Aqueous Integrated Process for the Recovery of Oil Bodies or Fatty Acid Emulsions from Sunflower Seeds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Integrated Process for the Release of Sunflower Seed Olesomes
2.2. Composition of Each Fraction
2.3. Modification of the Integrated Process to Obtain a New Complex Fatty Acid Emulsion
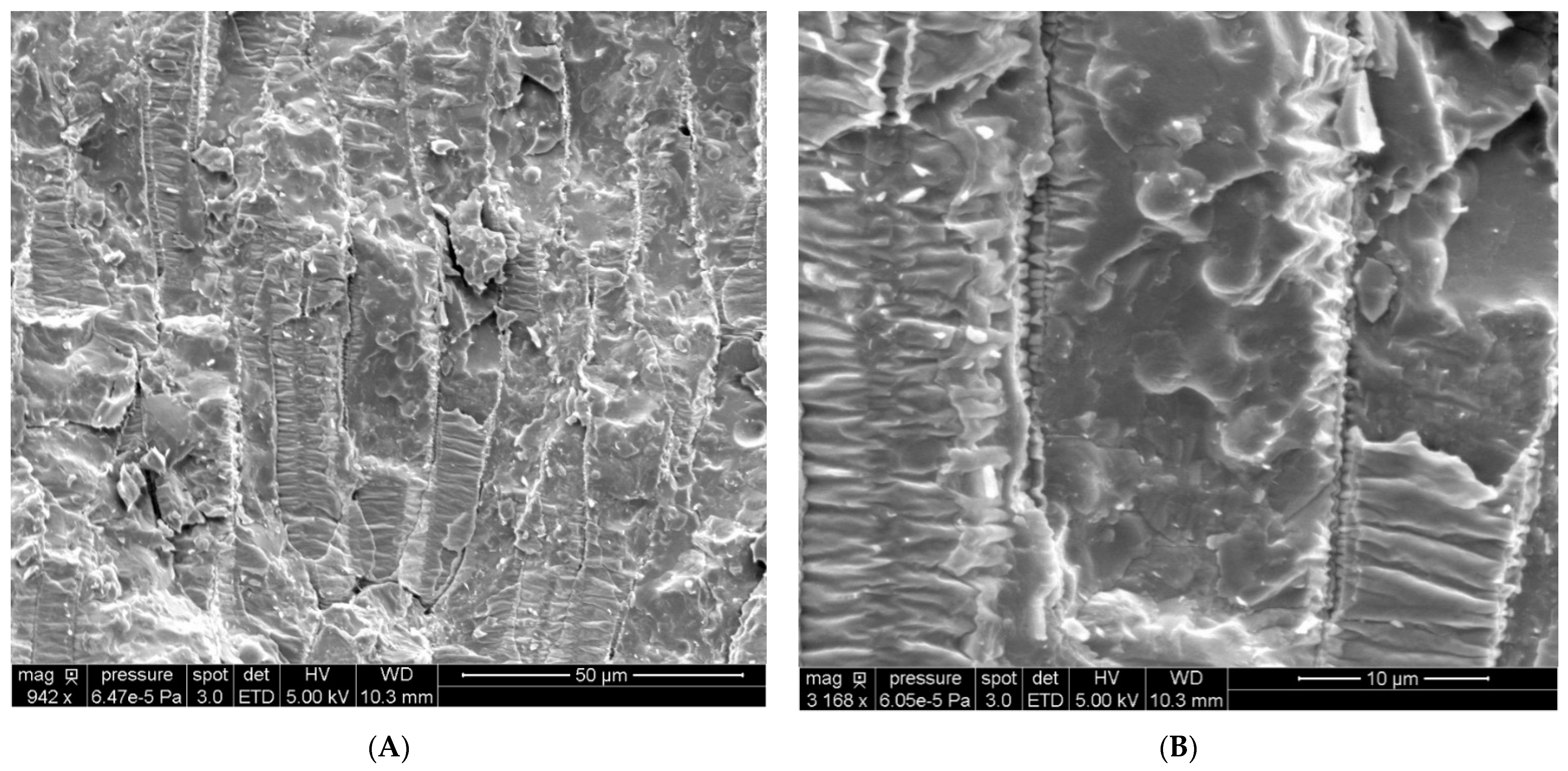
2.4. Observation of Sunflower Seeds and OBs by Cryo-SEM and Determination of the Size Distribution
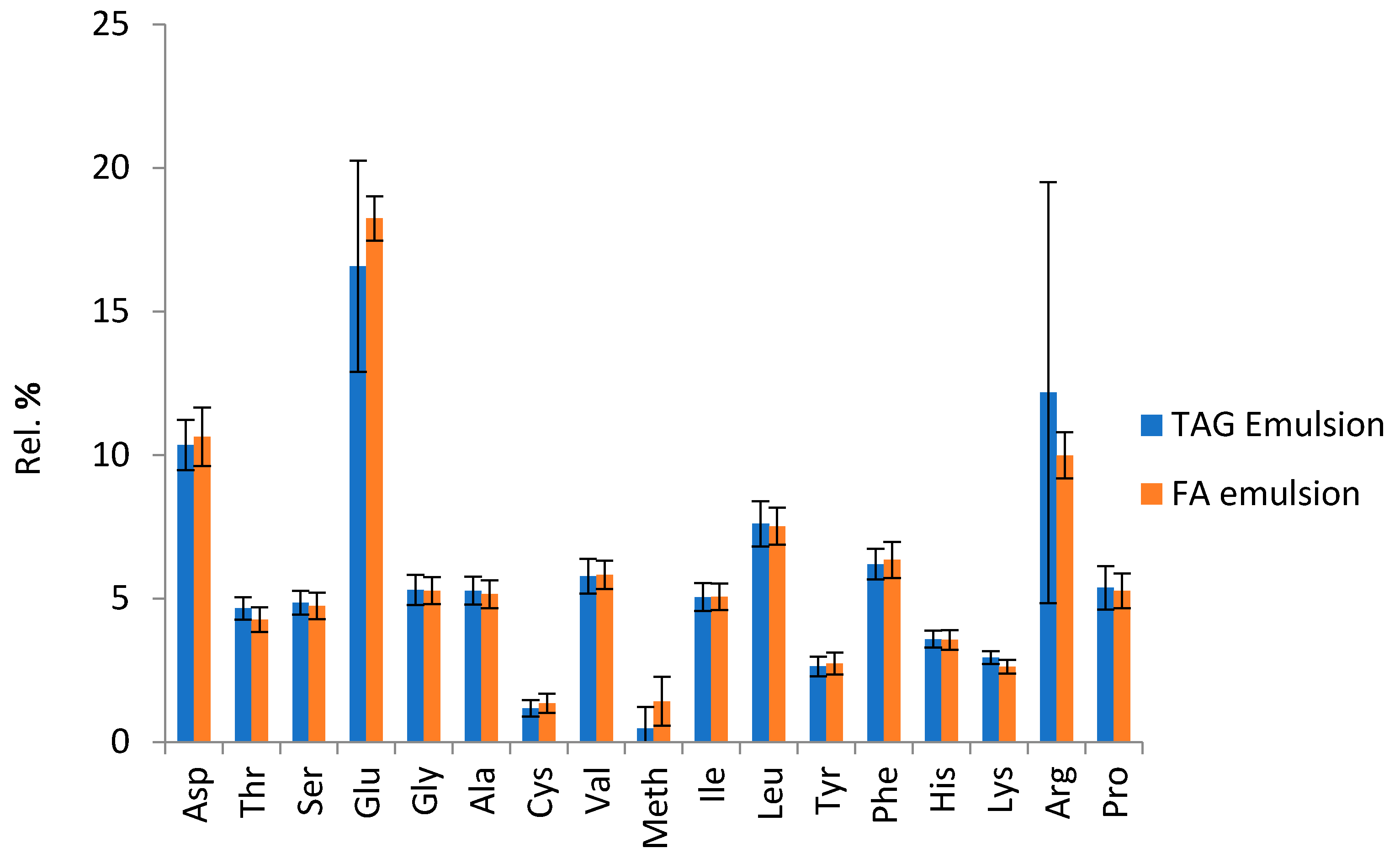
2.5. Lipids and Proteins of the Emulsions Obtained
3. Materials and Methods
3.1. Materials
3.2. Oil-Body Extraction in an Oil-in-Water Triglyceride Emulsion
3.3. Oil-Body Extraction in a Complex Fatty Acid Emulsion
3.4. Determination of the Composition of Each Fraction
3.5. Extraction of Lipids by Folch’s Method
3.6. Ultrasonic Assisted Extraction of Total Lipids from Fatty Acid Emulsion in Ethanol
3.7. Solid Phase Extraction (SPE)
3.8. Chromatography HPLC/ELSD
3.9. Chromatography GC/FID
3.10. Measurement of Oil Body Size Distribution
3.11. Cryo-Scanning Electron Microscopy Imaging
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzen, J.T.; Lie, G.C.; Huang, A.H. Characterization of the Charged Components and Their Topology on the Surface of Plant Seed Oil Bodies. J. Biol. Chem. 1992, 267, 15626–15634. [Google Scholar] [CrossRef]
- Tzen, J.; Huang, A. Surface Structure and Properties of Plant Seed Oil Bodies. J. Cell Biol. 1992, 117, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzen, J.; Cao, Y.; Laurent, P.; Ratnayake, C.; Huang, A. Lipids, Proteins, and Structure of Seed Oil Bodies from Diverse Species. Plant Physiol. 1993, 101, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Hao, L.; Chen, F.; Zhu, T. The Mechanism of Extraction of Peanut Protein and Oil Bodies by Enzymatic Hydrolysis of the Cell Wall. J. Oleo Sci. 2020, 69, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Deleu, M.; Vaca-Medina, G.; Fabre, J.-F.; Roïz, J.; Valentin, R.; Mouloungui, Z. Interfacial Properties of Oleosins and Phospholipids from Rapeseed for the Stability of Oil Bodies in Aqueous Medium. Colloids Surf. B Biointerfaces 2010, 80, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, A.; Herfellner, T.; Stäbler, A.; Menner, M.; Eisner, P. Influence of Process Conditions during Aqueous Protein Extraction upon Yield from Pre-Pressed and Cold-Pressed Rapeseed Press Cake. Ind. Crops Prod. 2018, 112, 236–246. [Google Scholar] [CrossRef]
