Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review
Abstract
:1. Introduction
- (a)
- (b)
- (c)
- (d)
- Agriculture, due to the creation of a thin coating on fruits and vegetables, which acts as a protective film preventing spoilage of agricultural products [51].
- (e)
- Medicine and biomedicine, depending on the chitosan purity it can be used in drug release and release systems, hemodialysis, artificial skin, linoleum, enzymatic immobilization, contact lenses, eye bandages, orthopedics, flossing, dentistry [52].
- (f)
- Medical engineering like wound healing, tissue engineering, manufacture of drug carriers, chitosan nanocarriers for anticancer drugs and vaccine release, gene therapy and bioimaging of vital organs [53].
2. Chitosan Composites Used in Implants and Dentistry
2.1. Composites of Chitosan/Carbon-Based Materials
2.2. Composites of Chitosan/Polymer-Based Materials
2.3. Composites of Chitosan/Metal or Metal Oxides
3. Synthesis Techniques of Chitosan Composites
3.1. Chitosan/Carbon-Based Composites Fabrication Techniques
3.2. Chitosan/Polymer-Based Composites Fabrication Techniques
3.3. Chitosan/Metal or Metal Oxide Composites Fabrication Techniques
4. Application of Chitosan Composites for Implant Scaffolds
5. Application of Chitosan Compositions for Dentistry
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, E.E.; Meffert, R.M.; Hermann, J.S.; Mellonig, J.T.; Cochran, D.L. Comparison of bioactive glass to demineralized freeze-dried bone allograft in the treatment of intrabony defects around implants in the canine mandible. J. Periodontol. 1999, 70, 526–535. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Boening, K.W.; Grychowska, N.; Paradowska-Stolarz, A. Clinical application of chitosan in dental specialities. Mini Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef]
- Lubin, G. Handbook of Composites; Springer Science & Business Media: Boston, MA, USA, 2013. [Google Scholar]
- Kmiec, M.; Pighinelli, L.; Tedesco, M.; Silva, M.; Reis, V. Chitosan-properties and applications in dentistry. Adv. Tissue Eng. Regen. Med. Open Access 2017, 2, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Moura, C.; Muszinski, P.; Schmidt, C.; Almeida, J.; Pinto, L. Quitina e quitosana produzidas a partir de resíduos de camarão e siri: Avaliação do processo em escala piloto. Vetor Rio Grande 2006, 16, 37–45. [Google Scholar]
- Rahimi, S.; SharifianJazi, F.; Esmaeilkhanian, A.; Moradi, M.; Samghabadi, A.H.S. Effect of SiO2 content on Y-TZP/Al2O3 ceramic-nanocomposite properties as potential dental applications. Ceram. Int. 2020, 46, 10910–10916. [Google Scholar] [CrossRef]
- Esmaeilkhanian, A.; Sharifianjazi, F.; Abouchenari, A.; Rouhani, A.; Parvin, N.; Irani, M. Synthesis and characterization of natural nano-hydroxyapatite derived from turkey femur-bone waste. Appl. Biochem. Biotechnol. 2019, 189, 919–932. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N. Application of chitosan in bone and dental engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Choi, S.H.; Moon, I.S.; Cho, K.S.; Chai, J.K.; Kim, C.K. Eight-week histological analysis on the effect of chitosan on surgically created one-wall intrabony defects in beagle dogs. J. Clin. Periodontol. 2003, 30, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A. A review of Polyvinyl alcohol/Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 69–76. [Google Scholar]
- Daraei, J. Production and characterization of PCL (Polycaprolactone) coated TCP/nanoBG composite scaffolds by sponge foam method for orthopedic applications. J. Compos. Compd. 2020, 2, 44–49. [Google Scholar] [CrossRef]
- Alam, M.A.; Asoushe, M.H.; Pourhakkak, P.; Gritsch, L.; Alipour, A.; Mohammadi, S. Preparation of bioactive polymer-based composite by different techniques and application in tissue engineering: A review. J. Compos. Compd. 2021, 3, 194–205. [Google Scholar]
- Magli, S.; Rossi, L.; Consentino, C.; Bertini, S.; Nicotra, F.; Russo, L. Combined Analytical Approaches to Standardize and Characterize Biomaterials Formulations: Application to Chitosan-Gelatin Cross-Linked Hydrogels. Biomolecules 2021, 11, 683. [Google Scholar] [CrossRef]
- Jafarigol, E.; Salehi, M.B.; Mortaheb, H.R. Synergetic effects of additives on structural properties of acrylamide-based hydrogel. J. Dispers. Sci. Technol. 2021, 42, 910–919. [Google Scholar] [CrossRef]
