Rapid and Highly Efficient Genetic Transformation and Application of Interleukin-17B Expressed in Duckweed as Mucosal Vaccine Adjuvant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Callus Induction
2.3. Agrobacterium-Mediated Transformation and Regeneration of L. minor
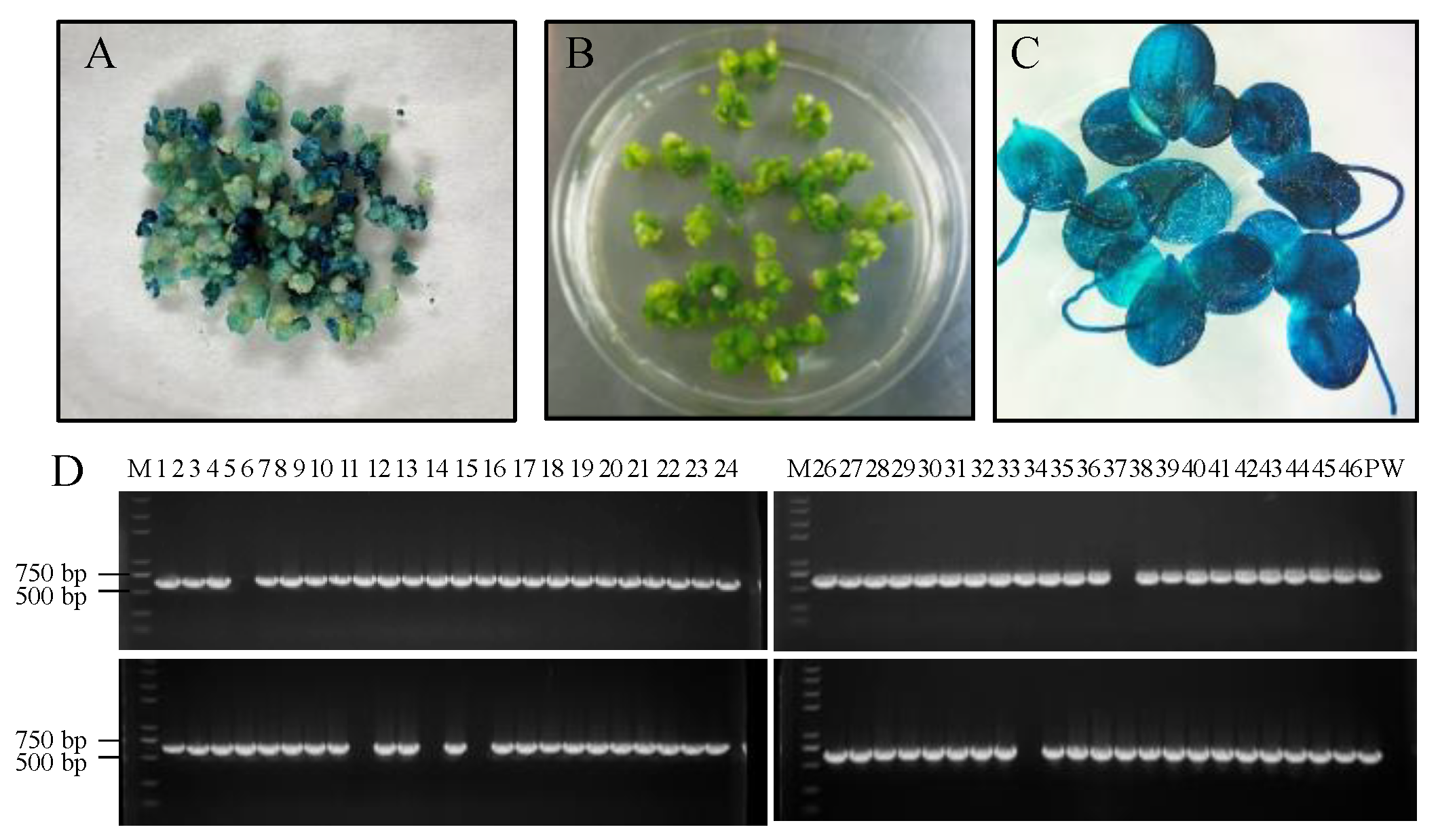
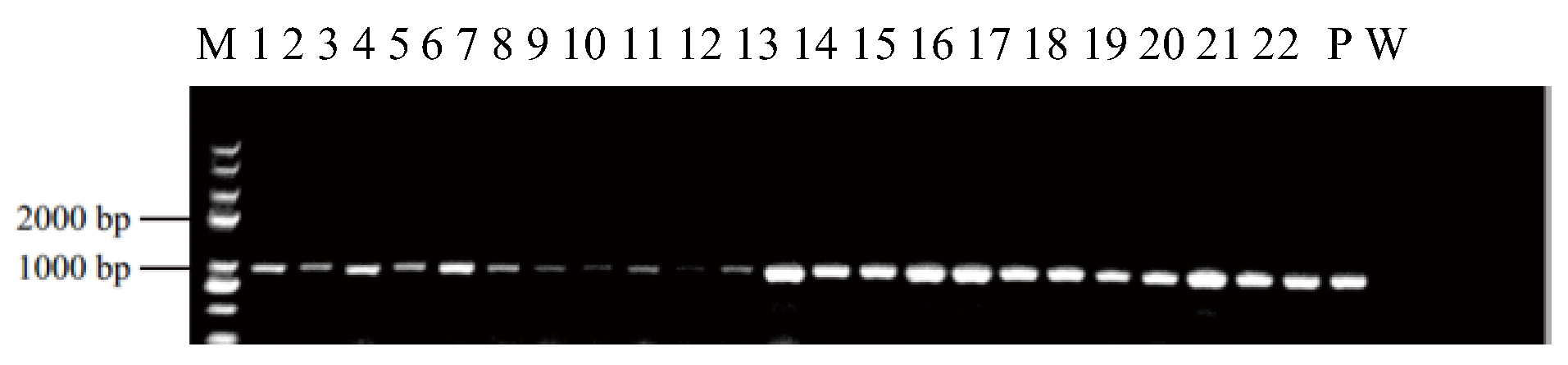
2.4. PCR Analysis, GUS Staining, and Transformation Efficiency
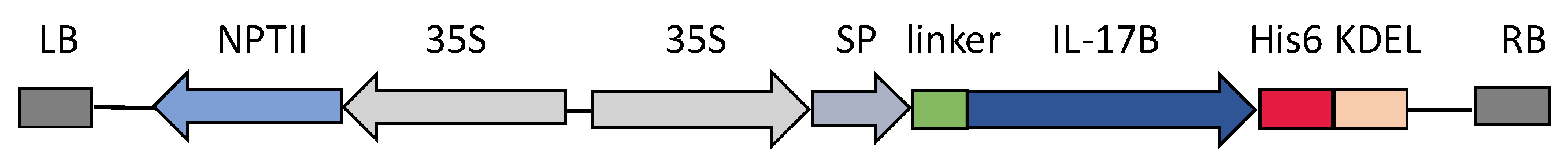
2.5. Construction of Plant Expression Vectors for chIL-17B
2.6. Detection and Quantification of the Recombinant Protein
2.7. Purification of Recombinant chIL-17B
2.8. Analysis of the Bioactivity of cIL-17B in DF-1 Cells
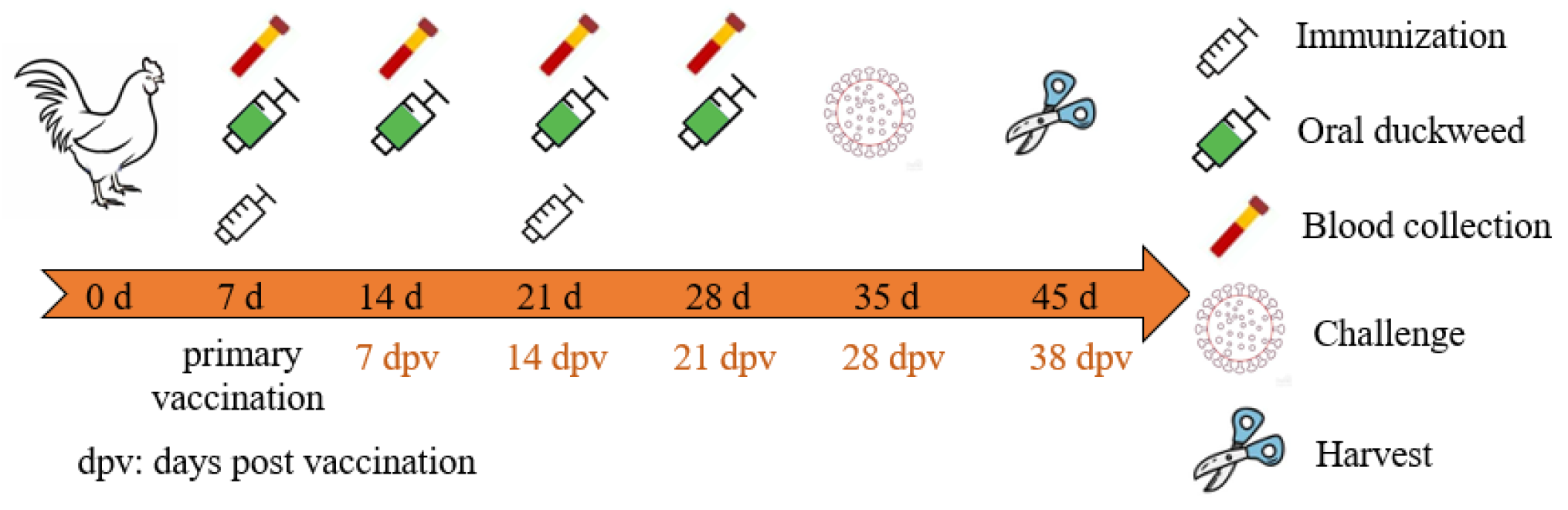
2.9. Animal Immunization
2.10. Evaluation of the Immunoadjuvant Effect of chIL17B in Chickens
2.11. Statistical Analysis
3. Results
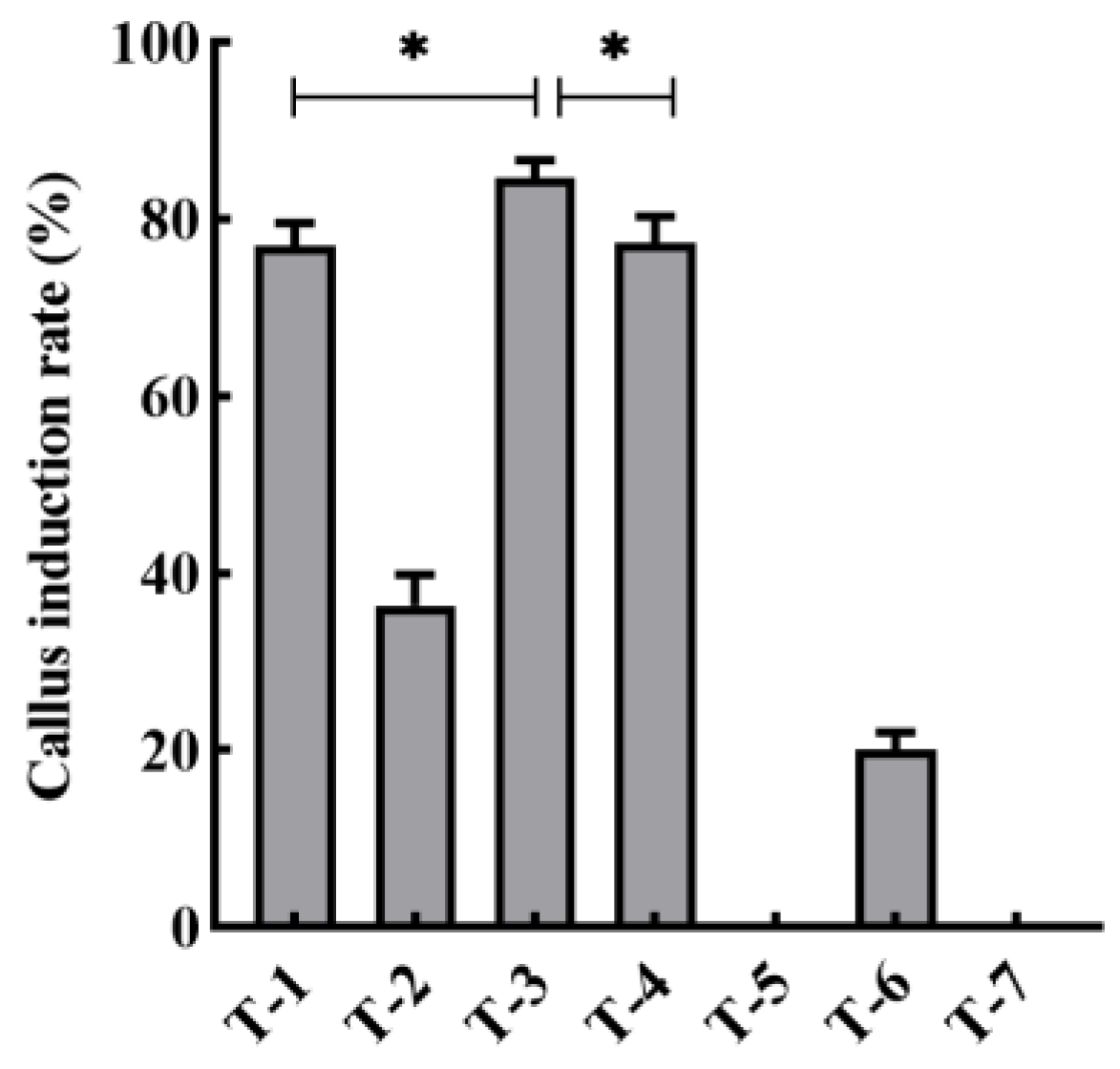
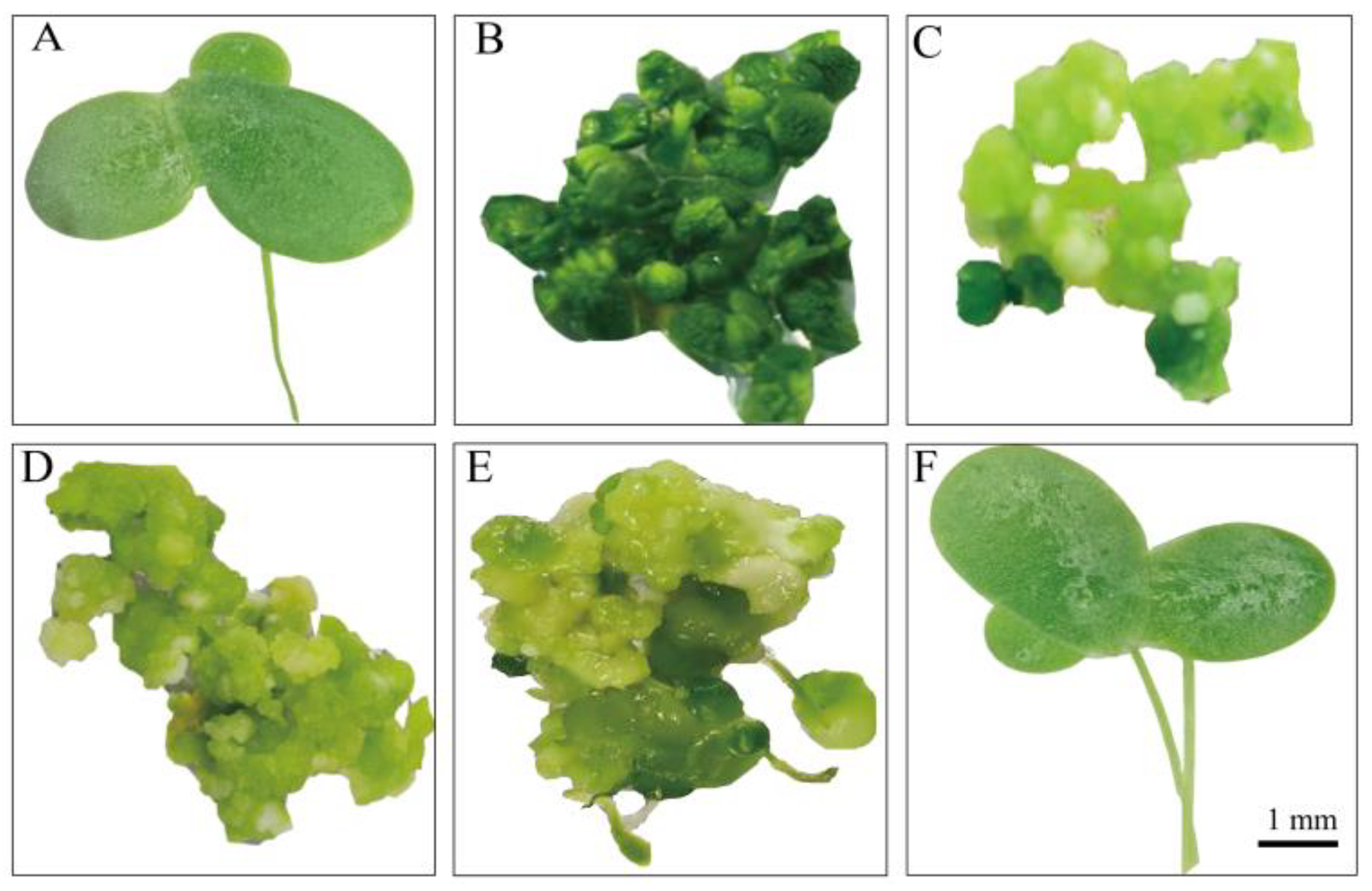
3.1. An Efficient and Stable Genetic Transformation System for L. minor
3.2. Expression of Bioactive chIL-17B in L. minor
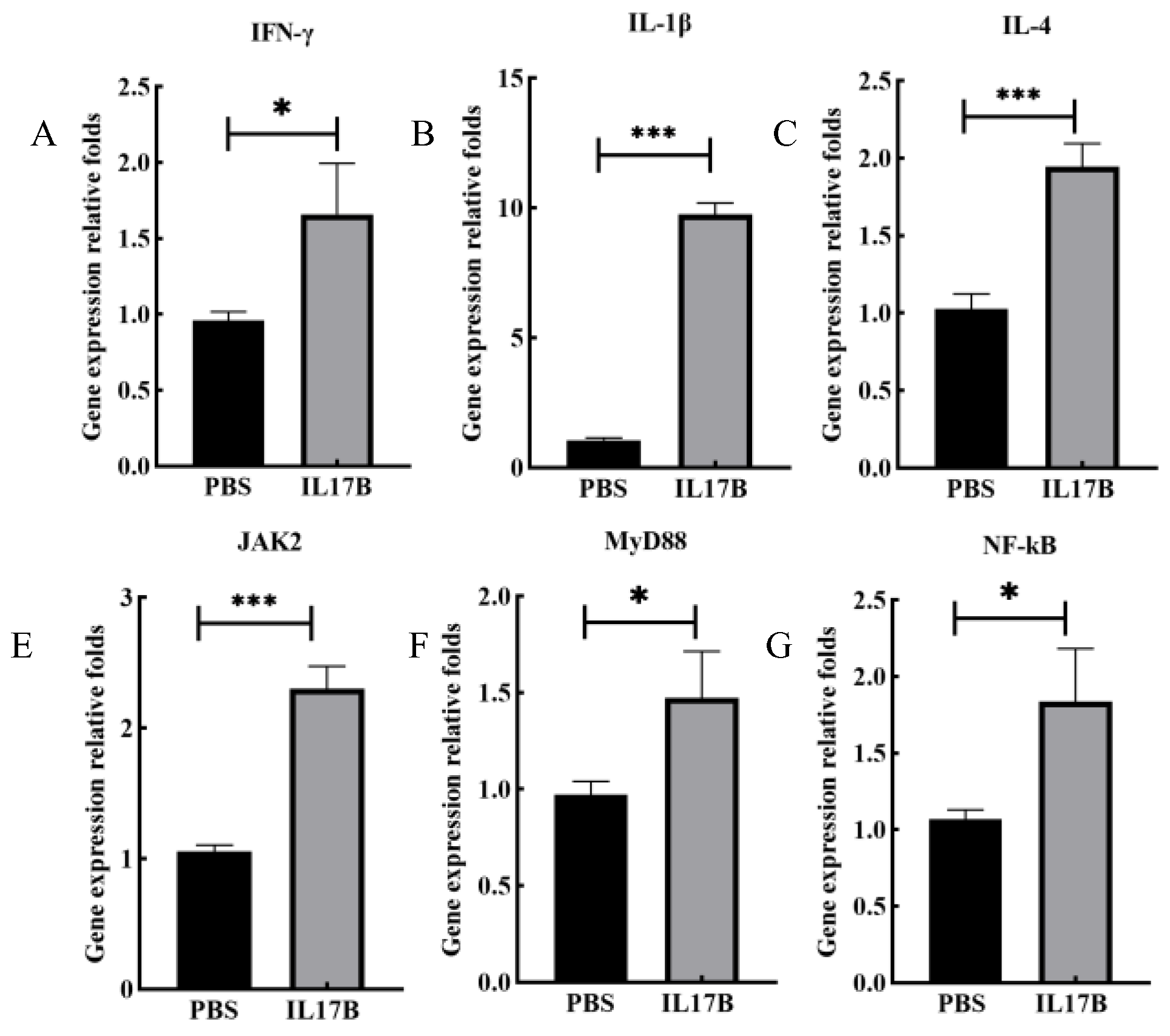
3.3. ChIL17B Transgenic Duckweed Exhibited Potent Immunoadjuvant Activities in Chickens
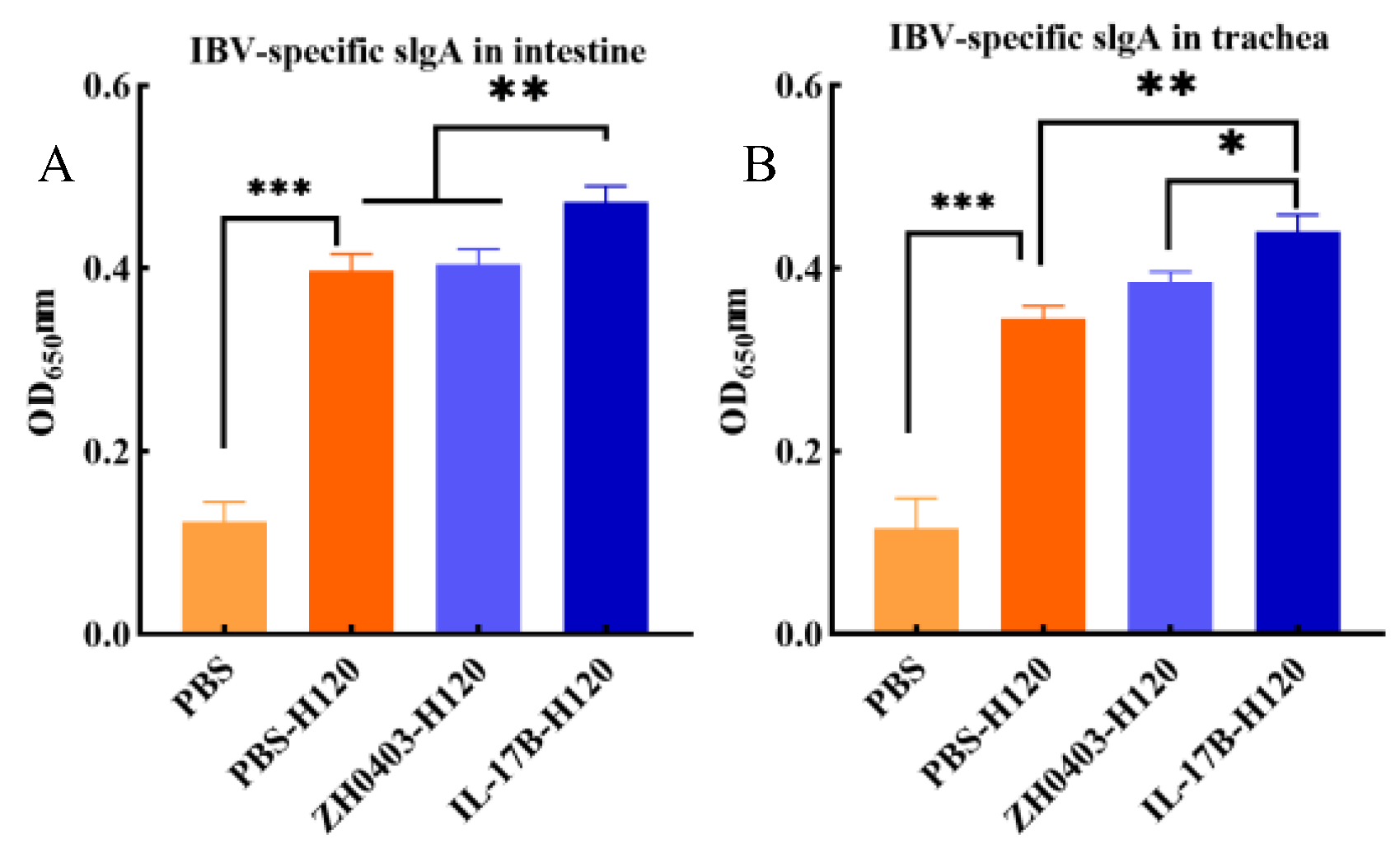