- Latif, S.; Diosady, L.L.; Anwar, F. Enzyme-assisted Aqueous Extraction of Oil and Protein from Canola (Brassica napus L.) Seeds. Eur. J. Lipid Sci. Technol. 2008, 110, 887–892. [Google Scholar] [CrossRef]
- Domínguez, H.; Sineiro, J.; Núñez, M.J.; Lema, J.M. Enzymatic Treatment of Sunflower Kernels before Oil Extraction. Food Res. Int. 1995, 28, 537–545. [Google Scholar] [CrossRef]
- Moradi, N.; Rahimi, M. Effect of Ultrasound- and Pulsed Electric Field-Assisted Enzymatic Treatment on the Recovery and Quality of Sunflower Oil. Sep. Sci. Technol. 2019, 54, 1043–1054. [Google Scholar] [CrossRef]
- Munder, S.; Latif, S.; Müller, J. Enzyme-Assisted Aqueous Oil Extraction from High Oleic Sunflower Seeds in a Scalable Prototype Reactor. Waste Biomass Valor 2020, 11, 899–908. [Google Scholar] [CrossRef]
- Aquino, D.S.; Fanhani, A.; Stevanato, N.; Silva, C. Sunflower Oil from Enzymatic Aqueous Extraction Process: Maximization of Free Oil Yield and Oil Characterization. J. Food Process Eng. 2019, 42. [Google Scholar] [CrossRef]
- Hou, L.X.; Shang, X.L.; Wang, X.; Liu, J. Application of Enzyme in Aqueous Extraction of Sesame Oil. Eur. Food Res. Technol. 2013, 236, 1027–1030. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Kiosseoglou, V. Aqueous Extraction of Oil Bodies from Maize Germ (Zea Mays) and Characterization of the Resulting Natural Oil-in-Water Emulsion. J. Agric. Food Chem. 2009, 57, 5591–5596. [Google Scholar] [CrossRef]
- Souza, T.S.P.; Dias, F.F.G.; Koblitz, M.G.B.; de M. Bell, J.M.L.N. Aqueous and Enzymatic Extraction of Oil and Protein from Almond Cake: A Comparative Study. Processes 2019, 7, 472. [Google Scholar] [CrossRef] [Green Version]
- Gibbins, R.D.; Aksoy, H.A.; Ustun, G. Enzyme-assisted Aqueous Extraction of Safflower Oil: Optimisation by Response Surface Methodology. Int. J. Food Sci. Technol. 2012, 47, 1055–1062. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K.; Gilmour, S.; Trinca, L. Combined Effect of Operational Variables and Enzyme Activity on Aqueous Enzymatic Extraction of Oil and Protein from Soybean. Enzym. Microb. Technol. 2001, 28, 499–509. [Google Scholar] [CrossRef]
- de Moura, J.M.L.N.; Campbell, K.; Mahfuz, A.; Jung, S.; Glatz, C.E.; Johnson, L. Enzyme-Assisted Aqueous Extraction of Oil and Protein from Soybeans and Cream De-Emulsification. J. Am. Oil Chem. Soc. 2008, 85, 985–995. [Google Scholar] [CrossRef]