- Jafarigol, E.; Salehi, M.B.; Mortaheb, H.R. Preparation and assessment of electro-conductive poly(acrylamide-co-acrylic acid) carboxymethyl cellulose/reduced graphene oxide hydrogel with high viscoelasticity. Chem. Eng. Res. Des. 2020, 162, 74–84. [Google Scholar] [CrossRef]
- Ahadi, F.; Khorshidi, S.; Karkhaneh, A. A hydrogel/fiber scaffold based on silk fibroin/oxidized pectin with sustainable release of vancomycin hydrochloride. Eur. Polym. J. 2019, 118, 265–274. [Google Scholar] [CrossRef]
- Karkhaneh, A.; Ahadi, F.; Khorshidi, S. Vancomycin Loaded Silk/Pectin Hydrogel for Bone Tissue Engineering. In Eco-Friendly and Smart Polymer Systems; Mirzadeh, H., Katbab, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 272–275. [Google Scholar]
- Sharifianjazi, F.; Irani, M.; Esmaeilkhanian, A.; Bazli, L.; Asl, M.S.; Jang, H.W.; Kim, S.Y.; Ramakrishna, S.; Shokouhimehr, M.; Varma, R.S. Polymer incorporated magnetic nanoparticles: Applications for magnetoresponsive targeted drug delivery. Mater. Sci. Eng. B 2021, 272, 115358. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan films and scaffolds for regenerative medicine applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Ikram, S. Chitosan: A natural antimicrobial agent—A review. J. Appl. Chem. 2014, 3, 493–503. [Google Scholar]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L.; Campana-Filho, S.P.; Pinto, L.A.D. Chitosan Based Materials and Its Applications; Bentham Science Publishers: Al Sharjah, United Arab Emirates, 2017. [Google Scholar]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Liu, J. Advance in chitosan hydrolysis by non-specific cellulases. Bioresour. Technol. 2008, 99, 6751–6762. [Google Scholar] [CrossRef]
- Khalilpour, H.; Shafiee, P.; Darbandi, A.; Yusuf, M.; Mahmoudi, S.; Goudarzi, Z.M.; Mirzamohammadi, S. Application of Polyoxometalate-based composites for sensor systems: A review. J. Compos. Compd. 2021, 3, 129–139. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Nasibi, S.; Alimohammadi, K.; Bazli, L.; Eskandarinezhad, S.; Mohammadi, A.; Sheysi, N. TZNT alloy for surgical implant applications: A systematic review. J. Compos. Compd. 2020, 2, 62–68. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Gea, S.; Ilyas, S.; Tamrin, T.; Radecka, I. Characterization of Bacterial Cellulose-Based Wound Dressing in Different Order Impregnation of Chitosan and Collagen. Biomolecules 2020, 10, 1511. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Thaweesest, W.; Bhuket, P.R.N.; Jantaratana, P.; Rojsitthisak, P.; Rojsitthisak, P. Polyethylene Glycol-Chitosan Oligosaccharide-Coated Superparamagnetic Iron Oxide Nanoparticles: A Novel Drug Delivery System for Curcumin Diglutaric Acid. Biomolecules 2020, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Niazvand, F.; Wagh, P.R.; Khazraei, E.; Dastjerdi, M.B.; Patil, C.; Najar, I.A. Application of carbon allotropes composites for targeted cancer therapy drugs: A review. J. Compos. Compd. 2021, 3, 140–151. [Google Scholar] [CrossRef]
- Bahmanpour, A.; Ghaffari, M.; Milan, P.B.; Moztarzadeh, F.; Mozafari, M. Synthesis and characterization of thermosensitive hydrogel based on quaternized chitosan for intranasal delivery of insulin. Biotechnol. Appl. Biochem. 2021, 68, 247–256. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Yi, T.; Zhou, C.; Ma, L.; Wu, L.; Xu, X.; Gu, L.; Fan, Y.; Xian, G.; Fan, H.; Zhang, X. Direct 3-D printing of Ti-6Al-4V/HA composite porous scaffolds for customized mechanical properties and biological functions. J. Tissue Eng. Regen. Med. 2020, 14, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Holland, I.; Logan, J.; Shi, J.; McCormick, C.; Liu, D.; Shu, W. 3D biofabrication for tubular tissue engineering. Bio-Des. Manuf. 2018, 1, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; van Le, Q. Bioactive glass coated zirconia for dental implants: A review. J. Compos. Compd. 2020, 2, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Zamani, Y.; Zareein, A.; Bazli, L.; NasrAzadani, R.; Mahammod, B.P.; Nasibi, S.; Chahardehi, A.M. Nanodiamond-containing composites for tissue scaffolds and surgical implants: A review. J. Compos. Compd. 2020, 2, 215–227. [Google Scholar] [CrossRef]
