Modulation of Anionic Lipid Bilayers by Specific Interplay of Protons and Calcium Ions
Abstract
1. Introduction
2. Materials and Methods
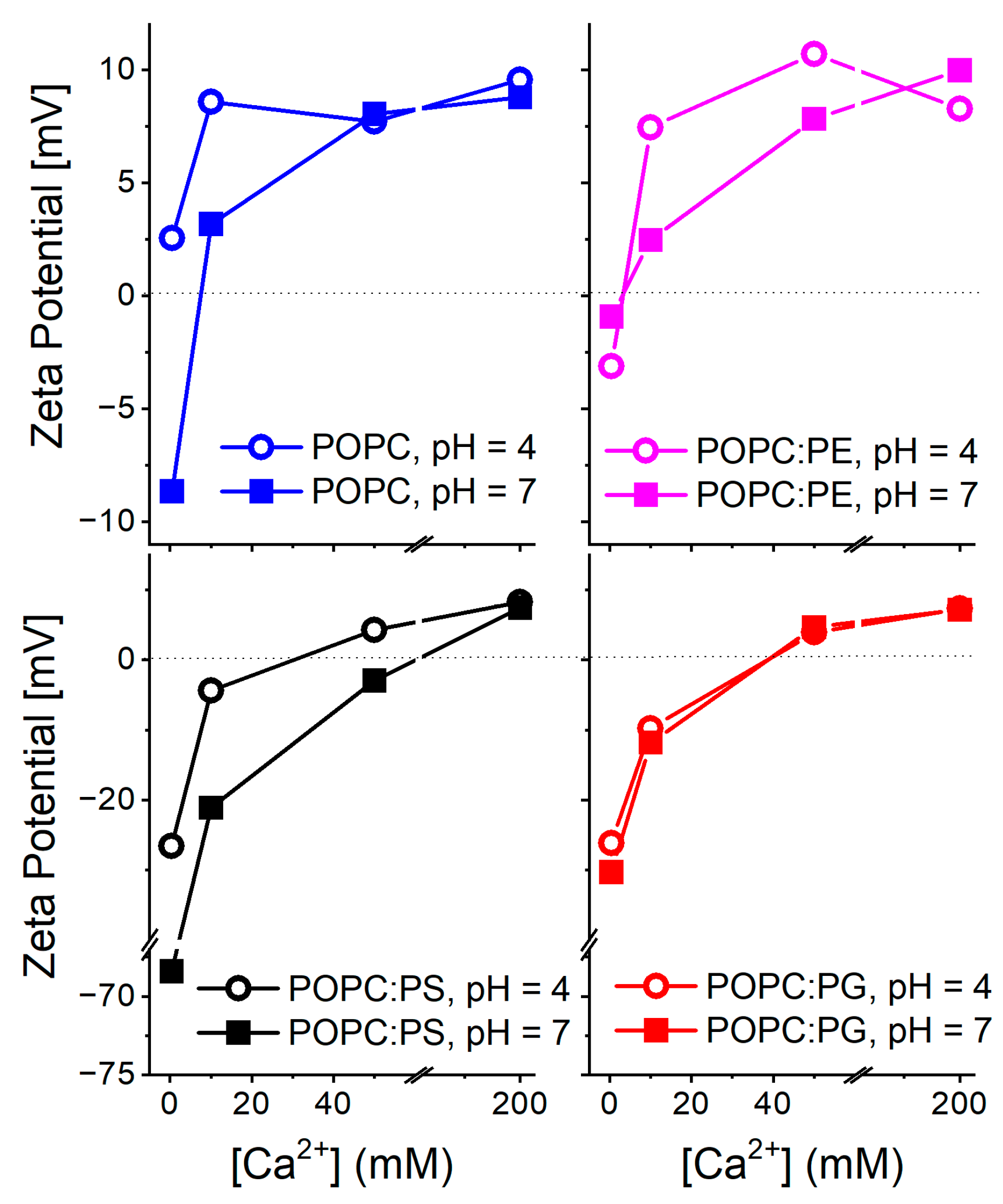
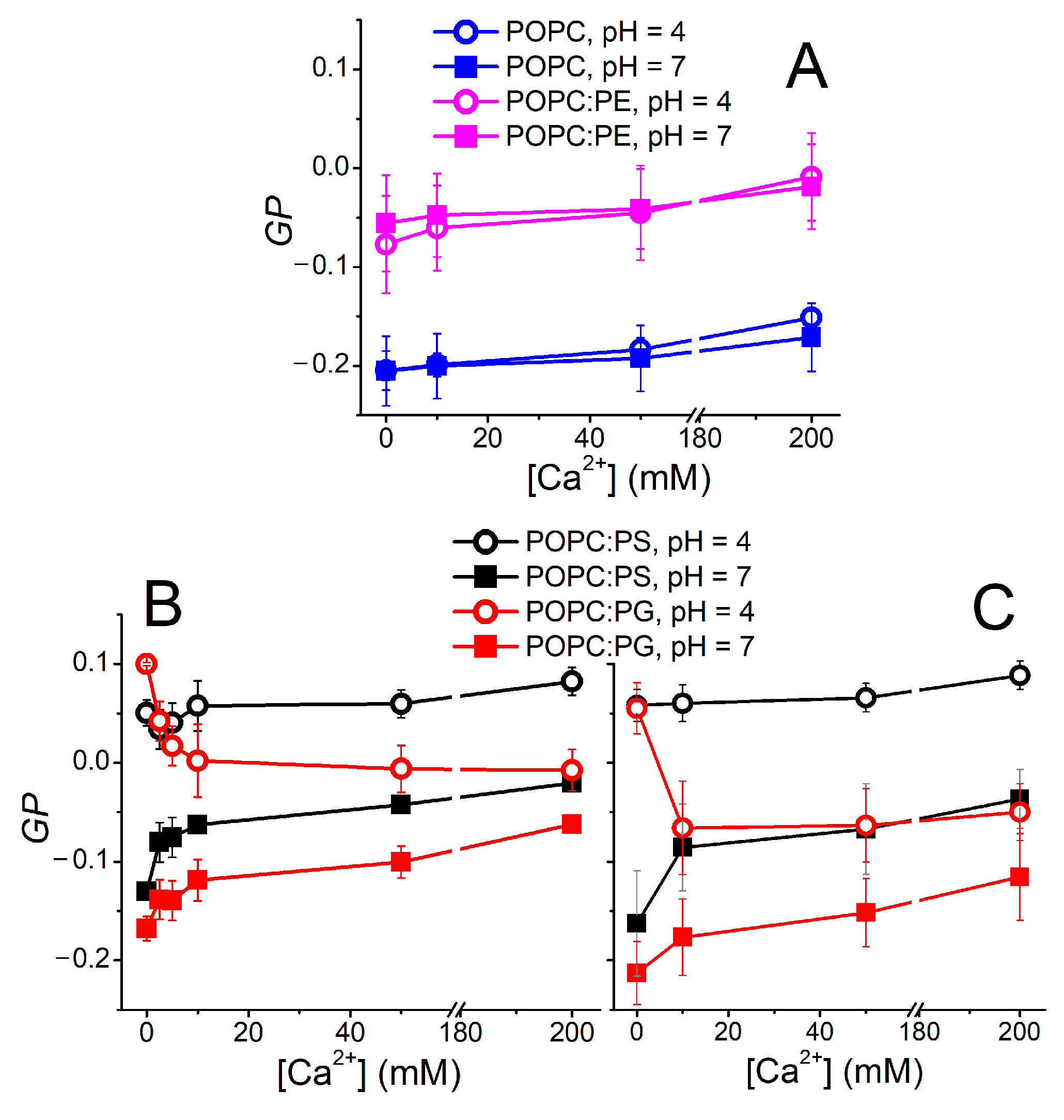
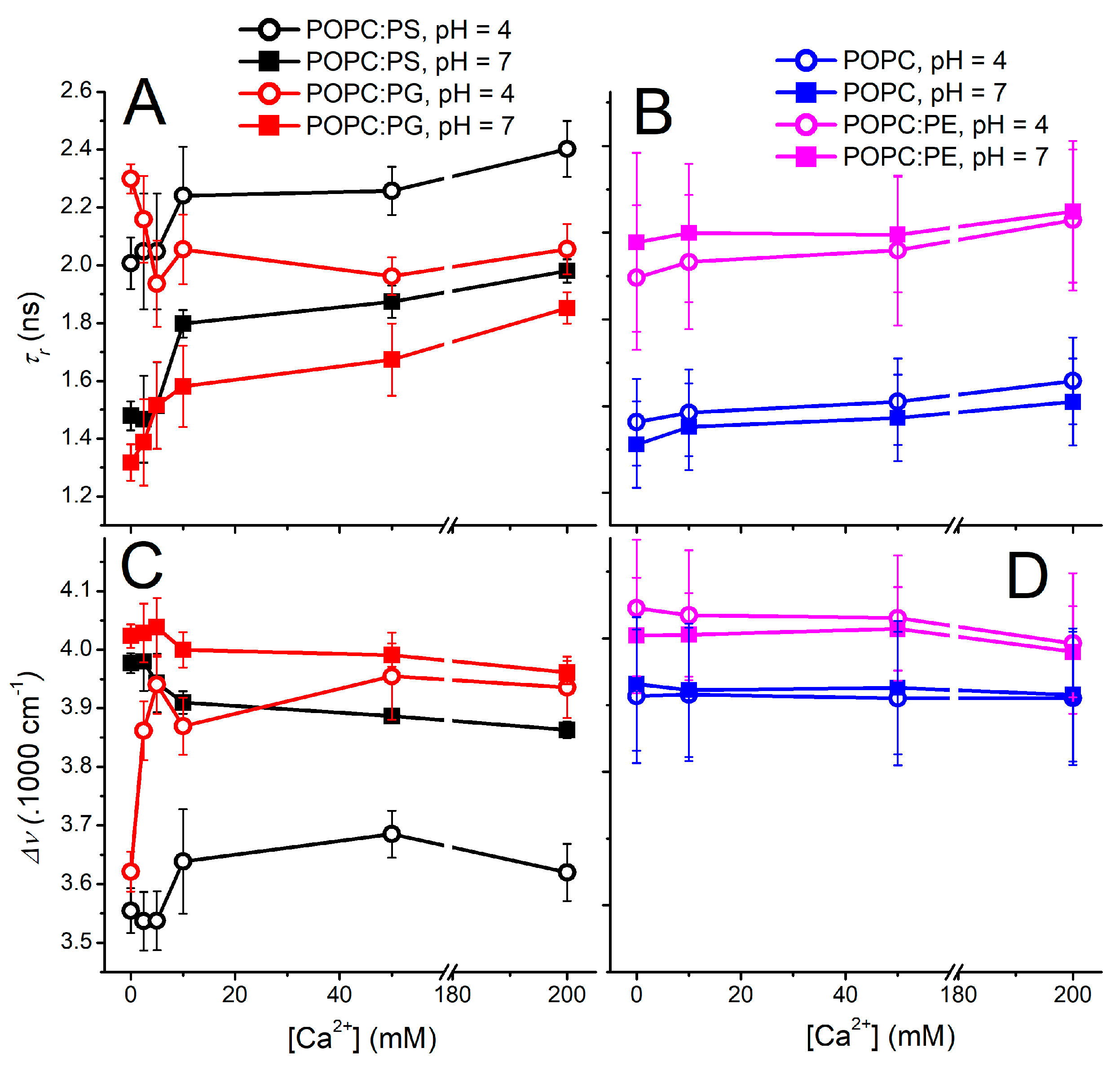
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Ghazvini, S.; Alonso, R.; Alhakamel, N.; Dhar, P. pH-Induced Changes in the Surface Viscosity of Unsaturated Phospholipids Monitored Using Active Interfacial Microrheology. Langmuir 2018, 34, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Edwardson, J.M. Phase separation in lipid bilayers triggered by low pH. Biochem. Biophys. Res. Commun. 2010, 399, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jaattela, M.; Liu, B. Lysosome as a Central Hub for Rewiring PH Homeostasis in Tumors. Cancers 2020, 12, 2437. [Google Scholar] [CrossRef] [PubMed]
- Conn, S.; Gilliham, M. Comparative physiology of elemental distributions in plants. Ann. Bot. 2010, 105, 1081–1102. [Google Scholar] [CrossRef]
- Venable, R.M.; Luo, Y.; Gawrisch, K.; Roux, B.; Pastor, R.W. Simulations of Anionic Lipid Membranes: Development of Interaction-Specific Ion Parameters and Validation Using NMR Data. J. Phys. Chem. B 2013, 117, 10183–10192. [Google Scholar] [CrossRef]
- Wilks, J.C.; Slonczewski, J.L. pH of the cytoplasm and periplasm of Escherichia coli: Rapid measurement by green fluorescent protein fluorimetry. J. Bacteriol. 2007, 189, 5601–5607. [Google Scholar] [CrossRef]
- McNulty, R.; Ulmschneider, J.P.; Luecke, H.; Ulmschneider, M.B. Mechanisms of molecular transport through the urea channel of Helicobacter pylori. Nat. Commun. 2013, 4, 2900. [Google Scholar] [CrossRef]
- Fuller, N.; Benatti, C.R.; Rand, R.P. Curvature and bending constants for phosphatidylserine-containing membranes. Biophys. J. 2003, 85, 1667–1674. [Google Scholar] [CrossRef]
- Seddon, J.M.; Kaye, R.D.; Marsh, D. Induction of the Lamellar-Inverted Hexagonal Phase-Transition in Cardiolipin by Protons and Mono-Valent Cations. Biochim. Biophys. Acta 1983, 734, 347–352. [Google Scholar] [CrossRef]