- Campbell, K.A.; Glatz, C.E. Mechanisms of Aqueous Extraction of Soybean Oil. J. Agric. Food Chem. 2009, 57, 10904–10912. [Google Scholar] [CrossRef] [Green Version]
- Towa, L.T.; Kapchie, V.N.; Hauck, C.; Murphy, P.A. Enzyme-Assisted Aqueous Extraction of Oil from Isolated Oleosomes of Soybean Flour. J. Am. Oil Chem. Soc. 2010, 87, 347–354. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Rosentrater, K.A.; Sekhon, J.; Wang, T.; Jung, S.; Johnson, L.A. Economic Feasibility of Soybean Oil Production by Enzyme-Assisted Aqueous Extraction Processing. Food Bioprocess Technol. 2019, 12, 539–550. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Q.; Che, L. Synergistic Effects of Ultrasound and Extraction Solvent on the Bioactive Compound in Kenaf Seed Oil. J. Food Sci. Technol. 2020, 57, 2118–2128. [Google Scholar] [CrossRef]
- Amigh, S.; Taghian Dinani, S. Combination of Ultrasound-Assisted Aqueous Enzymatic Extraction and Cooking Pretreatment for Date Seed Oil Recovery. Heat Mass Transf. 2020, 56, 2345–2354. [Google Scholar] [CrossRef]
- Datt, S.; Sidhu, G.K. Optimization of Ultrasound Assisted Aqueous Oil Extraction from (Zea mays L.) Germ Using Response Surface Methodology. J. Pharmacogn. Phytochem. 2019, 8, 451–456. [Google Scholar]
- Peng, L.; Ye, Q.; Liu, X.; Liu, S.; Meng, X. Optimization of Aqueous Enzymatic Method for Camellia Sinensis Oil Extraction and Reuse of Enzymes in the Process. J. Biosci. Bioeng. 2019, 128, 716–722. [Google Scholar] [CrossRef]
- Liu, Z.; Gui, M.; Xu, T.; Zhang, L.; Kong, L.; Qin, L.; Zou, Z. Efficient Aqueous Enzymatic-Ultrasonication Extraction of Oil from Sapindus Mukorossi Seed Kernels. Ind. Crops Prod. 2019, 134, 124–133. [Google Scholar] [CrossRef]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Comparison of Subcritical CO2 and Ultrasound-Assisted Aqueous Methods with the Conventional Solvent Method in the Extraction of Avocado Oil. J. Supercrit. Fluids 2018, 135, 45–51. [Google Scholar] [CrossRef]
- Goula, A.M.; Papatheodorou, A.; Karasavva, S.; Kaderides, K. Ultrasound-Assisted Aqueous Enzymatic Extraction of Oil from Pomegranate Seeds. Waste Biomass Valor 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Moradi, N.; Rahimi, M.; Moeini, A.; Parsamoghadam, M.A. Impact of Ultrasound on Oil Yield and Content of Functional Food Ingredients at the Oil Extraction from Sunflower. Sep. Sci. Technol. 2018, 53, 261–276. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Z.-G.; Gai, Q.-Y.; Li, X.-J.; Wei, F.-Y.; Fu, Y.-J.; Ma, W. Microwave-Assisted Aqueous Enzymatic Extraction of Oil from Pumpkin Seeds and Evaluation of Its Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. Food Chem. 2014, 147, 17–24. [Google Scholar] [CrossRef]