3.3.1. Enhancement of Humoral Immune Responses
3.3.2. Mucosal Immune Responses
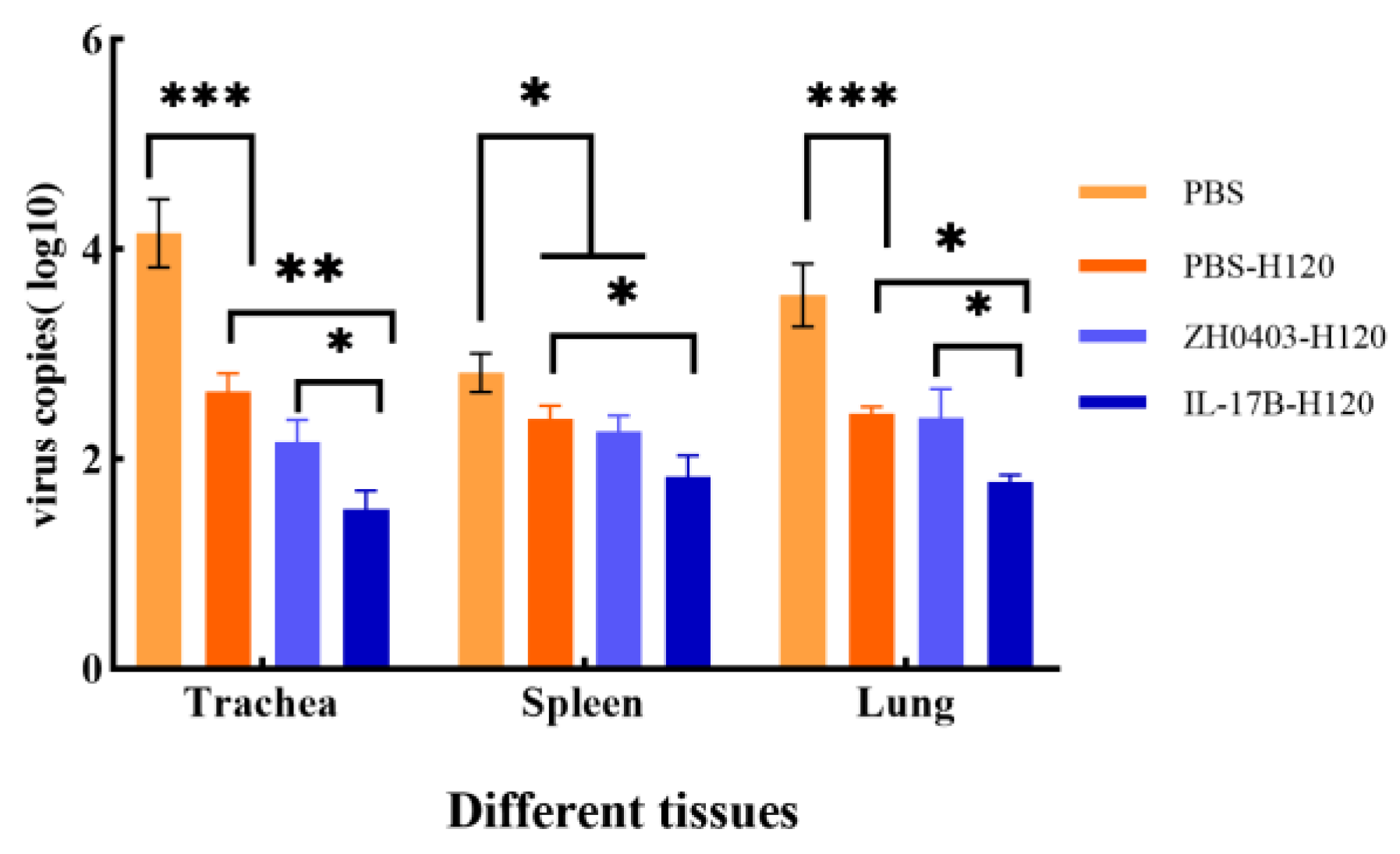
3.3.3. Challenges of Protective Immunity against Bronchitis Virus
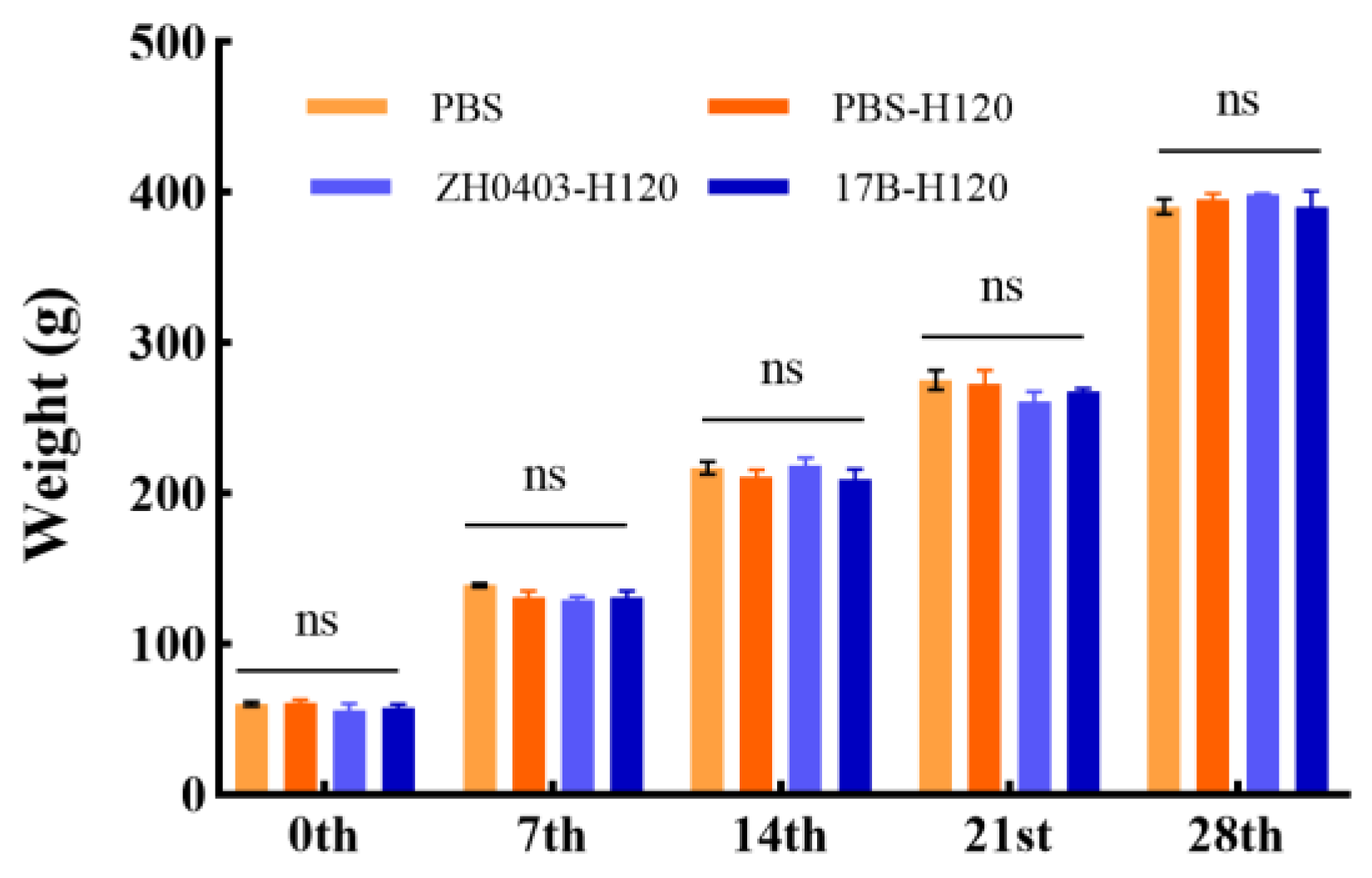
3.3.4. No Effect on Body Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hiatt, A.; Cafferkey, R.; Bowdish, K. Production of Antibodies in Transgenic Plants. Nature 1989, 342, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Buyel, J.F. Molecular farming—The slope of enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S.; Nieto-Gomez, R. Green Therapeutic Biocapsules: Using Plant Cells to Orally Deliver Biopharmaceuticals. Trends Biotechnol. 2018, 36, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 2022, 7, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.S.; Cherian, S.; Sumithra, T.G.; Raina, O.K.; Sankar, M. Edible vaccines against veterinary parasitic diseases—Current status and future prospects. Vaccine 2013, 31, 1879–1885. [Google Scholar] [CrossRef]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.J. Relative in vitro growth rates of duckweeds (Lemnaceae)—The most rapidly growing higher plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.K.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef]