- Zamani, Y.; Ghazanfari, H.; Erabi, G.; Moghanian, A.; Fakić, B.; Hosseini, S.M.; Mahammod, B.P. A review of additive manufacturing of Mg-based alloys and composite implants. J. Compos. Compd. 2021, 3, 71–83. [Google Scholar] [CrossRef]
- Abasian, P.; Radmansouri, M.; Jouybari, M.H.; Ghasemi, M.V.; Mohammadi, A.; Irani, M.; Jazi, F.S. Incorporation of magnetic NaX zeolite/DOX into the PLA/chitosan nanofibers for sustained release of doxorubicin against carcinoma cells death in vitro. Int. J. Biol. Macromol. 2019, 121, 398–406. [Google Scholar] [CrossRef]
- Sousa, C.F.V.; Saraiva, C.A.; Correia, T.R.; Pesqueira, T.; Patrício, S.G.; Rial-Hermida, M.I.; Borges, J.; Mano, J.F. Bioinstructive Layer-by-Layer-Coated Customizable 3D Printed Perfusable Microchannels Embedded in Photocrosslinkable Hydrogels for Vascular Tissue Engineering. Biomolecules 2021, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Marinho, S.; Illanes, M.; Ávila-Román, J.; Motilva, V.; Talero, E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef]
- Salama, A. Recent progress in preparation and applications of chitosan/calcium phosphate composite materials. Int. J. Biol. Macromol. 2021, 178, 240–252. [Google Scholar] [CrossRef]
- Lee, B.-S.; Lee, C.-C.; Wang, Y.-P.; Chen, H.-J.; Lai, C.-H.; Hsieh, W.-L.; Chen, Y.-W. Controlled-release of tetracycline and lovastatin by poly (D, L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int. J. Nanomed. 2016, 11, 285. [Google Scholar]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2021, 167, 1198–1210. [Google Scholar] [CrossRef]
- Carreno-Gomez, B.; Duncan, R. Evaluation of the biological properties of soluble chitosan and chitosan microspheres. Int. J. Pharm. 1997, 148, 231–240. [Google Scholar] [CrossRef]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajić, Z.; Hanke, T.; Bernhard, G.; Brunner, E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Khwaldia, K.; Arab-Tehrany, E.; Desobry, S. Biopolymer coatings on paper packaging materials. Compr. Rev. Food Sci. Food Saf. 2010, 9, 82–91. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Worley, S.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Clarification of fruit juice with chitosan. Process Biochem. 2004, 39, 2229–2232. [Google Scholar] [CrossRef]
- Cota-Arriola, O.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Controlled release matrices and micro/nanoparticles of chitosan with antimicrobial potential: Development of new strategies for microbial control in agriculture. J. Sci. Food Agric. 2013, 93, 1525–1536. [Google Scholar] [CrossRef]
- Lian, Q.; Li, D.; Jin, Z.; Wang, J.; Li, A.; Wang, Z.; Jin, Z. Fabrication and in vitro evaluation of calcium phosphate combined with chitosan fibers for scaffold structures. J. Bioact. Compat. Polym. 2009, 24, 113–124. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, X.-K.; Xue, X.-T.; Wu, D.-Y. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr. Polym. 2012, 88, 75–83. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chung, Y.-C. Antibacterial effect of water-soluble chitosan on representative dental pathogens Streptococcus mutans and Lactobacilli brevis. J. Appl. Oral Sci. 2012, 20, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, F.; Moghadamnia, A.A.; Kazemi, S.; Shirzad, A.; Motallebnejad, M. Effect of 0.5% Chitosan mouthwash on recurrent aphthous stomatitis: A randomized double-blind crossover clinical trial. Electron. Physician 2018, 10, 6912. [Google Scholar] [CrossRef] [PubMed]
- Mohire, N.C.; Yadav, A.V. Chitosan-based polyherbal toothpaste: As novel oral hygiene product. Indian J. Dent. Res. 2010, 21, 380. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Pakseresht, A.H.; Asl, M.S.; Esmaeilkhanian, A.; Jang, H.W.; Shokouhimehr, M. Hydroxyapatite consolidated by zirconia: Applications for dental implant. J. Compos. Compd. 2020, 2, 26–34. [Google Scholar] [CrossRef] [Green Version]
- di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Xu, H.H.; Simon, C.G., Jr. Fast setting calcium phosphate–chitosan scaffold: Mechanical properties and biocompatibility. Biomaterials 2005, 26, 1337–1348. [Google Scholar] [CrossRef]
