Development of a Gastrointestinal-Myoelectrical-Activity-Based Nomogram Model for Predicting the Risk of Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive Function Assessment
2.3. EGEG Records
2.4. Gastrointestinal Electrical Index
2.5. Statistical Analysis
3. Results
3.1. Clinical Baseline Information of Participants
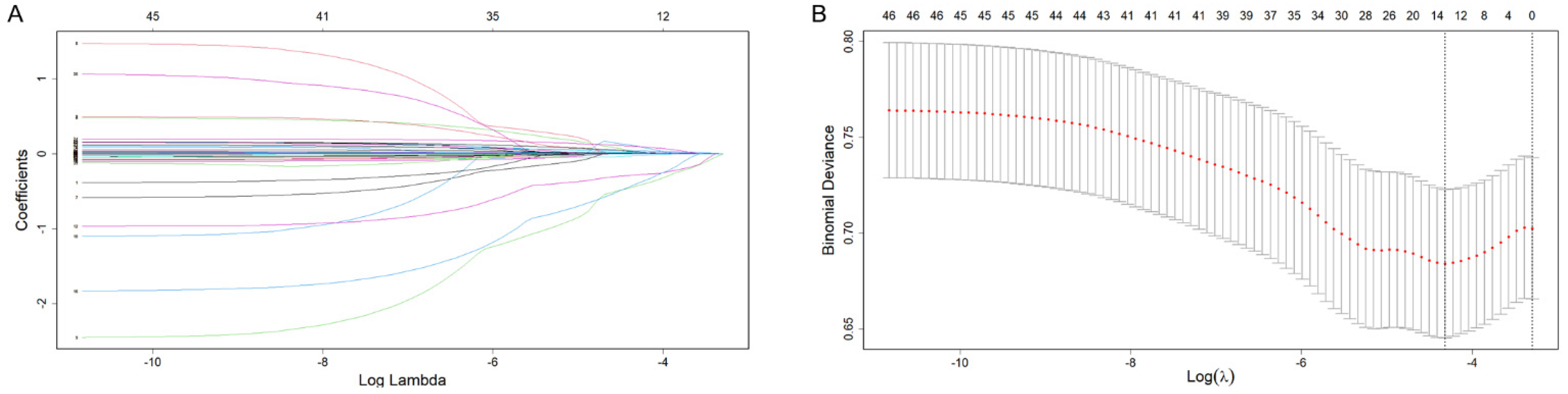
3.2. Independent Risk Factor Screening
3.3. Building the Predictive Model
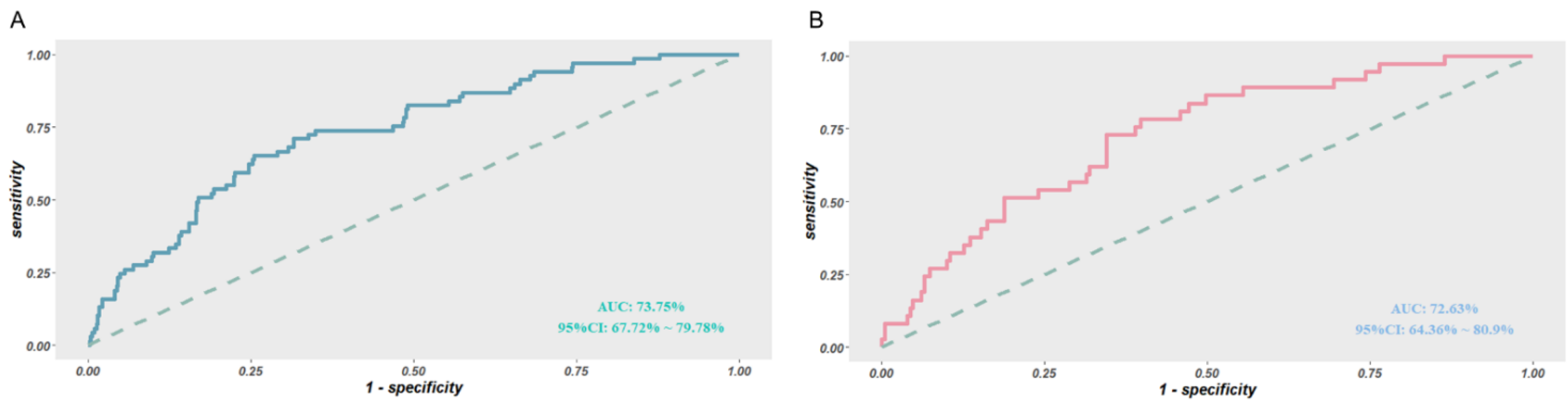
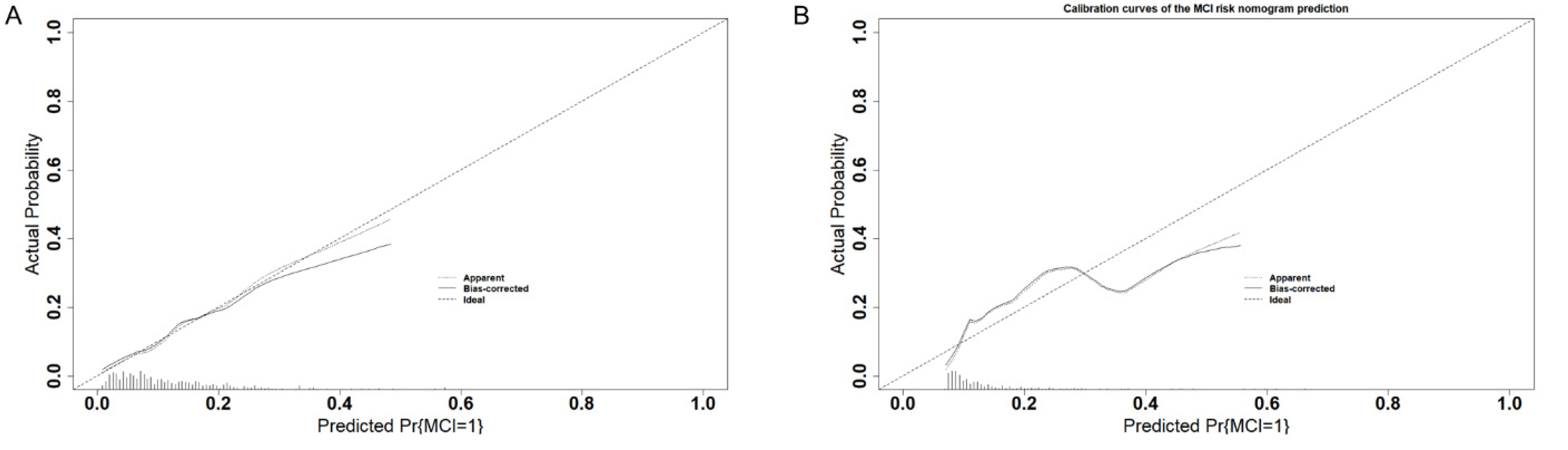
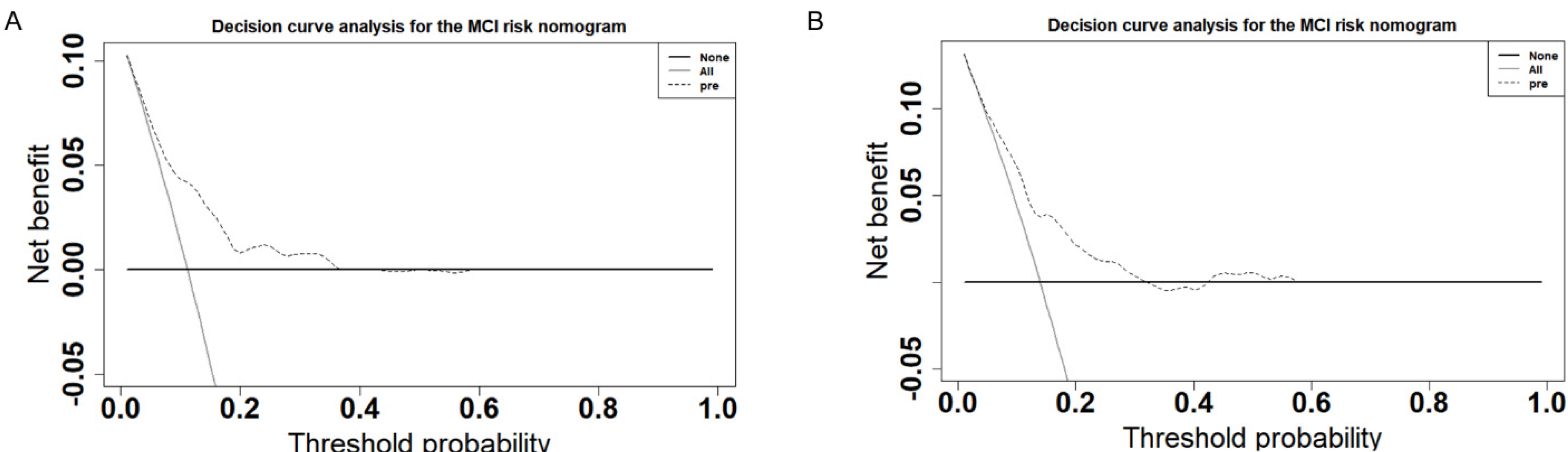
3.4. Validation of the Predictive Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Di Pasquale, V.; Buscemi, C.; Piccoli, T.; Giordano, C. Factors associated with mild cognitive impairment in a population-based cohort. Eur. J. Intern. Med. 2017, 43, e20–e21. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Chen, Z.L.; Strickland, S.; Norris, E.H. Increased Contact System Activation in Mild Cognitive Impairment Patients with Impaired Short-Term Memory. J. Alzheimers Dis. 2020, 77, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Fujishiro, H.; Takechi, H. Efficacy and Safety of Cholinesterase Inhibitors for Mild Cognitive Impairment:A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2019, 71, 513–523. [Google Scholar] [CrossRef]
- Chandler, M.J.; Parks, A.C.; Marsiske, M.; Rotblatt, L.J.; Smith, G.E. Everyday Impact of Cognitive Interventions in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2016, 26, 225–251. [Google Scholar] [CrossRef]
- Chang, Y.T. Physical Activity and Cognitive Function in Mild Cognitive Impairment. ASN Neuro 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Zhuang, L.; Yang, Y.; Gao, J. Cognitive assessment tools for mild cognitive impairment screening. J. Neurol. 2021, 268, 1615–1622. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Stewart, R. Mild cognitive impairment--the continuing challenge of its “real-world” detection and diagnosis. Arch. Med. Res. 2012, 43, 609–614. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Qin, Y.; Ai, D.; Jordan, A.E.; Guo, X.; Li, T.; Diao, S.; Zhao, H.; Liu, Y.; Xue, Q.; Wang, Y.; et al. Better Screening Value of Sylvian Fissure Ratio on Cognitive Decline Among Female Compared to Male: An Observational Study in Elderly Patients with Cerebral Small Vessel Disease in Soochow. Front. Neurosci. 2021, 15, 729782. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; Ray Chaudhuri, K.; Reynolds, R.; Tan, E.K.; Pettersson, S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain 2021, 144, 2571–2593. [Google Scholar] [CrossRef]
- Gorbovskaya, I.; Kanji, S.; Liu, J.C.W.; MacKenzie, N.E.; Agarwal, S.M.; Marshe, V.S.; Sriretnakumar, V.; Verdu, E.F.; Bercik, P.; De Palma, G.; et al. Investigation of the Gut Microbiome in Patients with Schizophrenia and Clozapine-Induced Weight Gain: Protocol and Clinical Characteristics of First Patient Cohorts. Neuropsychobiology 2020, 79, 5–12. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.E.; Bai, Y.M.; Tsai, S.J.; Su, T.P.; Chen, T.J.; Wang, Y.P.; Chen, M.H. Inflammatory bowel disease is associated with higher dementia risk: A nationwide longitudinal study. Gut 2021, 70, 85–91. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.D. Electrogastrography: Methodology, validation and applications. J. Neurogastroenterol. Motil. 2013, 19, 5–17. [Google Scholar] [CrossRef]
- Wang, T.; Yan, Y.F.; Yang, L.; Huang, Y.Z.; Duan, X.H.; Su, K.H.; Liu, W.L. Effects of Zuojin pill on depressive behavior and gastrointestinal function in rats with chronic unpredictable mild stress: Role of the brain-gut axis. J. Ethnopharmacol. 2020, 254, 112713. [Google Scholar] [CrossRef] [PubMed]
- Krygowska-Wajs, A.; Furgala, A.; Gorecka-Mazur, A.; Pietraszko, W.; Thor, P.; Potasz-Kulikowska, K.; Moskala, M. The effect of subthalamic deep brain stimulation on gastric motility in Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 26, 35–40. [Google Scholar] [CrossRef]