- Trewavas, A. The turnover of nucleic acids in Lemna minor. Plant Physiol 1970, 45, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Trewavas, A. Determination of the Rates of Protein Synthesis and Degradation in Lemna minor. Plant Physiol. 1972, 49, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Rapparini, F.; Cohen, J.D.; Slovin, J.P. Indole-3-acetic acid biosynthesis in Lemna gibba studied using stable isotope labeled anthranilate and tryptophan. Plant Growth Regul. 1999, 27, 139–144. [Google Scholar] [CrossRef]
- Tanaka, O.; Cleland, C.F. Comparison of the Ability of Salicylic-Acid and Ferricyanide to Induce Flowering in the Long-Day Plant, Lemna-Gibba-G3. Plant Physiol. 1980, 65, 1058–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.B.; Fang, Y.; Yi, Z.L.; Tian, X.P.; Li, J.M.; Jin, Y.L.; He, K.Z.; Liu, P.H.; Du, A.P.; Huang, Y.H.; et al. Determining the nutritional value and antioxidant capacity of duckweed (Wolffia arrhiza) under artificial conditions. LWT Food Sci. Technol. 2022, 153, 112477. [Google Scholar] [CrossRef]
- Yang, L.G.; Feng, D.; Liu, Y.T.; Lv, S.M.; Zheng, M.M.; Tan, A.J. Research Progress of a Potential Bioreactor: Duckweed. Biomolecules 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Khvatkov, P.; Firsov, A.; Shvedova, A.; Kozlov, O.; Chernobrovkina, M.; Pushin, A.; Shaloiko, L.; Dolgov, S. Wolffia arrhiza as a promising producer of recombinant hirudin. 3 Biotech 2021, 11, 209. [Google Scholar] [CrossRef]
- Firsov, A.; Tarasenko, I.; Mitiouchkina, T.; Ismailova, N.; Shaloiko, L.; Vainstein, A.; Dolgov, S. High-Yield Expression of M2e Peptide of Avian Influenza Virus H5N1 in Transgenic Duckweed Plants. Mol. Biotechnol. 2015, 57, 653–661. [Google Scholar] [CrossRef]
- Cox, K.M.; Sterling, J.D.; Regan, J.T.; Gasdaska, J.R.; Frantz, K.K.; Peele, C.G.; Black, A.; Passmore, D.; Moldovan-Loomis, C.; Srinivasan, M.; et al. Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat. Biotechnol. 2006, 24, 1591–1597. [Google Scholar] [CrossRef]
- Firsov, A.; Tarasenko, I.; Mitiouchkina, T.; Shaloiko, L.; Kozlov, O.; Vinokurov, L.; Rasskazova, E.; Murashev, A.; Vainstein, A.; Dolgov, S. Expression and Immunogenicity of M2e Peptide of Avian Influenza Virus H5N1 Fused to Ricin Toxin B Chain Produced in Duckweed Plants. Front. Chem. 2018, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.M.; Sun, H.J.; Oh, M.J.; Song, I.J.; Kim, M.J.; Sin, H.S.; Goh, C.H.; Kim, Y.W.; Lim, P.O.; Lee, H.Y.; et al. Expression of the Protective Antigen for PEDV in Transgenic Duckweed, Lemna minor. Hortic. Environ. Biotechnol. 2011, 52, 511–515. [Google Scholar] [CrossRef]