- Matsuda, A.; Ikoma, T.; Kobayashi, H.; Tanaka, J. Preparation and mechanical property of core-shell type chitosan/calcium phosphate composite fiber. Mater. Sci. Eng. C 2004, 24, 723–728. [Google Scholar] [CrossRef]
- Wang, S.-F.; Shen, L.; Zhang, W.-D.; Tong, Y.-J. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Kim, S.-K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [Green Version]
- Terada, M.; Abe, S.; Akasaka, T.; Uo, M.; Kitagawa, Y.; Watari, F. Development of a multiwalled carbon nanotube coated collagen dish. Dent. Mater. J. 2009, 28, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladiè, R.; Cosentino, C.; Tagliaro, I.; Antonini, C.; Bianchini, G.; Bertini, S. Supramolecular Structuring of Hyaluronan-Lactose-Modified Chitosan Matrix: Towards High-Performance Biopolymers with Excellent Biodegradation. Biomolecules 2021, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-based nanomaterials for tissue engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef]
- Nawrotek, K.; Tylman, M.; Rudnicka, K.; Gatkowska, J.; Balcerzak, J. Tubular electrodeposition of chitosan–carbon nanotube implants enriched with calcium ions. J. Mech. Behav. Biomed. Mater. 2016, 60, 256–266. [Google Scholar] [CrossRef]
- Venkatesan, J.; Ryu, B.; Sudha, P.; Kim, S.-K. Preparation and characterization of chitosan–carbon nanotube scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.; Valencia, C.H.; Zuluaga, F.; Valencia, M.E.; Mina, J.H.; Grande-Tovar, C.D. Synthesis and application of scaffolds of chitosan-graphene oxide by the freeze-drying method for tissue regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapata, M.E.; Tovar, C.D.; Hernandez, J.H. The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements. Biomolecules 2020, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Bahri, A.; Askarnia, R.; Esmaeilzadeh, J.; Fardi, S.R. Mechanical and electrochemical behaviors assessments of Aluminum-Graphene Oxide composites fabricated by mechanical milling and repetitive upsetting extrusion. J. Compos. Compd. 2021, 3, 152–158. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
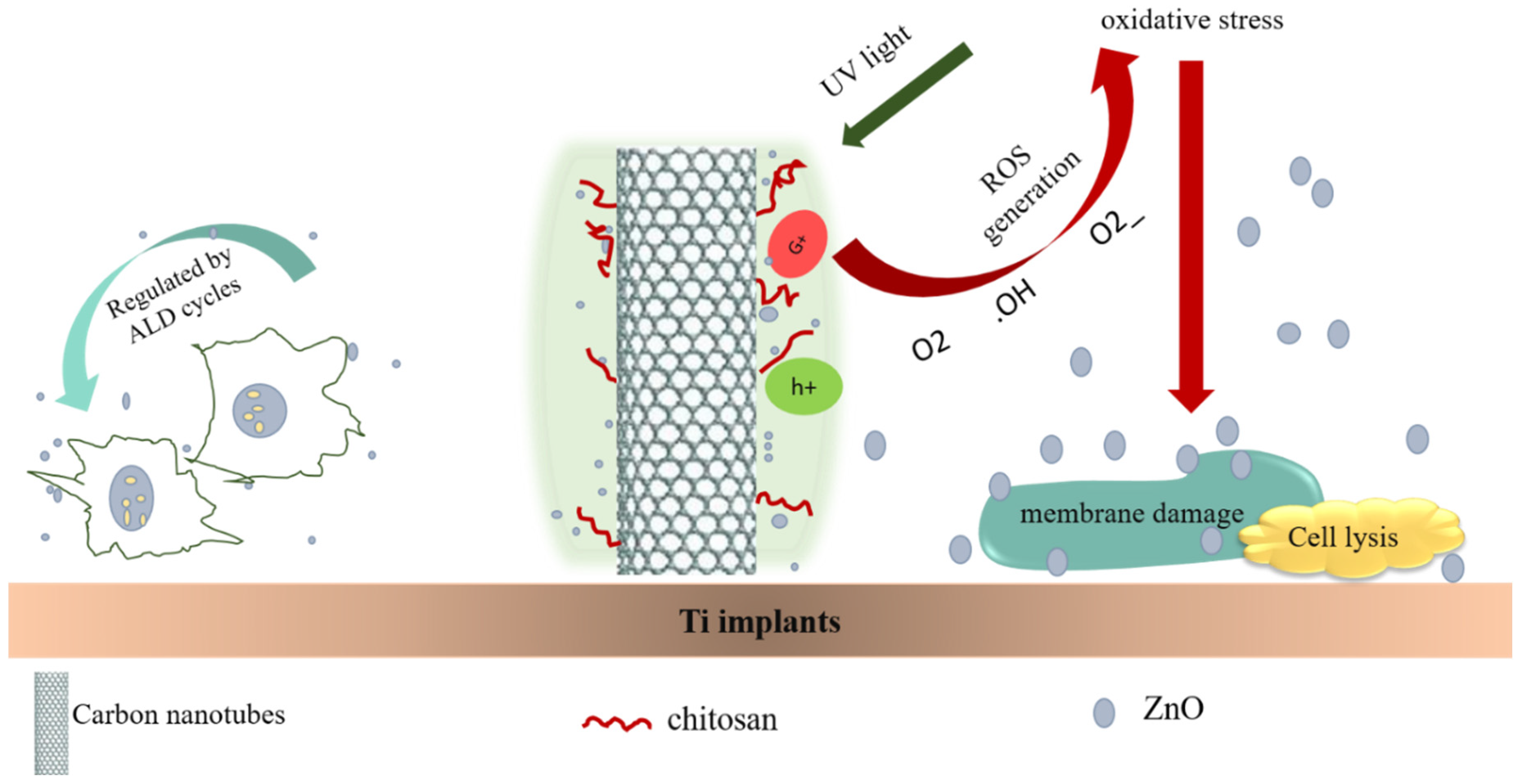
- Zhu, Y.; Liu, X.; Yeung, K.W.; Chu, P.K.; Wu, S. Biofunctionalization of carbon nanotubes/chitosan hybrids on Ti implants by atom layer deposited ZnO nanostructures. Appl. Surf. Sci. 2017, 400, 14–23. [Google Scholar] [CrossRef]
- Montazeri, A.; Karjibani, M. Preparation and Characterization of Chitosan-Double Walled Carbon Nanotubes Hydrogels. Int. J. Chemoinform. Chem. Eng. 2017, 6, 21–30. [Google Scholar] [CrossRef]
- Choo, K.; Ching, Y.C.; Chuah, C.H.; Julai, S.; Liou, N.-S. Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiryaghoubi, N.; Pesyan, N.N.; Fathi, M.; Omidi, Y. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 1338–1357. [Google Scholar] [CrossRef]
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Fourie, J.; Taute, F.; de Preez, L.; de Beer, D. Chitosan Composite Biomaterials for Bone Tissue Engineering—A Review. Regen. Eng. Transl. Med. 2020, 1–21. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. Evaluation of BMP-2 and VEGF loaded 3D printed hydroxyapatite composite scaffolds with enhanced osteogenic capacity in vitro and in vivo. Mater. Sci. Eng. C 2020, 112, 110893. [Google Scholar] [CrossRef] [PubMed]
- Bazli, L.; Yusuf, M.; Farahani, A.; Kiamarzi, M.; Seyedhosseini, Z.; Nezhadmansari, M.; Aliasghari, M.; Iranpoor, M. Application of composite conducting polymers for improving the corrosion behavior of various substrates: A Review. J. Compos. Compd. 2020, 2, 228–240. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Prakash, C.; Ramakrishna, S.; Lamberti, L.; Pruncu, C.I. 3D printed biodegradable composites: An insight into mechanical properties of PLA/chitosan scaffold. Polym. Test. 2020, 89, 106722. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Ilyas, K.; Dlouhý, I.; Siska, F.; Boccaccini, A.R. Electrophoretic Deposition of Copper (II)–Chitosan Complexes for Antibacterial Coatings. Int. J. Mol. Sci. 2020, 21, 2637. [Google Scholar] [CrossRef] [Green Version]
- Mirhashemi, A.; Akhondi, M.S.A.; Sodagar, A.; Jalali, Y.F.; Jazi, L. Effect of nano–zinc oxide and nano-chitosan particles on the shear bond strength of dental composites used as orthodontic adhesive. J. World Fed. Orthod. 2021, 10, 172–176. [Google Scholar] [CrossRef]
