon development in rat brain, optic nerve and retina. Toxicol. Appl. Pharmacol. 2013, 273, 242–258. [Google Scholar] [CrossRef]
- Jakobs, G.; Krüger, J.; Schüttler, A.; Altenburger, R.; Busch, W. Mixture toxicity analysis in zebrafish embryo: A time and concentration resolved study on mixture effect predictivity. Environ. Sci. Eur. 2020, 32, 1–21. [Google Scholar] [CrossRef]
- Lee, J.; Freeman, J. Zebrafish as a Model for Developmental Neurotoxicity Assessment: The Application of the Zebrafish in Defining the Effects of Arsenic, Methylmercury, or Lead on Early Neurodevelopment. Toxics 2014, 2, 464–495. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Freeman, J.L. Embryonic exposure to 10 μg L-1lead results in female-specific expression changes in genes associated with nervous system development and function and Alzheimer’s disease in aged adult zebrafish brain. Metallomics 2016, 8, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Freeman, J.L. Zebrafish as a model for investigating developmental lead (Pb) neurotoxicity as a risk factor in adult neurodegenerative disease: A mini-review. NeuroToxicology 2014, 43, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kiper, K.G.; Freeman, J.L. Zebrafish as a Tool to Assess Developmental Neurotoxicity; Humana: New York, NY, USA, 2019; pp. 169–193. [Google Scholar] [CrossRef]
- Peterson, S.M.; Zhang, J.; Freeman, J.L. Developmental reelin expression and time point-specific alterations from lead exposure in zebrafish. Neurotoxicol. Teratol. 2013, 38, 53–60. [Google Scholar] [CrossRef]
- Peterson, S.M.; Zhang, J.; Weber, G.; Freeman, J.L. Global Gene Expression Analysis Reveals Dynamic and Developmental Stage–Dependent Enrichment of Lead-Induced Neurological Gene Alterations. Environ. Health Perspect. 2011, 119, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Wirbisky, S.E.; Weber, G.J.; Lee, J.W.; Cannon, J.R.; Freeman, J.L. Novel dose-dependent alterations in excitatory GABA during embryonic development associated with lead (Pb) neurotoxicity. Toxicol. Lett. 2014, 229, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Peterson, S.M.; Weber, G.J.; Zhu, X.; Zheng, W.; Freeman, J.L. Decreased axonal density and altered expression profiles of axonal guidance genes underlying lead (Pb) neurodevelopmental toxicity at early embryonic stages in the zebrafish. Neurotoxicol. Teratol. 2011, 33, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Tilton, F.A.; Tilton, S.C.; Bammler, T.K.; Beyer, R.P.; Stapleton, P.L.; Scholz, N.L.; Gallagher, E.P. Transcriptional impact of organophosphate and metal mixtures on olfaction: Copper dominates the chlorpyrifos-induced response in adult zebrafish. Aquat. Toxicol. 2011, 102, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Chen, Y.; Liu, W.; Bai, C.; Liu, X.; Liu, K.; Li, R.; Zhu, J.-H.; Huang, C. Developmental lead acetate exposure induces embryonic toxicity and memory deficit in adult zebrafish. Neurotoxicol. Teratol. 2012, 34, 581–586. [Google Scholar] [CrossRef]
- Dipp, V.R.; Valles, S.; Ortiz-Kerbertt, H.; Suarez, J.V.; Bardullas, U. Neurobehavioral Alterations in Zebrafish Due to Long-Term Exposure to Low Doses of Inorganic Arsenic. Zebrafish 2018, 15, 575–585. [Google Scholar] [CrossRef]
- Ashok, A.; Rai, N.K.; Tripathi, S.; Bandyopadhyay, S. Exposure to As-, Cd-, and Pb-Mixture Induces Aβ, Amyloidogenic APP Processing and Cognitive Impairments via Oxidative Stress-Dependent Neuroinflammation in Young Rats. Toxicol. Sci. 2015, 143, 64–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCollum, C.W.; Hans, C.; Shah, S.; Merchant, F.A.; Gustafsson, J.Å.; Bondesson, M. Embryonic exposure to sodium arsenite perturbs vascular development in zebrafish. Aquat. Toxicol. 2014, 152, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Hou, S.; Barton, C.H.; Vaziri, N.D. Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells. Kidney Int. 2004, 66, 2329–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, D.; Kishimoto, T.; Dekio, S.; Tada, M. Inhibitory effect of lead on tube formation by cultured human vascular endothelial cells. Hum. Cell. 1997, 10, 283–291. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9573489 (accessed on 1 May 2019).