- Maroušek, J. Use of Continuous Pressure Shockwaves Apparatus in Rapeseed Oil Processing. Clean Technol. Environ. Policy 2013, 15, 721–725. [Google Scholar] [CrossRef]
- Do, L.D.; Sabatini, D.A. Aqueous Extended-Surfactant Based Method for Vegetable Oil Extraction: Proof of Concept. J. Am. Oil Chem. Soc. 2010, 87, 1211–1220. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Liu, J.; Zhao, W.; Yang, R. Surfactant-Assisted Aqueous Extraction Processing of Camellia Seed Oil by Cyclic Utilization of Aqueous Phase. Eur. J. Lipid Sci. Technol. 2019, 121, 1800504. [Google Scholar] [CrossRef]
- Mat Yusoff, M.; Gordon, M.H.; Niranjan, K. Aqueous Enzyme Assisted Oil Extraction from Oilseeds and Emulsion De-Emulsifying Methods: A Review. Trends Food Sci. Technol. 2015, 41, 60–82. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, B.; Yang, G.; Bi, Y.; Chen, F. Rapid Salt-Assisted Microwave Demulsification of Oil-Rich Emulsion Obtained by Aqueous Enzymatic Extraction of Peanut Seeds. Eur. J. Lipid Sci. Technol. 2020, 122, 1900120. [Google Scholar] [CrossRef]
- White, D.A.; Fisk, I.D.; Mitchell, J.R.; Wolf, B.; Hill, S.E.; Gray, D.A. Sunflower-Seed Oil Body Emulsions: Rheology and Stability Assessment of a Natural Emulsion. Food Hydrocoll. 2008, 22, 1224–1232. [Google Scholar] [CrossRef]
- Karkani, O.A.; Nenadis, N.; Nikiforidis, C.V.; Kiosseoglou, V. Effect of Recovery Methods on the Oxidative and Physical Stability of Oil Body Emulsions. Food Chem. 2013, 139, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Nikiforidis, C.V.; Matsakidou, A.; Kiosseoglou, V. Composition, Properties and Potential Food Applications of Natural Emulsions and Cream Materials Based on Oil Bodies. RSC Adv. 2014, 4, 25067–25078. [Google Scholar] [CrossRef] [Green Version]
- Nikiforidis, C.V.; Scholten, E. High Internal Phase Emulsion Gels (HIPE-Gels) Created through Assembly of Natural Oil Bodies. Food Hydrocoll. 2015, 43, 283–289. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Donsouzi, S.; Kiosseoglou, V. The Interplay between Diverse Oil Body Extracts and Exogenous Biopolymers or Surfactants. Food Res. Int. 2016, 83, 14–24. [Google Scholar] [CrossRef]