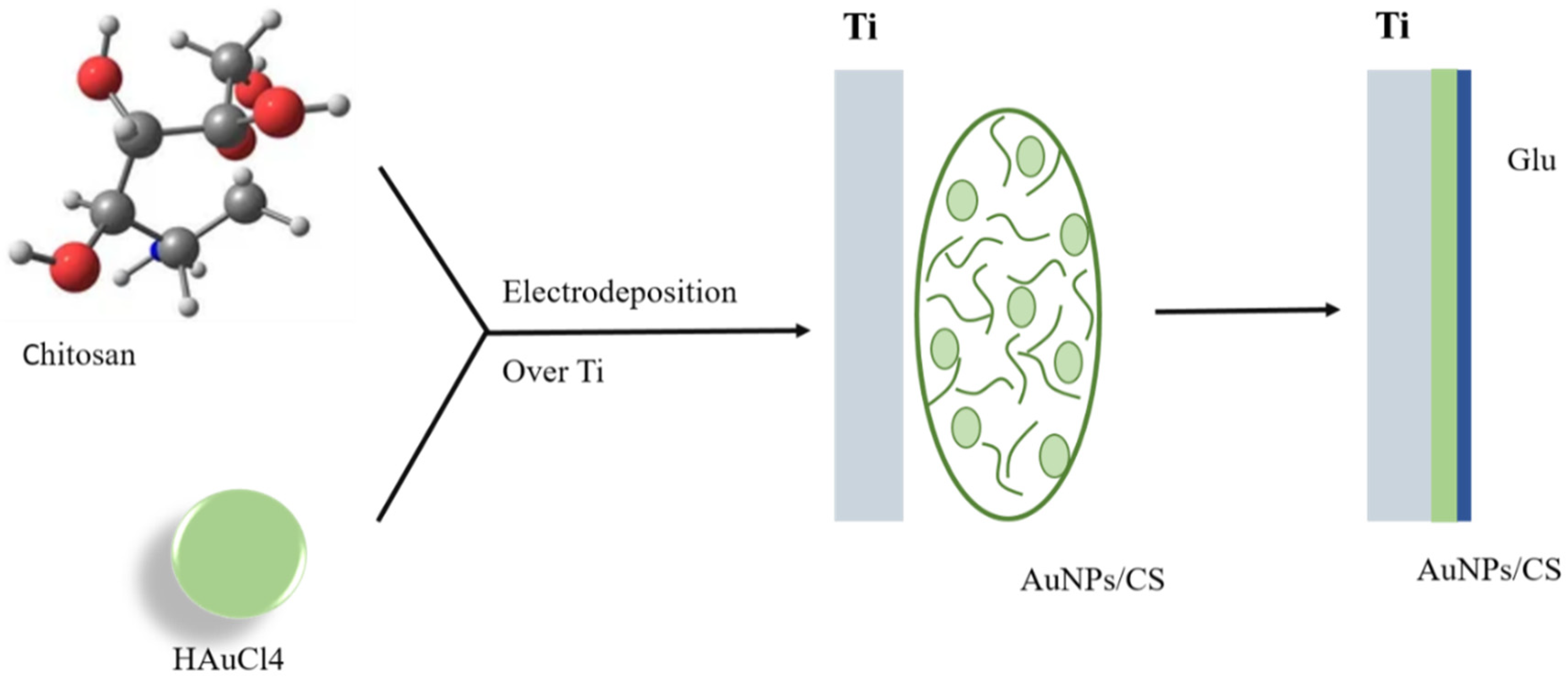
- Hussein, M.A.M.; Baños, F.G.D.; Grinholc, M.; Dena, A.S.A.; El-Sherbiny, I.M.; Megahed, M. Exploring the physicochemical and antimicrobial properties of gold-chitosan hybrid nanoparticles composed of varying chitosan amounts. Int. J. Biol. Macromol. 2020, 162, 1760–1769. [Google Scholar] [CrossRef]
- Farasat, M.; Niazvand, F.; Khorsandi, L. Zinc oxide nanoparticles induce necroptosis and inhibit autophagy in MCF-7 human breast cancer cells. Biologia 2020, 75, 161–174. [Google Scholar] [CrossRef]
- Nourmohammadi, M.; Rouhani, S.; Azizi, S.; Maaza, M.; Msagati, T.A.M.; Rostamnia, S.; Hatami, M.; Khaksar, S.; Zarenezhad, E.; Jang, H.W.; et al. Magnetic nanocomposite of crosslinked chitosan with 4,6-diacetylresorcinol for gold immobilization (Fe3O4@CS/DAR-Au) as a catalyst for an efficient one-pot synthesis of propargylamine. Mater. Today Commun. 2021, 29, 102798. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Gholipour, B.; Rostamnia, S.; Eftekhari, A.; Tanomand, A.; Khaksar, S.; Khalilov, R. Biosynthesis of AgNPs onto the urea-based periodic mesoporous organosilica (AgxNPs/Ur-PMO) for antibacterial and cell viability assay. J. Colloid Interface Sci. 2021, 585, 676–683. [Google Scholar] [CrossRef]
- Farghali, R.; Fekry, A.; Ahmed, R.A.; Elhakim, H. Corrosion resistance of Ti modified by chitosan–gold nanoparticles for orthopedic implantation. Int. J. Biol. Macromol. 2015, 79, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Gawad, S.A.; Nasr, A.; Fekry, A.M.; Filippov, L.O. Electrochemical and hydrogen evolution behaviour of a novel nano-cobalt/nano-chitosan composite coating on a surgical 316L stainless steel alloy as an implant. Int. J. Hydrog. Energy 2021, 46, 18233–18241. [Google Scholar] [CrossRef]
- Narayanan, V.; Sumathi, S.; Narayanasamy, A.N.R. Tricomponent composite containing copper–hydroxyapatite/chitosan/polyvinyl pyrrolidone for bone tissue engineering. J. Biomed. Mater. Res. Part A 2020, 108, 1867–1880. [Google Scholar] [CrossRef]
- Asl, M.S.; Razavizadeh, H. Synergistic effects of ferric sulfate addition and mechanical activation on leaching of Sarcheshmeh copper sulfide concentrate. J. Compos. Compd. 2021, 3, 171–175. [Google Scholar]
- Javed, R.; Rais, F.; Kaleem, M.; Jamil, B.; Ahmad, M.A.; Yu, T.; Qureshi, S.W.; Ao, Q. Chitosan capping of CuO nanoparticles: Facile chemical preparation, biological analysis, and applications in dentistry. Int. J. Biol. Macromol. 2021, 167, 1452–1467. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Kumar, M.K.; Alinaghibeigi, M.; Moolayadukkam, S.; Eskandarinejad, S.; Mahmoudi, S.; Mirzamohammadi, S.; Rezaei-khamseh, M. A review on zinc oxide composites for energy storage applications: Solar cells, batteries, and supercapacitors. J. Compos. Compd. 2021, 3, 182–193. [Google Scholar]
- Hu, X.; He, J.; Yong, X.; Lu, J.; Xiao, J.; Liao, Y.; Li, Q.; Xiong, C. Biodegradable poly (lactic acid-co-trimethylene carbonate)/chitosan microsphere scaffold with shape-memory effect for bone tissue engineering. Colloids Surf. B Biointerfaces 2020, 195, 111218. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhang, L.; Wang, J.; Wang, S.; Zou, Y.; Wang, X. Novel fabrication of morphology tailored nanostructures with Gelatin/Chitosan Co-polymeric bio-composited hydrogel system to accelerate bone fracture healing and hard tissue nursing care management. Process Biochem. 2020, 90, 177–183. [Google Scholar] [CrossRef]
- Deen, I.; Selopal, G.S.; Wang, Z.M.; Rosei, F. Electrophoretic deposition of collagen/chitosan films with copper-doped phosphate glasses for orthopaedic implants. J. Colloid Interface Sci. 2022, 607, 869–880. [Google Scholar] [CrossRef]
- Gouveia, Z.; Perinpanayagam, H.; Zhu, J. Development of robust chitosan–silica class II hybrid coatings with antimicrobial properties for titanium implants. Coatings 2020, 10, 534. [Google Scholar] [CrossRef]
- Askarnia, R.; Fardi, S.R.; Sobhani, M.; Staji, H. Ternary Hydroxyapatite/Chitosan/Graphene Oxide Composite Coating on AZ91D Magnesium Alloy by Electrophoretic Deposition. Ceram. Int. 2021, 47, 27071–27081. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Onder, O.C.; Sevgili, I.; Yilgor, E.; Kavakli, I.H.; Yilgor, I. 3D printed poly (lactic acid) scaffolds modified with chitosan and hydroxyapatite for bone repair applications. Mater. Today Commun. 2020, 25, 101515. [Google Scholar] [CrossRef]