- Heenatigala, P.P.M.; Sun, Z.; Yang, J.; Zhao, X.; Hou, H. Expression of LamB Vaccine Antigen in Wolffia globosa (Duck Weed) Against Fish Vibriosis. Front. Immunol 2020, 11, 1857. [Google Scholar] [CrossRef]
- Khvatkov, P.; Firsov, A.; Shvedova, A.; Shaloiko, L.; Kozlov, O.; Chernobrovkina, M.; Pushin, A.; Tarasenko, I.; Chaban, I.; Dolgov, S. Development of Wolffia arrhiza as a Producer for Recombinant Human Granulocyte Colony-Stimulating Factor. Front. Chem. 2018, 6, 304. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Tobin, E.M. Deletion Analysis of a Phytochrome-Regulated Monocot Rbcs Promoter in a Transient Assay System. Proc. Natl. Acad. Sci. USA 1991, 88, 2683–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelman, M.; Perl, A.; Flaishman, M.; Blumenthal, A. Transgenic Lemnaceae; WO 99/19497; Yeda Research and Development Co. Ltd.: Rehovot, Israel, 1999. [Google Scholar]
- Stomp, A.M.; Rajbhandari, N. Genetically Engineered Duckweed; WO 1999007210; United States Environmental Protection Agency: Washington, DC, USA, 1999.