- Valentine, M.L.; Cardenas, A.E.; Elber, R.; Baiz, C.R. Calcium-Lipid Interactions Observed with Isotope-Edited Infrared Spectroscopy. Biophys. J. 2020, 118, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Branden, M.; Sanden, T.; Brzezinski, P.; Widengren, J. Localized proton microcircuits at the biological membrane-water interface. Proc. Natl. Acad. Sci. USA 2006, 103, 19766–19770. [Google Scholar] [CrossRef] [PubMed]
- Cranfield, C.G.; Berry, T.; Holt, S.A.; Hossain, K.R.; Le Brun, A.P.; Carne, S.; Al Khamici, H.; Coster, H.; Valenzuela, S.M.; Cornell, B. Evidence of the Key Role of H3O+ in Phospholipid Membrane Morphology. Langmuir 2016, 32, 10725–10734. [Google Scholar] [CrossRef] [PubMed]
- Melcrova, A.; Pokorna, S.; Vosahlikova, M.; Sykora, J.; Svoboda, P.; Hof, M.; Cwiklik, L.; Jurkiewicz, P. Concurrent Compression of Phospholipid Membranes by Calcium and Cholesterol. Langmuir 2019, 35, 11358–11368. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Voth, G.A. Properties of Hydrated Excess Protons near Phospholipid Bilayers. J. Phys. Chem. B 2010, 114, 592–603. [Google Scholar] [CrossRef]
- Mao, Y.Y.; Du, Y.; Cang, X.H.; Wang, J.A.; Chen, Z.X.; Yang, H.Y.; Jiang, H.L. Binding Competition to the POPG Lipid Bilayer of Ca2+, Mg2+, Na+, and K+ in Different Ion Mixtures and Biological Implication. J. Phys. Chem. B 2013, 117, 850–858. [Google Scholar] [CrossRef]
- Deplazes, E.; White, J.; Murphy, C.; Cranfield, C.G.; Garcia, A.A.-O. Competing for the same space: Protons and alkali ions at the interface of phospholipid bilayers. Biophys. Rev. 2019, 11, 483–490. [Google Scholar] [CrossRef]
- Deplazes, E.; Poger, D.; Cornell, B.; Cranfield, C.G. The effect of hydronium ions on the structure of phospholipid membranes. Phys. Chem. Chem. Phys. 2018, 20, 357–366. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Nakahara, H.; Phan, C.M. Surface Potential of the Air/Water Interface. J. Oleo Sci. 2020, 69, 519–528. [Google Scholar] [CrossRef]
- Melcrova, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035. [Google Scholar] [CrossRef]