- Furse, S.; Liddell, S.; Ortori, C.A.; Williams, H.; Neylon, D.C.; Scott, D.J.; Barrett, D.A.; Gray, D.A. The Lipidome and Proteome of Oil Bodies from Helianthus Annuus (Common Sunflower). J. Chem. Biol. 2013, 6, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah; Weiss, J.; Zhang, H. Recent Advances in the Composition, Extraction and Food Applications of Plant-Derived Oleosomes. Trends Food Sci. Technol. 2020, 106, 322–332. [Google Scholar] [CrossRef]
- Ding, J.; Wen, J.; Wang, J.; Tian, R.; Yu, L.; Jiang, L.; Zhang, Y.; Sui, X. The Physicochemical Properties and Gastrointestinal Fate of Oleosomes from Non-Heated and Heated Soymilk. Food Hydrocoll. 2020, 100, 105418. [Google Scholar] [CrossRef]
- Romero-Guzmán, M.J.; Köllmann, N.; Zhang, L.; Boom, R.M.; Nikiforidis, C.V. Controlled Oleosome Extraction to Produce a Plant-Based Mayonnaise-like Emulsion Using Solely Rapeseed Seeds. LWT 2020, 123, 109120. [Google Scholar] [CrossRef]
- Fisk, I.D.; Linforth, R.S.T.; Taylor, A.J.; Gray, D.A. Aroma Encapsulation and Aroma Delivery by Oil Body Suspensions Derived from Sunflower Seeds (Helianthus annus). Eur Food Res Technol 2011, 232, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Karefyllakis, D.; Jan van der Goot, A.; Nikiforidis, C.V. The Behaviour of Sunflower Oleosomes at the Interfaces. Soft Matter 2019, 15, 4639–4646. [Google Scholar] [CrossRef] [PubMed]
- Matsakidou, A.; Tsimidou, M.Z.; Kiosseoglou, V. Storage Behavior of Caseinate-Based Films Incorporating Maize Germ Oil Bodies. Food Res. Int. 2019, 116, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Lásztity, R.; Morsi, A.E.E.; Samei, M.B.A.; Ramadan, M.E. Solubility of Sunflower Proteins and Gel Filtration Chromatography of Their Water-Soluble Fractions. Period. Polytech. Chem. Eng. 1984, 28. [Google Scholar]
- Iwanaga, D.; Gray, D.A.; Fisk, I.D.; Decker, E.A.; Weiss, J.; McClements, D.J. Extraction and Characterization of Oil Bodies from Soy Beans: A Natural Source of Pre-Emulsified Soybean Oil. J. Agric. Food Chem. 2007, 55, 8711–8716. [Google Scholar] [CrossRef]
- Lacey, D.J.; Wellner, N.; Beaudoin, F.; Napier, J.A.; Shewry, P.R. Secondary Structure of Oleosins in Oil Bodies Isolated from Seeds of Safflower (Carthamus tinctorius L.) and Sunflower (Helianthus annuus L.). Biochem. J. 1998, 334, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforidis, C.V.; Kiosseoglou, V.; Scholten, E. Oil Bodies: An Insight on Their Microstructure—Maize Germ vs. Sunflower Seed. Food Res. Int. 2013, 52, 136–141. [Google Scholar] [CrossRef]