- Weimer, K.; Sauer, H.; Horing, B.; Valitutti, F.; Mazurak, N.; Zipfel, S.; Stengel, A.; Enck, P.; Mack, I. Impaired Gastric Myoelectrical Reactivity in Children and Adolescents with Obesity Compared to Normal-Weight Controls. Nutrients 2018, 10, 699. [Google Scholar] [CrossRef]
- Obbels, J.; Vansteelandt, K.; Verwijk, E.; Dols, A.; Bouckaert, F.; Oudega, M.L.; Vandenbulcke, M.; Stek, M.; Sienaert, P. MMSE Changes During and After ECT in Late-Life Depression: A Prospective Study. Am. J. Geriatr. Psychiatry 2019, 27, 934–944. [Google Scholar] [CrossRef]
- Peng, A.; Ji, S.; Li, W.; Lai, W.; Qiu, X.; He, S.; Dong, B.; Huang, C.; Chen, L. Gastric Electrical Dysarrhythmia in Probable Rapid Eye Movement Sleep Behavior Disorder. Front. Neurol. 2021, 12, 687215. [Google Scholar] [CrossRef]
- Lim, S.J.; Lee, Z.; Kwon, L.N.; Chun, H.W. Medical Health Records-Based Mild Cognitive Impairment (MCI) Prediction for Effective Dementia Care. Int. J. Environ. Res. Public Health 2021, 18, 9223. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–218. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Montesi, F.; Lucicesare, A.; Pisacane, N.; Rietti, E.; Dalmonte, E.; Bianchin, M.; Mecocci, P. Mild cognitive impairment: Epidemiology and dementia risk in an elderly Italian population. J. Am. Geriatr. Soc. 2008, 56, 51–58. [Google Scholar] [CrossRef]
- Artero, S.; Ancelin, M.L.; Portet, F.; Dupuy, A.; Berr, C.; Dartigues, J.F.; Tzourio, C.; Rouaud, O.; Poncet, M.; Pasquier, F.; et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J. Neurol. Neurosurg. Psychiatry 2008, 79, 979–984. [Google Scholar] [CrossRef]
- Tsoi, K.K.F.; Chan, J.Y.C.; Hirai, H.W.; Wong, A.; Mok, V.C.T.; Lam, L.C.W.; Kwok, T.C.Y.; Wong, S.Y.S. Recall Tests Are Effective to Detect Mild Cognitive Impairment: A Systematic Review and Meta-analysis of 108 Diagnostic Studies. J. Am. Med. Dir. Assoc. 2017, 18, 807. [Google Scholar] [CrossRef]
- De Roeck, E.E.; De Deyn, P.P.; Dierckx, E.; Engelborghs, S. Brief cognitive screening instruments for early detection of Alzheimer's disease: A systematic review. Alzheimers Res. Ther. 2019, 11, 21. [Google Scholar] [CrossRef]