- Allolio, C.; Harries, D. Calcium Ions Promote Membrane Fusion by Forming Negative-Curvature Inducing Clusters on Specific Anionic Lipids. ACS Nano 2021, 15, 12880–12887. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.T.; Lane, N.J.; Miller, N.G.A.; Bangham, A.D. Fusion of Liposome Membranes by the Normal-Alkyl Bromides. J. Membr. Biol. 1980, 55, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Uster, P.S.; Deamer, D.W. Fusion competence of phosphatidylserine-containing liposomes quantitatively measured by a fluorescence resonance energy transfer assay. Arch. Biochem. Biophys. 1981, 209, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Nir, S.; Duzgunes, N. Molecular Mechanisms of Calcium-Induced Membrane-Fusion. J. Bioenerg. Biomembr. 1990, 22, 157–179. [Google Scholar] [CrossRef]
- Murzyn, K.; Rog, T.; Pasenkiewicz-Gierula, M. Phosphatidylethanolamine-phosphatidylglycerol bilayer as a model of the inner bacterial membrane: A molecular modeling study. Biophys. J. 2005, 88, 1091–1103. [Google Scholar] [CrossRef]
- Urbina, J.A.; Moreno, B.; Arnold, W.; Taron, C.H.; Orlean, P.; Oldfield, E. A carbon-13 nuclear magnetic resonance spectroscopic study of inter-proton pair order parameters: A new approach to study order and dynamics in phospholipid membrane systems. Biophys. J. 1998, 75, 1372–1383. [Google Scholar] [CrossRef]
- Hubner, W.; Blume, A. Interactions at the lipid-water interface. Chem. Phys. Lipids 1998, 96, 99–123. [Google Scholar] [CrossRef]
- Pandit, S.A.; Bostick, D.; Berkowitz, M.L. Mixed bilayer containing dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylserine: Lipid complexation, ion binding, and electrostatics. Biophys. J. 2003, 85, 3120–3131. [Google Scholar] [CrossRef]
- Dickey, A.; Faller, R. Examining the contributions of lipid shape and headgroup charge on bilayer behavior. Biophys. J. 2008, 95, 2636–2646. [Google Scholar] [CrossRef]
- Mattai, J.; Hauser, H.; Demel, R.A.; Shipley, G.G. Interactions of Metal-Ions with Phosphatidylserine Bilayer-Membranes—Effect of Hydrocarbon Chain Unsaturation. Biochemistry 1989, 28, 2322–2330. [Google Scholar] [CrossRef]