- Li, D.; Lu, C.; Wang, J.; Hu, W.; Cao, Z.; Sun, D.; Xia, H.; Ma, X. Developmental mechanisms of arsenite toxicity in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2009, 91, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Phan, N.-N.; Ho, S.-H.; Lai, Y.-H.; Tsai, C.-H.; Yang, C.-H.; Yu, H.-G.; Wang, J.-C.; Huang, P.-L.; Lin, Y.-C. Metabolomic assessment of arsenite toxicity and novel biomarker discovery in early development of zebrafish embryos. Toxicol. Lett. 2018, 290, 116–122. [Google Scholar] [CrossRef]
- Olivares, C.I.; Field, J.A.; Simonich, M.; Tanguay, R.L.; Sierra-Alvarez, R. Arsenic (III, V), indium (III), and gallium (III) toxicity to zebrafish embryos using a high-throughput multi-endpoint in vivo developmental and behavioral assay. Chemosphere 2016, 148, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Carlson, P.; Smalley, D.M.; van Beneden, R.J. Proteomic Analysis of Arsenic-Exposed Zebrafish (Danio rerio) Identifies Altered Expression in Proteins Involved in Fibrosis and Lipid Uptake in a Gender-Specific Manner. Toxicol. Sci. 2013, 134, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Beaver, L.M.; Truong, L.; Barton, C.L.; Chase, T.T.; Gonnerman, G.D.; Wong, C.P.; Tanguay, R.L.; Ho, E. Combinatorial effects of zinc deficiency and arsenic exposure on zebrafish (Danio rerio) development. PLoS ONE 2017, 12, e0183831. [Google Scholar] [CrossRef] [Green Version]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Wasel, O.; Thompson, K.M.; Gao, Y.; Godfrey, A.E.; Gao, J.; Mahapatra, C.T.; Lee, L.S.; Sepulvda, M.S.; Freeman, J.L. Comparison of zebrafish in vitro and in vivo developmental toxicity assessments of perfluoroalkyl acids (PFAAs). J. Toxicol. Environ. Health Part A 2021, 84, 125–136. [Google Scholar] [CrossRef]
- Kabir, T.; Anwar, S.; Mourosi, J.T.; Hossain, J.; Rabbane, G.; Rahman, M.; Tahsin, T.; Hasan, N.; Shill, M.C.; Hosen, M.J. Arsenic hampered embryonic development: An in vivo study using local Bangladeshi Danio rerio model. Toxicol. Rep. 2020, 7, 155–161. [Google Scholar] [CrossRef]
- Yin, J.; Wang, A.P.; Li, W.F.; Shi, R.; Jin, H.T.; Wei, J.F. Sensitive biomarkers identification for differentiating Cd and Pb induced toxicity on zebrafish embryos. Environ. Toxicol. Pharmacol. 2017, 56, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Deneer, J.W. Toxicity of mixtures of pesticides in aquatic systems. Pest Manag. Sci. 2000, 56, 516–520. [Google Scholar] [CrossRef]