- Aydemir, T.; Pastore, J.I.; Jimenez-Pique, E.; Roa, J.J.; Boccaccini, A.R.; Ballarre, J. Morphological and mechanical characterization of chitosan/gelatin/silica-gentamicin/bioactive glass coatings on orthopaedic metallic implant materials. Thin Solid Films 2021, 732, 138780. [Google Scholar] [CrossRef]
- Lin, M.-H.; Wang, Y.-H.; Kuo, C.-H.; Ou, S.-F.; Huang, P.-Z.; Song, T.-Y.; Chen, Y.-C.; Chen, S.-T.; Wu, C.-H.; Hsueh, Y.-H. Hybrid ZnO/chitosan antimicrobial coatings with enhanced mechanical and bioactive properties for titanium implants. Carbohydr. Polym. 2021, 257, 117639. [Google Scholar] [CrossRef]
- Niazvand, F.; Cheshmi, A.; Zand, M.; NasrAzadani, R.; Kumari, B.; Raza, A.; Nasibi, S. An overview of the development of composites containing Mg and Zn for drug delivery. J. Compos. Compd. 2020, 2, 193–204. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Sivashankari, P.; Prabaharan, M. Chitosan/carbon-based nanomaterials as scaffolds for tissue engineering. In Biopolymer-Based Composites; Elsevier: Cambridge, MA, USA, 2017; pp. 381–397. [Google Scholar]
- Hamghavandi, M.R.; Montazeri, A.; Daryakenari, A.A.; Pishvaei, M. Preparation and characterization of chitosan/graphene oxide nanocomposite coatings on Mg–2 wt% Zn scaffold by pulse electrodeposition process. Biomed. Mater. 2021, 16, 065005. [Google Scholar] [CrossRef]
- Murugan, S.S.; Anil, S.; Sivakumar, P.; Shim, M.S.; Venkatesan, J. 3D-Printed Chitosan Composites for Biomedical Applications. In Chitosan for Biomaterials IV; Jayakumar, R., Prabaharan, M., Eds.; Advances in Polymer Science; Springer: Cham, Switzerland, 2021; Volume 288. [Google Scholar]
- Zadeh, M.H.R.; Seifi, M.; Abdolrahimi, M.; Hadavi, M. A comprehensive in vitro study of the carbon nanotube enhanced chitosan scaffolds for cancellous bone regeneration. Biomed. Phys. Eng. Express 2018, 4, 035027. [Google Scholar] [CrossRef]
- Valencia-Llano, C.H.; Solano, M.A.; Grande-Tovar, C.D. Nanocomposites of Chitosan/Graphene Oxide/Titanium Dioxide Nanoparticles/Blackberry Waste Extract as Potential Bone Substitutes. Polymers 2021, 13, 3877. [Google Scholar] [CrossRef]
- Daramola, O.; Olajide, J.; Oladele, I.; Adediran, A.; Adewuyi, B.; Muhammed, A.; Sadiku, E. Mechanical and wear behaviour of polylactic acid matrix composites reinforced with crab-shell synthesized chitosan microparticles. Mater. Today Proc. 2021, 38, 999–1005. [Google Scholar] [CrossRef]
- Torres-Hernández, Y.G.; Ortega-Díaz, G.M.; Téllez-Jurado, L.; Castrejón-Jiménez, N.S.; Altamirano-Torres, A.; García-Pérez, B.E.; Balmori-Ramírez, H. Biological compatibility of a polylactic acid composite reinforced with natural chitosan obtained from shrimp waste. Materials 2018, 11, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mania, S.; Ryl, J.; Jinn, J.-R.; Wang, Y.-J.; Michałowska, A.; Tylingo, R. The Production possibility of the antimicrobial filaments by co-extrusion of the PLA pellet with chitosan powder for FDM 3D printing technology. Polymers 2019, 11, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini, Z.; Rudsary, S.S.; Shahraeini, S.S.; Dizaji, B.F.; Goleij, P.; Bakhtiari, A.; Irani, M.; Sharifianjazi, F. Magnetic bioactive glasses/Cisplatin loaded-chitosan (CS)-grafted-poly (ε-caprolactone) nanofibers against bone cancer treatment. Carbohydr. Polym. 2021, 258, 117680. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper–zinc for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar] [CrossRef]
- Saravanan, S.; Nethala, S.; Pattnaik, S.; Tripathi, A.; Moorthi, A.; Selvamurugan, N. Preparation, characterization and antimicrobial activity of a bio-composite scaffold containing chitosan/nano-hydroxyapatite/nano-silver for bone tissue engineering. Int. J. Biol. Macromol. 2011, 49, 188–193. [Google Scholar] [CrossRef]
- Nouri, A.; Dizaji, B.F.; Kianinejad, N.; Rad, A.J.; Rahimi, S.; Irani, M.; Jazi, F.S. Simultaneous linear release of folic acid and doxorubicin from ethyl cellulose/chitosan/g-C3N4/MoS2 core-shell nanofibers and its anticancer properties. J. Biomed. Mater. Res. Part A 2021, 109, 903–914. [Google Scholar] [CrossRef]