- Pasenkiewicz-Gierula, M.; Takaoka, Y.; Miyagawa, H.; Kitamura, K.; Kusumi, A. Charge pairing of headgroups in phosphatidylcholine membranes: A molecular dynamics simulation study. Biophys. J. 1999, 76, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Scollo, F.; Evci, H.; Amaro, M.; Jurkiewicz, P.; Sykora, J.; Hof, M. What Does Time-Dependent Fluorescence Shift (TDFS) in Biomembranes (and Proteins) Report on? Front. Chem. 2021, 9, 738350. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, S.; Holsaeter, A.M.; Skar, M.; Frantzen, C.B.; Brandl, M. Liposome size analysis by dynamic/static light scattering upon size exclusion-/field flow-fractionation. J. Nanosci. Nanotechnol. 2006, 6, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; Loiero, M.; Raimondi, M.; Ravagnan, G.; Gratton, E. Effect of Cholesterol on Phospholipid Phase Domains as Detected by Laurdan Generalized Polarization. Biophys. J. 1993, 64, A72. [Google Scholar]
- Horng, M.L.; Gardecki, J.A.; Papazyan, A.; Maroncelli, M. Subpicosecond Measurements of Polar Solvation Dynamics—Coumarin-153 Revisited. J. Phys. Chem. 1995, 99, 17311–17337. [Google Scholar] [CrossRef]
- Jurkiewicz, P.; Sykora, J.; Olzynska, A.; Humplickova, J.; Hof, M. Solvent relaxation in phospholipid bilayers: Principles and recent applications. J. Fluoresc. 2005, 15, 883–894. [Google Scholar] [CrossRef]
- Fee, R.S.; Maroncelli, M. Estimating the Time-Zero Spectrum in Time-Resolved Emission Measurements of Solvation Dynamics. Chem. Phys. 1994, 183, 235–247. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D.; Pastor, R.W. Update of the CHARMM All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single-Crystals—A New Molecular-Dynamics Method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Nose, S. A Unified Formulation of the Constant Temperature Molecular-Dynamics Methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald—An N.Log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Lee, J.; Patel, D.S.; Stahle, J.; Park, S.J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI Membrane Builder for Complex Biological Membrane Simulations with Glycolipids and Lipoglycans. J. Chem. Theory Comput. 