- Alexander, L.; Sessions, R.; Clarke, A.; Tatham, A.; Shewry, P.; Napier, J. Characterization and Modelling of the Hydrophobic Domain of a Sunflower Oleosin. Planta 2002, 214, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Sosulski, F.W.; Imafidon, G.I. Amino Acid Composition and Nitrogen-to-Protein Conversion Factors for Animal and Plant Foods. J. Agric. Food Chem. 1990, 38, 1351–1356. [Google Scholar] [CrossRef]
- Mills, C.T.; Goldhaber, M.B. On Silica-Based Solid Phase Extraction Techniques for Isolating Microbial Membrane Phospholipids: Ensuring Quantitative Recovery of Phosphatidylcholine-Derived Fatty Acids. Soil Biol. Biochem. 2010, 42, 1179–1182. [Google Scholar] [CrossRef]
- Silversand, C.; Haux, C. Improved High-Performance Liquid Chromatographic Method for the Separation and Quantification of Lipid Classes: Application to Fish Lipids. J. Chromatogr. B Biomed. Sci. Appl. 1997, 703, 7–14. [Google Scholar] [CrossRef]
- Tan, F.; Qiao, X.; Chen, J. Removal of Chemisorbed Lubricant on the Surface of Silver Flakes by Chemicals. Appl. Surf. Sci. 2006, 253, 703–707. [Google Scholar] [CrossRef]
- Carrillo Pérez, C.; Cavia Camarero, M.D.M.; Alonso de la Torre, S. Antitumor Effect of Oleic Acid; Mechanisms of Action. A Review. Nutr. Hosp. 2012, 1860–1865. [Google Scholar] [CrossRef] [Green Version]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Oleic and Undecylenic Acids as Renewable Feedstocks in the Synthesis of Polyols and Polyurethanes. Polymers 2010, 2, 440–453. [Google Scholar] [CrossRef] [Green Version]




| % (w/w) | Triglyceride Emulsion | Aqueous Phase | Bottom Residue |
|---|---|---|---|
| Dry matter (%) | 46.24 ± 1.18 | 3.07 ± 0.07 | 36.77 ± 2.04 |
| Water and volatile compounds (%) 1 | 53.76 ± 1.18 | 96.93 ± 0.07 | 63.23 ± 2.04 |
| Ash (%) | 0.89 ± 0.07 | n.d. | 1.15 ± 0.08 |
| Lipid (%) | 34.19 ± 2.04 | 0.14 ± 0.05 | 22.54 ± 1.19 |
| Total nitrogen (%) | 1.17 ± 0.12 | 0.14 ± 0.01 | 0.75 ± 0.05 |
| Protein (%) 2 | 6.07 ± 0.65 | 0.76 ± 0.02 | 3.98 ± 0.28 |
| Extraneous matter (%) | 5.09 ± 1.01 | 2.18 ± 0.05 | 9.11 ± 0.79 |
| % (w/w) | Fatty Acid Emulsion | Aqueous Phase | Bottom Residue |
|---|---|---|---|
| Dry matter (%) | 56.99 ± 1.34 | 4.07 ± 0.07 | 32.50 ± 1.88 |
| Water and volatile compounds (%) 1 | 43.01 ± 1.34 | 95.93 ± 0.07 | 67.50 ± 1.88 |
| Ash (%) | 0.48 ± 0.06 | <0.1 | 0.72 ± 0.04 |
| Lipid (%) | 39.55 ± 0.76 | 0.17 ± 0.02 | 18.46 ± 0.99 |
| Total nitrogen (%) | 1.29 ± 0.08 | 0.15 ± 0.01 | 0.70 ± 0.03 |
| Protein (%) 2 | 6.82 ± 0.44 | 0.81 ± 0.02 | 3.71 ± 0.17 |