- Poscente, M.D.; Mintchev, M.P. Enhanced electrogastrography: A realistic way to salvage a promise that was never kept? World J. Gastroenterol. 2017, 23, 4517–4528. [Google Scholar] [CrossRef]
- Tokmakçi, M. Analysis of the electrogastrogram using discrete wavelet transform and statistical methods to detect gastric dysrhythmia. J. Med. Syst. 2007, 31, 295–302. [Google Scholar] [CrossRef]
- Zhao, Q.; Ning, B.F.; Zhou, J.Y.; Wang, J.; Yao, Y.J.; Peng, Z.Y.; Yuan, Z.L.; Chen, J.D.Z.; Xie, W.F. Transcutaneous Electrical Acustimulation Ameliorates Motion Sickness Induced by Rotary Chair in Healthy Subjects: A Prospective Randomized Crossover Study. Neuromodulation 2021, 25, 1421–1430. [Google Scholar] [CrossRef]
- Li, M.; Xu, F.; Liu, M.; Li, Y.; Zheng, J.; Zhu, Y.; Lin, L.; Chen, J. Effects and Mechanisms of Transcutaneous Electrical Acustimulation on Postoperative Recovery After Elective Cesarean Section. Neuromodulation 2020, 23, 838–846. [Google Scholar] [CrossRef]


| Variables | Training Set (N = 620) | Validation Set (N = 266) | ||||
|---|---|---|---|---|---|---|
| With MCI | Without MCI | p Value | With MCI | Without MCI | p Value | |
| Gender | ||||||
| Female | 45 (65.217) | 378 (68.603) | 0.666 | 26 (70.270) | 164 (71.616) | 1.000 |
| Male | 24 (34.783) | 173 (31.397) | 11 (29.730) | 65 (28.384) | ||
| Smoke | 13 (18.841) | 69 (12.523) | 0.203 | 4 (10.811) | 23 (10.044) | 0.776 |
| Alcohol | 22 (31.884) | 128 (23.230) | 0.152 | 8 (21.622) | 44 (19.214) | 0.905 |
| Age | 58.072 ± 6.434 | 55.713 ± 6.236 | 0.005 | 61.000 ± 6.637 | 55.764 ± 6.296 | <0.001 |
| BMI | 23.694 ± 2.811 | 24.298 ± 3.104 | 0.100 | 24.099 ± 2.396 | 24.464 ± 3.385 | 0.423 |
| Glucose | 5.392 ± 1.191 | 5.449 ± 1.155 | 0.705 | 5.811 ± 1.973 | 5.427 ± 1.313 | 0.260 |
| TG | 1.588 ± 0.758 | 1.575 ± 1.014 | 0.900 | 1.632 ± 0.781 | 1.781 ± 1.861 | 0.405 |
| TCH | 5.477 ± 0.836 | 5.372 ± 0.976 | 0.337 | 5.196 ± 0.932 | 5.397 ± 1.010 | 0.235 |