2019, 15, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Bonthuis, D.J.; Mamatkulov, S.I.; Netz, R.R. Optimization of classical nonpolarizable force fields for OH- and H3O+. J. Chem. Phys. 2016, 144, 104503. [Google Scholar] [CrossRef]
- Mamatkulov, S.I.; Allolio, C.; Netz, R.R.; Bonthuis, D.J. Frontispiece: Orientation-Induced Adsorption of Hydrated Protons at the Air–Water Interface. Angew. Chem. Int. Ed. 2017, 56, 15846–15851. [Google Scholar] [CrossRef]
- Marsh, D. Handbook of Lipid Bilayers, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Klein, J.W.; Ware, B.R.; Barclay, G.; Petty, H.R. Phospholipid Dependence of Calcium-Ion Effects on Electrophoretic Mobilities of Liposomes. Chem. Phys. Lipids 1987, 43, 13–23. [Google Scholar] [CrossRef]
- Kubickova, A.; Krizek, T.; Coufal, P.; Vazdar, M.; Wernersson, E.; Heyda, J.; Jungwirth, P. Overcharging in Biological Systems: Reversal of Electrophoretic Mobility of Aqueous Polyaspartate by Multivalent Cations. Phys. Rev. Lett. 2012, 108, 186101. [Google Scholar] [CrossRef]
- Ribeiro, M.M.B.; Domingues, M.M.; Freire, J.M.; Santos, N.C.; Castanho, M.A.R.B. Translocating the blood-brain barrier using electrostatics. Front. Cell. Neurosci. 2012, 6, 44. [Google Scholar] [CrossRef]
- Mclaughlin, S.; Mulrine, N.; Gresalfi, T.; Vaio, G.; Mclaughlin, A. Adsorption of Divalent-Cations to Bilayer-Membranes Containing Phosphatidylserine. J. Gen. Physiol. 1981, 77, 445–473. [Google Scholar] [CrossRef] [PubMed]
- Brockman, H. Dipole potential of lipid membranes. Chem. Phys. Lipids 1994, 73, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Smaby, J.M.; Brockman, H.L. Surface dipole-moments of lipids at the argon-water interface—Similarities among glycerol-ester-based lipids. Biophys. J. 1990, 58, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Disalvo, A.; Frias, M.A. Surface Characterization of Lipid Biomimetic Systems. Membranes 2021, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Artukhov, V.Y.; Zharkova, O.M.; Morozova, J.P. Features of absorption and fluorescence spectra of prodan. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 36–42. [Google Scholar] [CrossRef]
- Parasassi, T.; Destasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of Lipid Phases in Phospholipid-Vesicles by the Generalized Polarization of Laurdan Fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef]
- Bagatolli, L.A. LAURDAN Fluorescence Properties in Membranes: A Journey from the Fluorometer to the Microscope. In Fluorescent Methods to Study Biological Membranes; Mély, Y., Duportail, G., Eds.; Springer Series on Fluorescence; Volume 13, Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Sanchez, S.A.; Tricerri, M.A.; Gratton, E. Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 7314–7319. [Google Scholar] [CrossRef]
- Sezgin, E.; Sadowski, T.; Simons, K. Measuring Lipid Packing of Model and Cellular Membranes with Environment Sensitive Probes. Langmuir 2014, 30, 8160–8166. [Google Scholar] [CrossRef]
- Vallejo, A.A.; Velazquez, J.B.; Fernandez, M.S. Lateral organization of mixed, two-phosphatidylcholine liposomes as investigated by GPS, the slope of Laurdan generalized polarization spectra. Arch. Biochem. Biophys. 2007, 466, 145–154. [Google Scholar] [CrossRef]
- Amaro, M.; Sachl, R.; Jurkiewicz, P.; Coutinho, A.; Prieto, M.; Hof, M. Time-Resolved Fluorescence in Lipid Bilayers: Selected Applications and Advantages over Steady State. Biophys. J. 2014, 107, 2751–2760. [Google Scholar] [CrossRef]
- Tempra, C.; Ollila, O.H.S.; Javanainen, M. Accurate Simulations of Lipid Monolayers Require a Water Model with Correct Surface Tension. J. Chem. Theory Comput. 2022, 18, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Kucerka, N.; Nieh, M.P.; Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Melcr, J.; Ferreira, T.M.; Jungwirth, P.; Ollila, O.H.S. Improved Cation Binding to Lipid Bilayers with Negatively Charged POPS by Effective Inclusion of Electronic Polarization. J. Chem. Theory Comput. 