- Yang, L.; Han, Y.J.; Wu, D.; Yong, W.; Liu, M.M.; Wang, S.T.; Liu, W.X.; Lu, M.Y.; Wei, Y.; Sun, J.S. Salt and cadmium stress tolerance caused by overexpression of the Glycine Max Na+/H+ Antiporter (GmNHX1) gene in duckweed (Lemna turionifera 5511). Aquat. Toxicol. 2017, 192, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Xu, S.; Tang, X.; Zhao, J.; Yu, C.; He, G.; Xu, H.; Wang, S.; Tang, Y.; et al. Efficient genetic transformation and CRISPR/Cas9-mediated genome editing in Lemna aequinoctialis. Plant Biotechnol. J. 2019, 17, 2143–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Fu, L.; Sun, X.; Di, R.; Zhang, J. Rapid and highly efficient callus induction and plant regeneration in the starch-rich duckweed strains of Landoltia punctata. Acta Physiol. Plant. 2016, 38, 122. [Google Scholar] [CrossRef]
- Canto-Pastor, A.; Molla-Morales, A.; Ernst, E.; Dahl, W.; Zhai, J.; Yan, Y.; Meyers, B.C.; Shanklin, J.; Martienssen, R. Efficient transformation and artificial miRNA gene silencing in Lemna minor. Plant Biol. 2015, 17, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Na, Y.J.; Yang, B.G.; Choi, J.P.; Seo, Y.B.; Hong, C.P.; Yun, C.H.; Kim, D.H.; Sohn, E.J.; Kim, J.H.; et al. Oral immunization of haemaggulutinin H5 expressed in plant endoplasmic reticulum with adjuvant saponin protects mice against highly pathogenic avian influenza A virus infection. Plant Biotechnol. J. 2015, 13, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Rhee, J.H.; Lee, S.E.; Kim, S.Y. Mucosal vaccine adjuvants update. Clin. Exp. Vaccine Res. 2012, 1, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Bhadouriya, S.; Sharma, B.K.; Kakker, N.K.; Chhabra, R. Toll like receptors and cytokines as immunostimulatory adjuvants in poultry vaccines: Current status and future trends. World’s Poult. Sci. J. 2019, 75, 417–428. [Google Scholar] [CrossRef]
- Wang, X.; Meng, D. Innate endogenous adjuvants prime to desirable immune responses via mucosal routes. Protein Cell 2015, 6, 170–184. [Google Scholar] [CrossRef]
- Maier, C.; Fuchs, J.; Irrgang, P.; Wissing, M.H.; Beyerlein, J.; Tenbusch, M.; Lapuente, D. Mucosal immunization with an adenoviral vector vaccine confers superior protection against RSV compared to natural immunity. Front. Immunol. 2022, 13, 920256. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, W.; Zhang, H.; Zhang, S.; Cui, L.; Wang, M.; Bai, X.; Yang, W.; Sun, L.; Yang, L.; et al. Interferon as a Mucosal Adjuvant for an Influenza Vaccine in Pigs. Virol. Sin. 2019, 34, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, D.; Taniai, M.; Ariyasu, H.; Taniguchi, M.; Aga, M.; Ariyasu, T.; Ohta, T.; Fukuda, S. Cholesteryl Pullulan Encapsulated TNF-α Nanoparticles Are an Effective Mucosal Vaccine Adjuvant against Influenza Virus. BioMed Res. Int. 2015, 2015, 471468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Peng, J.; Xiao, Y.; Liu, Y.; Hao, W.; Yang, X.; Wang, H.; Gao, R. The Construction and Immunoadjuvant Activities of the Oral Interleukin-17B Expressed by Lactobacillus plantarum NC8 Strain in the Infectious Bronchitis Virus Vaccination of Chickens. Vaccines 2020, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Laconi, A.; Weerts, E.A.W.S.; Bloodgood, J.C.G.; Marrero, J.P.D.; Berends, A.J.; Cocciolo, G.; de Wit, J.J.; Verheije, M.H. Attenuated live infectious bronchitis virus QX vaccine disseminates slowly to target organs distant from the site of inoculation. Vaccine 2020, 38, 1486–1493. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and Techniques for Induction and Growth of Monocotyledonous and Dicotyledonous Plant Cell Cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient Requirements of Suspension Cultures of Soybean Root Cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Do, P.T.; Lee, H.; Mookkan, M.; Folk, W.R.; Zhang, Z.Y.J. Rapid and efficient Agrobacterium-mediated transformation of sorghum (Sorghum bicolor) employing standard binary vectors and bar gene as a selectable marker. Plant Cell Rep. 2016, 35, 2065–2076. [Google Scholar] [CrossRef]
- Lin, Y.J.; Zhang, Q. Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep. 2005, 23, 540–547. [Google Scholar] [CrossRef]
- Khvatkov, P.; Chernobrovkina, M.; Okuneva, A.; Pushin, A.; Dolgov, S. Transformation of Wolffia arrhiza (L.) Horkel ex Wimm. Plant Cell Tissue Organ Cult. 2015, 123, 299–307. [Google Scholar] [CrossRef]
- Lu, Y.T.; Li, M.Y.; Cheng, K.T.; Tan, C.M.; Su, L.W.; Lin, W.Y.; Shih, H.T.; Chiou, T.J.; Yang, J.Y. Transgenic Plants That Express the Phytoplasma Effector SAP11 Show Altered Phosphate Starvation and Defense Responses. Plant Physiol. 2014, 164, 1456–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redkiewicz, P.; Wiesyk, A.; Gora-Sochacka, A.; Sirko, A. Transgenic tobacco plants as production platform for biologically active human interleukin 2 and its fusion with proteinase inhibitors. Plant Biotechnol. J. 2012, 10, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jain, M.; Vunsh, R.; Vishnevetsky, J.; Hanania, U.; Flaishman, M.; Perl, A.; Edelman, M. Callus induction and regeneration in Spirodela and Lemna. Plant Cell Rep. 2004, 22, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Heenatigala, P.P.M.; Yang, J.J.; Bishopp, A.; Sun, Z.L.; Li, G.J.; Kumar, S.; Hu, S.Q.; Wu, Z.G.; Lin, W.; Yao, L.G.; et al. Development of Efficient Protocols for Stable and Transient Gene Transformation for Wolffia globosa Using Agrobacterium. Front. Chem. 2018, 6, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Li, G.; Hu, S.; Bishopp, A.; Heenatigala, P.P.M.; Kumar, S.; Duan, P.; Yao, L.; Hou, H. A protocol for efficient callus induction and stable transformation of Spirodela polyrhiza (L.) Schleiden using Agrobacterium tumefaciens. Aquat. Bot. 2018, 151, 80–86. [Google Scholar] [CrossRef]
- Yang, G.L.; Fang, Y.; Xu, Y.L.; Tan, L.; Li, Q.; Liu, Y.; Lai, F.; Jin, Y.L.; Du, A.P.; He, K.Z.; et al. Frond transformation system mediated by Agrobacterium tumefaciens for Lemna minor. Plant Mol. Biol. 2018, 98, 319–331. [Google Scholar] [CrossRef]