| Extraneous matter (%) | 10.14 ± 1.55 | 3.11 ± 0.06 | 9.61 ± 0.79 |
| Fatty Acids (%) | Kernel | TAG Emulsion | FA Emulsion | |||
|---|---|---|---|---|---|---|
| NL | PL | NL | PL | NL | PL | |
| C14:0 | 0.1 ± 0.0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C16:0 | 3.1 ± 0.0 | 8.5 ± 0.6 | 3.2 ± 0.0 | 12.6 ± 1.4 | 3.3 ± 0.0 | 10.6 ± 1.1 |
| C18:0 | 1.4 ± 0.0 | 2 ± 0.3 | 1.4 ± 0.0 | 5.3 ± 1.1 | 1.5 ± 0.0 | 5.5 ± 0.5 |
| C20:0 | 0.1 ± 0.0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C22:0 | 0.7 ± 0.0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| SATURATED | 5.5 ± 0.0 | 10.5 | 4.6 ± 0.0 | 17.9 ± 2.5 | 4.8 ± 0.0 | 16.1 ± 1.6 |
| C16:1n7 | 0.1 ± 0.0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| C18:1n9 | 90.9 ± 0.0 | 84.2 ± 0.9 | 91.5 ± 0.0 | 77.2 ± 2.3 | 92.0 ± 0.0 | 78.9 ± 2.0 |
| C18:1n7c | 1 ± 0.0 | 0.9 ± 0.0 | n.d. | 2.2 ± 0.1 | n.d. | 1.5 ± 0.2 |
| C20:1n9 | 0.4 ± 0.0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| MONOUNSATURATED | 92.4 ± 0.0 | 85.1 ± 0.9 | 91.5 ± 0.0 | 79.4 ± 2.4 | 92 ± 0.0 | 80.4 ± 2.2 |
| C18:2n6 | 2.2 ± 0.0 | 4.3 ± 0.0 | 2.2 ± 0.0 | 2.7 ± 0.1 | 2.0 ± 0.0 | 3.5 ± 0.2 |
| Seed and Emulsions | Total (mg/100 g Lipids) | PA (rel %) | PE (rel %) | PC (rel %) | PI (rel %) |
|---|---|---|---|---|---|
| Sunflower seed | 1811.2 ± 122.2 | 5.2 ± 0.9 | 11.6 ± 1.2 a | 68 ± 3.7 | 15.2 ± 2.2 b |
| TAG emulsion | 144.4 ± 6 | 23.4 ± 1.2 | 12.4 ± 0.5 a | 53.4 ± 3.4 | 10.8 ± 1.9 b |
| FA emulsion | 17.1 ± 4.4 | 32 ± 2.2 | 52.8 ± 0.3 | 15.2 ± 2.4 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassen, A.; Fabre, J.-F.; Lacroux, E.; Cerny, M.; Vaca-Medina, G.; Mouloungui, Z.; Merah, O.; Valentin, R. Aqueous Integrated Process for the Recovery of Oil Bodies or Fatty Acid Emulsions from Sunflower Seeds. Biomolecules 2022, 12, 149. https://doi.org/10.3390/biom12020149
Cassen A, Fabre J-F, Lacroux E, Cerny M, Vaca-Medina G, Mouloungui Z, Merah O, Valentin R. Aqueous Integrated Process for the Recovery of Oil Bodies or Fatty Acid Emulsions from Sunflower Seeds. Biomolecules. 2022; 12(2):149. https://doi.org/10.3390/biom12020149
Chicago/Turabian StyleCassen, Audrey, Jean-François Fabre, Eric Lacroux, Muriel Cerny, Guadalupe Vaca-Medina, Zéphirin Mouloungui, Othmane Merah, and Romain Valentin. 2022. "Aqueous Integrated Process for the Recovery of Oil Bodies or Fatty Acid Emulsions from Sunflower Seeds" Biomolecules 12, no. 2: 149. https://doi.org/10.3390/biom12020149