- Belden, J.B.; Gilliom, R.J.; Lydy, M.J. How well can we predict the toxicity of pesticide mixtures to aquatic life? Integr. Environ. Assess. Manag. 2007, 3, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Vijver, M.G.; Elliott, E.G.; Peijnenburg, W.J.G.M.; de Snoo, G.R. Response predictions for organisms water-exposed to metal mixtures: A meta-analysis. Environ. Toxicol. Chem. 2011, 30, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Nys, C.; van Regenmortel, T.; Janssen, C.R.; Oorts, K.; Smolders, E.; de Schamphelaere, K.A.C. A framework for ecological risk assessment of metal mixtures in aquatic systems. Environ. Toxicol. Chem. 2018, 37, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.; Cedergreen, N.; Skovgaard, I.M.; Streibig, J.C. An isobole-based statistical model and test for synergism/antagonism in binary mixture toxicity experiments. Environ. Ecol. Stat. 2007, 14, 383–397. [Google Scholar] [CrossRef]



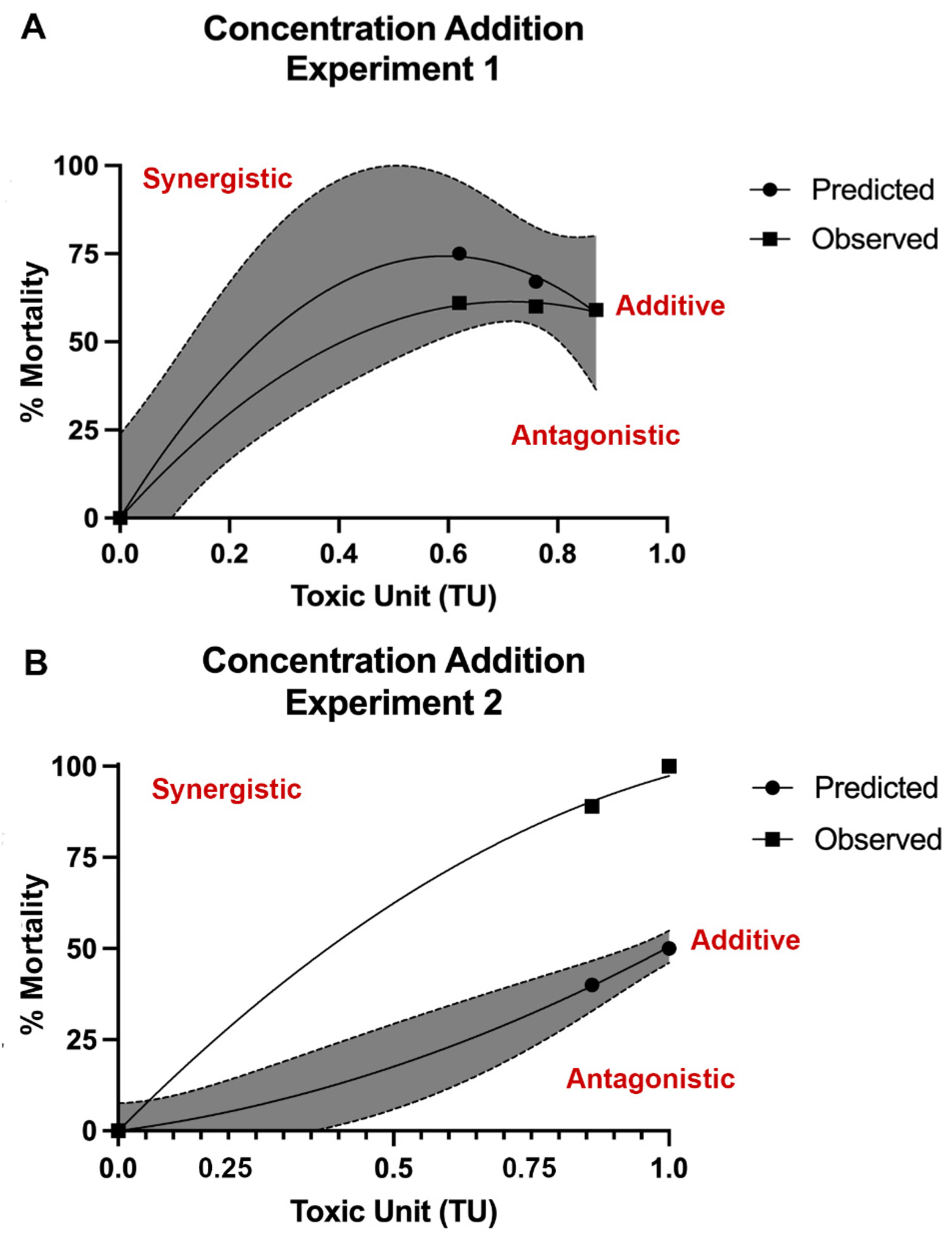

| Experiment | Mixture 1 | Mixture 2 | Mixture 3 |
|---|---|---|---|
| Experiment 1 | Pb LC25 + As LC25 | Pb LC25 + As LC50 | Pb LC25 + As LC75 |
| Experiment 2 | Pb LC50 + As LC25 | Pb LC50 + As LC50 | Pb LC50 + As LC75 |
| Experiment 3 | Pb LC75 + As LC25 | Pb LC75 + As LC50 | Pb LC75 + As LC75 |
| Mixture Treatments | Observed vs. CA | Observed vs. IA | ||
|---|---|---|---|---|
| x2 | p | x2 | p | |
| Pb LC25 + As LC25 | 22.427 | 0.0001 | 20.74 | 0.0001 |
| Pb LC25 + As LC50 | 3.209 | 0.0733 | - | - |
| Pb LC25 + As LC75 | 0.328 | 0.5666 | - | - |
| Pb LC50 + As LC25 | 48 | <0.0001 | - | - |
| Pb LC50 + As LC50 | 50 | <0.0001 | 50 | 0.0001 |
| Pb LC50 + As LC75 | 33.333 | <0.0001 | - | - |
| Pb LC75 + As LC25 | 50 | <0.0001 | - | - |
| Pb LC75+ As LC50 | 25 | <0.0001 | - | - |
| Pb LC75 + As LC75 | 15.97 | <0.0001 | 15.789 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiper, K.; Freeman, J.L. Joint Action Toxicity of Arsenic (As) and Lead (Pb) Mixtures in Developing Zebrafish. Biomolecules 2022, 12, 1833. https://doi.org/10.3390/biom12121833
Kiper K, Freeman JL. Joint Action Toxicity of Arsenic (As) and Lead (Pb) Mixtures in Developing Zebrafish. Biomolecules. 2022; 12(12):1833. https://doi.org/10.3390/biom12121833
Chicago/Turabian StyleKiper, Keturah, and Jennifer L. Freeman. 2022. "Joint Action Toxicity of Arsenic (As) and Lead (Pb) Mixtures in Developing Zebrafish" Biomolecules 12, no. 12: 1833. https://doi.org/10.3390/biom12121833