- Chhabra, G.; Chaudhary, D.; Sainger, M.; Jaiwal, P.K. Genetic transformation of Indian isolate of Lemna minor mediated by Agrobacterium tumefaciens and recovery of transgenic plants. Physiol. Mol. Biol. Plants 2011, 17, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Vunsh, R.; Li, J.H.; Hanania, U.; Edelman, M.; Flaishman, M.; Perl, A.; Wisniewski, J.P.; Freyssinet, G. High expression of transgene protein in Spirodela. Plant Cell Rep. 2007, 26, 1511–1519. [Google Scholar] [CrossRef]
- Wang, K.T.; Hong, M.C.; Wu, Y.S.; Wu, T.M. Agrobacterium-Mediated Genetic Transformation of Taiwanese Isolates of Lemna aequinoctialis. Plants 2021, 10, 1576. [Google Scholar] [CrossRef]
- Krishnan, S.R.; Priya, A.M.; Ramesh, M. Rapid regeneration and ploidy stability of ‘cv IR36’ indica rice (Oryza sativa L.) confers efficient protocol for in vitro callus organogenesis and Agrobacterium tumefaciens mediated transformation. Bot. Stud. 2013, 54, 47. [Google Scholar] [CrossRef] [Green Version]
- Feng, M.F.; Cang, J.; Wang, J.H.; Sun, J.; Yu, J.; Xu, Q.H.; Zhang, D.; Yang, N.; Lu, Q.W.; Lv, Y. Regeneration and Agrobacterium-Mediated Transformation of Japonica Rice Varieties Developed for a Cold Region. Czech J. Genet. Plant Breed. 2018, 54, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Supartana, P.; Shimizu, T.; Shioiri, H.; Nogawa, M.; Nozue, M.; Kojima, M. Development of simple and efficient in Planta transformation method for rice (Oryza sativa L.) using Agrobacterium tumefaciens. J. Biosci. Bioeng. 2005, 100, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.N.; Zheng, S.S.; Ling, H.Q. An efficient regeneration system and Agrobacterium-mediated transformation of Chinese upland rice cultivar Handao297. Plant Cell Tissue Organ Cult. 2011, 106, 475–483. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Alves, S.C.; Smedley, M.; Snape, J.W.; Harwood, W.A. High-throughput Agrobacterium-mediated barley transformation. Plant Methods 2008, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Murray, F.; Brettell, R.; Matthews, P.; Bishop, D.; Jacobsen, J. Comparison of Agrobacterium-mediated transformation of four barley cultivars using the GFP and GUS reporter genes. Plant Cell Rep. 2004, 22, 397–402. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Cai, T.S.; Tagliani, L.; Miller, M.; Wang, N.; Pang, H.; Rudert, M.; Schroeder, S.; Hondred, D.; Seltzer, J.; et al. Agrobacterium-mediated sorghum transformation. Plant Mol. Biol. 2000, 44, 789–798. [Google Scholar] [CrossRef]
- Hatamoto, H.; Boulter, M.E.; Shirsat, A.H.; Croy, E.J.; Ellis, J.R. Recovery of morphologically normal transgenic tobacco from hairy roots co-transformed with Agrobacterium rhizogenes and a binary vector plasmid. Plant Cell Rep. 1990, 9, 88–92. [Google Scholar] [CrossRef]
- Rachmat, A.; Chairunisa; Maulana, B.S. Efficiency of Agrobacterium tumefaciens-mediated transformation of tobacco (Nicotiana tabacum L.) with rice OsNAC6 gene. IOP Conf. Ser. Earth Environ. Sci. 2021, 762, 012062. [Google Scholar] [CrossRef]
- Wang, Z.N.; Liu, H.J.; Li, L.H.; Li, Q.L.; Wang, X.R.; Jiang, Y.; Fu, Y.Q.; Lu, C.L. Ultrasonic-assisted Mesoporous Silica Nanoparticle-mediated Exogenous Gene Stable Expression in Tobacco. Chem. Res. Chin. Univ. 2017, 33, 912–916. [Google Scholar] [CrossRef]
- Supartana, P.; Shimizu, T.; Nogawa, M.; Shioiri, H.; Nakajima, T.; Haramoto, N.; Nozue, M.; Kojima, M. Development of simple and efficient in planta transformation method for wheat (Triticum aestivum L.) using Agrobacterium tumefaciens. J. Biosci. Bioeng. 2006, 102, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.; Thistleton, J.; Higgins, T.J.; Howitt, C.; Ayliffe, M. Efficient Agrobacterium transformation of elite wheat germplasm without selection. Plant Cell Tissue Organ Cult. 2014, 119, 647–659. [Google Scholar] [CrossRef]
- Moon, H.-K.; Yang, M.-S. Nodular Somatic Embryosenesis and Frond Regeneration in Duckweed, Lemna gibha G3. J. Plant Biol. 2002, 45, 154–160. [Google Scholar] [CrossRef]
- Wang, Y. Callus Induction and Frond Regeneration in Spirodela polyrhiza. Czech J. Genet. Plant Breed. 2016, 52, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.T.; Rajbhandari, N.; Lin, X.H.; Bergmann, B.A.; Nishimura, Y.; Stomp, A.M. Genetic transformation of duckweed Lemna gibba and Lemna minor. In Vitro Cell Dev. Biol. Plant 2001, 37, 349–353. [Google Scholar] [CrossRef]
- Dennehey, B.K.; Retersen, W.L.; Fordsantino, C.; Pajeau, M.; Armstrong, C.L. Comparison of Selective Agents for Use with the Selectable Marker Gene Bar in Maize Transformation. Plant Cell Tissue Organ Cult. 1994, 36, 1–7. [Google Scholar] [CrossRef]
- Dekeyser, R.; Claes, B.; Marichal, M.; Vanmontagu, M.; Caplan, A. Evaluation of Selectable Markers for Rice Transformation. Plant Physiol. 1989, 90, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Oota, M.; Nakano, M. Embryogenic callus induction from leaf explants of the Liliaceous ornamental plant, Agapanthus praecox ssp. orientalis (Leighton) Leighton: Histological study and response to selective agents. Sci. Hortic. 2002, 95, 123–132. [Google Scholar] [CrossRef]
- Meng, Z.H.; Liang, A.H.; Yang, W.C. Effects of hygromycin on cotton cultures and its application in Agrobacterium-mediated cotton transformation. In Vitro Cell. Dev. Biol. Plant 2007, 43, 111–118. [Google Scholar] [CrossRef]
- Yan, W.J.; Qiu, R.B.; Wang, F.Y.; Fu, X.; Li, H.; Cui, P.F.; Zhai, Y.; Li, C.; Zhang, L.; Gu, K.; et al. Genetic and pathogenic characterization of a novel recombinant avian infectious bronchitis virus derived from GI-1, GI-13, GI-28, and GI-19 strains in Southwestern China. Poult. Sci. 2021, 100, 101210. [Google Scholar] [CrossRef]
- Granell, A.; Fernandez-del-Carmen, A.; Orzaez, D. In planta production of plant-derived and non-plant-derived adjuvants. Expert Rev. Vaccines 2010, 9, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.V.; Golovchenko, V.V.; Ovodova, R.G.; Smirnov, V.V.; Khramova, D.S.; Popova, G.Y.; Ovodov, Y.S. Characterisation of the oral adjuvant effect of lemnan, a pectic polysaccharide of Lemna minor L. Vaccine 2006, 24, 5413–5419. [Google Scholar] [CrossRef] [PubMed]