- Dizaji, B.F.; Azerbaijan, M.H.; Sheisi, N.; Goleij, P.; Mirmajidi, T.; Chogan, F.; Irani, M.; Sharafian, F. Synthesis of PLGA/chitosan/zeolites and PLGA/chitosan/metal organic frameworks nanofibers for targeted delivery of Paclitaxel toward prostate cancer cells death. Int. J. Biol. Macromol. 2020, 164, 1461–1474. [Google Scholar] [CrossRef]
- Radmansouri, M.; Bahmani, E.; Sarikhani, E.; Rahmani, K.; Sharifianjazi, F.; Irani, M. Doxorubicin hydrochloride—Loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int. J. Biol. Macromol. 2018, 116, 378–384. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S. Fabrication, characterization of chitosan/nanosilver film and its potential antibacterial application. J. Biomater. Sci. Polym. Ed. 2009, 20, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, A.; Pramanik, N.; Jana, P.; Mitra, T.; Gnanamani, A.; Das, M.; Kundu, P.P. Development of bone-like zirconium oxide nanoceramic modified chitosan based porous nanocomposites for biomedical application. Int. J. Biol. Macromol. 2017, 95, 348–356. [Google Scholar] [CrossRef]
- Bordini, E.A.F.; Cassiano, F.B.; Silva, I.S.P.; Usberti, F.R.; Anovazzi, G.; Pacheco, L.E.; Pansani, T.N.; Leite, M.L.; Hebling, J.; de Costa, C.A. Synergistic potential of 1α, 25-dihydroxyvitamin D3 and Calcium–Aluminate–Chitosan scaffolds with dental pulp cells. Clin. Oral Investig. 2020, 24, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Omid, S.O.; Goudarzi, Z.; Kangarshahi, L.M.; Mokhtarzade, A.; Bahrami, F. Self-expanding stents based on shape memory alloys and shape memory polymers. J. Compos. Compd. 2020, 2, 92–98. [Google Scholar]
- Chehrehsaz, Y.; Hajizadeh, K.; Hadjizadeh, A.; Moradi, L.; Mahshid, S. Effect of ECAP on Physicochemical and Biological Properties of TiO2 Nanotubes Anodized on Commercially Pure Titanium. Met. Mater. Int. 2021. [Google Scholar] [CrossRef]
- Ahmed, R.A.; Fekry, A.M.; Farghali, R. A study of calcium carbonate/multiwalled-carbon nanotubes/chitosan composite coatings on Ti–6Al–4V alloy for orthopedic implants. Appl. Surf. Sci. 2013, 285, 309–316. [Google Scholar] [CrossRef]
- Torghabeh, N.A.; Raissi, B.; Riahifar, R.; Sahbayaghmaee, M.; Bidgoli, Z.M. Investigation of the flocculation and sedimentation of TiO2 nanoparticles in different alcoholic environments through turbidity measurements. J. Compos. Compd. 2021, 3, 159–163. [Google Scholar] [CrossRef]
- Santos, E.M.; Ramos, F.J.H.T.V.; da Silveira, P.H.P.M.; Monteiro, S.N.; Gomes, A.V. Chemical, thermal, and microstructural characterization of polyethylene terephthalate composites reinforced with steel slag geopolymer waste. J. Compos. Compd. 2021, 3, 164–170. [Google Scholar]
- Clavijo, S.; Membrives, F.; Quiroga, G.; Boccaccini, A.R.; Santillán, M.J. Electrophoretic deposition of chitosan/Bioglass® and chitosan/Bioglass®/TiO2 composite coatings for bioimplants. Ceram. Int. 2016, 42, 14206–14213. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł.; Zieliński, A.; Mielewczyk-Gryń, A.; Strugała, G.; Cieślik, B. Electrophoretic Deposition and Characteristics of Chitosan–Nanosilver Composite Coatings on a Nanotubular TiO2 Layer. Coatings 2020, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Bonifacio, M.A.; Cometa, S.; Dicarlo, M.; Baruzzi, F.; de Candia, S.; Gloria, A.; Giangregorio, M.M.; Mattioli-Belmonte, M.; de Giglio, E. Gallium-modified chitosan/poly (acrylic acid) bilayer coatings for improved titanium implant performances. Carbohydr. Polym. 2017, 166, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Chozhanathmisra, M.; Pandian, K.; Govindaraj, D.; Karthikeyan, P.; Mitu, L.; Rajavel, R. Halloysite nanotube-reinforced ion-incorporated hydroxyapatite-chitosan composite coating on Ti-6Al-4 V alloy for implant application. J. Chem. 2019, 2019, 7472058. [Google Scholar] [CrossRef] [Green Version]
- Doench, I.; Tran, T.A.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N. Cellulose nanofiber-reinforced chitosan hydrogel composites for intervertebral disc tissue repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Ballarre, J.; Aydemir, T.; Liverani, L.; Roether, J.A.; Goldmann, W.; Boccaccini, A.R. Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: Improving early stage performance of titanium implants. Surf. Coat. Technol. 2020, 381, 125138. [Google Scholar] [CrossRef]