| HDL | 1.612 ± 0.398 | 1.792 ± 0.505 | 0.001 | 1.477 ± 0.379 | 1.777 ± 0.477 | <0.001 |
| LDL | 3.145 ± 0.729 | 2.990 ± 0.718 | 0.098 | 3.046 ± 0.743 | 2.978 ± 0.732 | 0.605 |
| Variables | β | Std Error | Z Value | p Value | OR | 95%CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 0.068 | 0.021 | 3.257 | 0.001 | 1.071 | 1.027 | 1.116 |
| BMI | −0.090 | 0.047 | −1.942 | 0.052 | 0.914 | 0.832 | 0.999 |
| HDL | −0.920 | 0.318 | −2.895 | 0.004 | 0.399 | 0.208 | 0.726 |
| LDL | 0.323 | 0.180 | 1.797 | 0.072 | 1.382 | 0.972 | 1.972 |
| TDIA | 0.177 | 0.090 | 1.970 | 0.049 | 1.193 | 0.992 | 1.428 |
| DFGA | −1.141 | 0.559 | −2.042 | 0.041 | 0.320 | 0.104 | 0.938 |
| RDGA | 0.122 | 0.044 | 2.749 | 0.006 | 1.130 | 1.036 | 1.233 |
| DPGA | 0.091 | 0.029 | 3.143 | 0.002 | 1.096 | 1.036 | 1.161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Ji, S.; Peng, A.; Yang, N.; Zhao, X.; Feng, P.; Zhang, Y.; Chen, L. Development of a Gastrointestinal-Myoelectrical-Activity-Based Nomogram Model for Predicting the Risk of Mild Cognitive Impairment. Biomolecules 2022, 12, 1861. https://doi.org/10.3390/biom12121861
Li B, Ji S, Peng A, Yang N, Zhao X, Feng P, Zhang Y, Chen L. Development of a Gastrointestinal-Myoelectrical-Activity-Based Nomogram Model for Predicting the Risk of Mild Cognitive Impairment. Biomolecules. 2022; 12(12):1861. https://doi.org/10.3390/biom12121861
Chicago/Turabian StyleLi, Baichuan, Shuming Ji, Anjiao Peng, Na Yang, Xia Zhao, Peimin Feng, Yunwu Zhang, and Lei Chen. 2022. "Development of a Gastrointestinal-Myoelectrical-Activity-Based Nomogram Model for Predicting the Risk of Mild Cognitive Impairment" Biomolecules 12, no. 12: 1861. https://doi.org/10.3390/biom12121861
APA StyleLi, B., Ji, S., Peng, A., Yang, N., Zhao, X., Feng, P., Zhang, Y., & Chen, L. (2022). Development of a Gastrointestinal-Myoelectrical-Activity-Based Nomogram Model for Predicting the Risk of Mild Cognitive Impairment. Biomolecules, 12(12), 1861. https://doi.org/10.3390/biom12121861