2020, 16, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Subedi, K.P.; Paudel, O.; Sham, J.S. Detection of differentially regulated subsarcolemmal calcium signals activated by vasoactive agonists in rat pulmonary artery smooth muscle cells. Am. J. Physiol. Cell Physiol. 2014, 306, C659–C669. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Hicks, G.R.; Raikhel, N.V. Molecular Composition of Plant Vacuoles: Important but Less Understood Regulations and Roles of Tonoplast Lipids. Plants 2015, 4, 320–333. [Google Scholar] [CrossRef]
- Casares, D.; Escriba, P.V.; Rossello, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef]
- Miller, S.I.; Salama, N.R. The gram-negative bacterial periplasm: Size matters. PLoS Biol. 2018, 16, e2004935. [Google Scholar] [CrossRef]
- Perez-Isidoro, R.; Ruiz-Suarez, J.C. Calcium and protons affect the interaction of neurotransmitters and anesthetics with anionic lipid membranes. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2215–2222. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abhinav; Jurkiewicz, P.; Hof, M.; Allolio, C.; Sýkora, J. Modulation of Anionic Lipid Bilayers by Specific Interplay of Protons and Calcium Ions. Biomolecules 2022, 12, 1894. https://doi.org/10.3390/biom12121894
Abhinav, Jurkiewicz P, Hof M, Allolio C, Sýkora J. Modulation of Anionic Lipid Bilayers by Specific Interplay of Protons and Calcium Ions. Biomolecules. 2022; 12(12):1894. https://doi.org/10.3390/biom12121894
Chicago/Turabian StyleAbhinav, Piotr Jurkiewicz, Martin Hof, Christoph Allolio, and Jan Sýkora. 2022. "Modulation of Anionic Lipid Bilayers by Specific Interplay of Protons and Calcium Ions" Biomolecules 12, no. 12: 1894. https://doi.org/10.3390/biom12121894
APA StyleAbhinav, Jurkiewicz, P., Hof, M., Allolio, C., & Sýkora, J. (2022). Modulation of Anionic Lipid Bilayers by Specific Interplay of Protons and Calcium Ions. Biomolecules, 12(12), 1894. https://doi.org/10.3390/biom12121894