- Foss, D.L.; Murtaugh, M.P. Mechanisms of vaccine adjuvanticity at mucosal surfaces. Anim. Health Res. Rev. 2000, 1, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.P.; Gupta, P.N. Implication of nanoparticles/microparticles in mucosal vaccine delivery. Expert Rev. Vaccines 2007, 6, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Fujkuyama, Y.; Tokuhara, D.; Kataoka, K.; Gilbert, R.S.; McGhee, J.R.; Yuki, Y.; Kiyono, H.; Fujihashi, K. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev. Vaccines 2012, 11, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.S.; Puth, S.; Tan, W.Z.; Verma, V.; Jeong, K.; Lee, S.E.; Rhee, J.H. More robust gut immune responses induced by combining intranasal and sublingual routes for prime-boost immunization. Hum. Vaccines Immunother. 2018, 14, 2194–2202. [Google Scholar] [CrossRef]
- Liebowitz, D.; Gottlieb, K.; Kolhatkar, N.S.; Garg, S.J.; Asher, J.M.; Nazareno, J.; Kim, K.; McIlwain, D.R.; Tucker, S.N. Efficacy, immunogenicity, and safety of an oral influenza vaccine: A placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect. Dis. 2020, 20, 435–444. [Google Scholar] [CrossRef]
- Langel, S.N.; Johnson, S.; Martinez, C.I.; Tedjakusuma, S.N.; Peinovich, N.; Dora, E.G.; Kuehl, P.J.; Irshad, H.; Barrett, E.G.; Werts, A.D.; et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 2022, 14, eabn6868. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Amin, Z.; Rodrigues, K.F.; Saallah, S.; Shaarani, S.M.; Sarker, S.; Siddiquee, S. Infectious Bronchitis Virus (Gammacoronavirus) in Poultry Farming: Vaccination, Immune Response and Measures for Mitigation. Vet. Sci. 2021, 8, 273. [Google Scholar] [CrossRef]

| Varian | Induction Medium | Subculture Medium | pH | Culture Condition | ||
|---|---|---|---|---|---|---|
| Basal Medium | Phytohormones | Basal Medium | Phytohormones | |||
| T-1 | B5 + 10 g/L su + 0.35% Ge | 2,4-D 45 μM + TDZ 5 μM | B5 + 10 g/L Su + 0.35% Ge | 2,4-D10 45 μM + TDZ 5 μM | 5.8 | 16 h day/8 h night |
| T-2 | B5 + 10 g/L su + 0.35% Ge | 2,4-D 0.45 μM + 2-IP 5 μM | B5 + 10 g/L Su + 0.35% Ge | 2,4-D 1 0.45 μM + 2-IP 5 μM | 5.8 | 16 h day/8 h night |
| T-3 | MS + 30 g/L su + 0.35% Ge | 2,4-D 5 μM + TDZ 0.5 μM | MS + 30 g/L Su + 0.35% Ge | 2,4-D 1 μM + 6-BA 2 μM | 5.6 | 16 h day/8 h night |
| T-4 | MS + 30 g/L su + 0.35% Ge | 2,4-D 4.5 μM + TDZ 0.45 μM | MS + 30 g/L Su + 0.35% Ge | 2,4-D 4.5 μM + T DZ 0.45 μM | 5.9 | 24 h night |
| T-5 | MS + 30 g/L su + 0.35% Ge | TDZ 0.45 μM | MS + 30 g/L Su + 0.35% Ge | 2,4-D 0.9 μM | 5.6 | 16 h day/8 h night |
| T-6 | SH + 20 g/L gl + 10 g/L So + 10 g/L Ma + 0.35% Ge | 2,4-D 22.5 μM + 6-BA 2.2 μM | SH + 20 g/L Gl + 10 g/L Sor + 10 g/L Ma + 0.35% Ge | 2,4-D 22.5 μM + 6-BA 2.2 μM | 5.6 | 16 h day/8 h night |
| T-7 | MS + 30 g/L Su + 0.35% Ge | NAA 10 μM + TDZ 0.5 μM | MS + 30 g/L Su + 0.35% Ge | 2,4-D 1 μM + 6-BA 2 μM | 5.6 | 16 h day/8 h night |
| Species | Explant | Gene | Selection Time | Regeneration Time | Callus/Frond Transient Efficiency | Stable Transformation Efficiency in DNA Level | Stable Transformation Efficiency in Protein Level | Reference |
|---|---|---|---|---|---|---|---|---|
| Lemna minor ZH0403 | Callus | GUS | 3–4 weeks | 4 weeks | 95% | 88% | 86% | This study |
| Lemna aequinoctialis 6002 | Callus | GUS | 5–6 weeks | 94% | - | - | [25] | |
| Lemna minor L. | Callus | GUS-M2e | 8–10 weeks | - | 85% | 85% | [15] | |
| Spirodela punctata 8717 | Frond | GUS | 8 weeks | 92% | - | 80% | [22] | |
| Wolffia globosa 5563 | Frond | GUS | - | - | - | - | 21.8% | [47] |
| Lenna gibba G3 | Frond | GUS | - | - | 100% | - | 17% | [23] |
| Spirodela polyrhiza 5543 | Callus | GUS | - | - | - | - | 13% | [48] |
| Lemna minor strain ZH0055 | Callus | GUS | 36 weeks | 80% | - | 4% | [49] | |
| Lemna minor | Callus | GUS | - | - | 89% | - | 3.8% | [50] |
| Lemna minor | Frond | GUS | 9 weeks | 30–40% | - | 2–6% | [49] | |
| Spirodela oligorrhiza | Callus | GFP | 4–6 weeks | - | - | 0.5–5% | [51] | |
| Lemna aequinoctialis | Callus | GFP | 10 weeks | 4 weeks | 49% | - | 3% | [52] |
| Spirodela punctata | Callus | GUS | - | - | - | - | 2.4% | [22] |
| Lemna minor | Callus | GFP | 5 weeks | 59% | - | - | [27] | |
| Wolffia arrhiza | Callus | Hpt | 6–8 weeks | - | - | - | 0.2–0.4% | [43] |
| Wolffia globosa 5563 | Callus | GUS | 4 weeks | - | - | - | 0.14% | [47] |
| Indica rice | Callus/frond | GUS | - | - | 99%/49.5% | - | - | [53] |
| Japonica rice | Callus | GUS | - | - | 10–91% | - | - | [54] |
| Oryza sativa L. | Seed | GUS | - | - | - | 40% | 43% | [55] |
| Indica rice | Callus | GUS | - | - | - | 9.3–23.4% | 7–18.9% | [42] |
| Japonica rice | Callus | GUS | - | - | - | - | 0–20% | [56] |
| Barley | Immature embryo | Hpt | - | - | - | - | 4–33% | [57] |
| Barley | Callus | GFP | - | - | 47–76% | - | 0.6–4.4% | [58] |
| Sorghum | Immature embryo | GUS | - | - | 41.3% | - | 14.2% | [41] |
| Sorghum | Immature embryo | GUS | - | - | 21–63% | - | 0.71–10.1% | [59] |
| Nicotiana tabacum | Leaf | GUS | - | - | - | - | 53% | [60] |
| Nicotiana tabacum | Callus | GUS | - | - | - | 15% | - | [61] |
| Nicotiana tabacum | Callus | GUS | - | - | - | 41.79–53.13% | - | [62] |
| Triticum aestivum L. | Seed | GUS | - | - | - | 29–38% | - | [63] |
| Wheat | Embryo | GUS | - | - | - | 68% | 41% | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Chen, S.; Fang, Y.; Liu, P.; Hu, Z.; Jin, Y.; Yi, Z.; He, K.; Li, X.; Zhao, L.; et al. Rapid and Highly Efficient Genetic Transformation and Application of Interleukin-17B Expressed in Duckweed as Mucosal Vaccine Adjuvant. Biomolecules 2022, 12, 1881. https://doi.org/10.3390/biom12121881
Tan X, Chen S, Fang Y, Liu P, Hu Z, Jin Y, Yi Z, He K, Li X, Zhao L, et al. Rapid and Highly Efficient Genetic Transformation and Application of Interleukin-17B Expressed in Duckweed as Mucosal Vaccine Adjuvant. Biomolecules. 2022; 12(12):1881. https://doi.org/10.3390/biom12121881
Chicago/Turabian StyleTan, Xiao, Shuang Chen, Yang Fang, Penghui Liu, Zhubin Hu, Yanling Jin, Zhuolin Yi, Kaize He, Xing Li, Leyi Zhao, and et al. 2022. "Rapid and Highly Efficient Genetic Transformation and Application of Interleukin-17B Expressed in Duckweed as Mucosal Vaccine Adjuvant" Biomolecules 12, no. 12: 1881. https://doi.org/10.3390/biom12121881