- Francis, A.; Abdel-Gawad, S.; Shoeib, M. Toward CNT-reinforced chitosan-based ceramic composite coatings on biodegradable magnesium for surgical implants. J. Coat. Technol. Res. 2021, 18, 971–988. [Google Scholar] [CrossRef]
- Kjalarsdóttir, L.; Dýrfjörd, A.; Dagbjartsson, A.; Laxdal, E.H.; Örlygsson, G.; Gíslason, J.; Einarsson, J.M.; Ng, C.-H.; Jónsson, H., Jr. Bone remodeling effect of a chitosan and calcium phosphate-based composite. Regen. Biomater. 2019, 6, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.M.; Al-Rashidy, Z.M.; Ghany, N.A.A.; Ahmed, H.Y.; Omar, A.E.; Farag, M.M. Bioactive and antibacterial metal implant composite coating based on Ce-doped nanobioactive glass and chitosan by electrophoretic deposition method. J. Mater. Res. 2021, 36, 1899–1913. [Google Scholar] [CrossRef]
- Vafa, E.; Bazargan-Lari, R.; Bahrololoom, M.E. Electrophoretic deposition of polyvinyl alcohol/natural chitosan/bioactive glass composite coatings on 316L stainless steel for biomedical application. Prog. Org. Coat. 2021, 151, 106059. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Synthesis and characterization of iron oxide-hydroxyapatite-chitosan composite coating and its biological assessment for biomedical applications. Prog. Org. Coat. 2021, 150, 106011. [Google Scholar] [CrossRef]
- Aqib, R.; Kiani, S.; Bano, S.; Wadood, A.; Rehman, M.A.U. Ag–Sr doped mesoporous bioactive glass nanoparticles loaded chitosan/gelatin coating for orthopedic implants. Int. J. Appl. Ceram. Technol. 2021, 18, 544–562. [Google Scholar] [CrossRef]
- Muşat, V.; Anghel, E.M.; Zaharia, A.; Atkinson, I.; Mocioiu, O.C.; Buşilă, M.; Alexandru, P. A Chitosan–Agarose Polysaccharide-Based Hydrogel for Biomimetic Remineralization of Dental Enamel. Biomolecules 2021, 11, 1137. [Google Scholar] [CrossRef]
- Yang, X.; Han, G.; Pang, X.; Fan, M. Chitosan/collagen scaffold containing bone morphogenetic protein-7 DNA supports dental pulp stem cell differentiation in vitro and in vivo. J. Biomed. Mater. Res. Part A 2020, 108, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Xu, W.; Xue, Y.; Chen, L.; Ye, H.; Zhong, E.; Ye, Z.; Gao, J.; Yan, Y. The use of chitosan/PLA nano-fibers by emulsion eletrospinning for periodontal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaram, M.N.; Pradeep, A.; Varma, P.K.; Jayakumar, R. Different Forms of Chitosan and Its Derivatives as Hemostatic Agent and Tissue Sealants. In Chitosan for Biomaterials IV.; Jayakumar, R., Prabaharan, M., Eds.; Advances in Polymer Science; Springer: Cham, Switzerland, 2021; Volume 288. [Google Scholar]

| Composite | The Fabrication Method | In-Vitro Biological Achievement | Application | Ref. |
|---|---|---|---|---|
| poly (lactic acid-co-trimethylene carbonate)/chitosan (PLA-TMC/Chitosan) | freeze-drying and solvent/nonsolvent sintering method | ALP activity assay and CCK8 cell proliferation assay showed that the scaffolds were conductive to cell adhesion and non-toxic. The scaffolds were potentially usable in bone repair and bone regeneration applications | 3D scaffolds | [93] |
| TiO2/Gel-CS (titanium dioxide/gelatin-chitosan) hydrogel | simple chemical approach | The measurements of cytotoxicity and cell attachment by (Live/Dead) and Actin/DAPI staining estimation of hydrogel on cells were preformed. Thus, good biocompatibility, biodegradability and thermal stability of the hydrogel materials have shown that the prepared hydrogel has a good potential for the bone tissue engineering and nursing care applications | orthopedic implants | [94] |
| collagen, chitosan, and copper-doped phosphate glass composite | co-deposited cathodically | Collagen CS coatings incorporating copper oxide-doped phosphate glass promise to allow the cells to permeate throughout the coatings, create coatings that resemble the extracellular matrix of native bone tissue, enhance the mineralization rate of natural hydroxyapatite, and increase the anti-bacterial properties of the coating. Therefore it will improve surgery procedures and ultimately the quality of life, of patients requiring orthopaedic implants. | orthopedic implants | [95] |
| AgNP-loaded chitosan–silica class II hybrid | thin film coatings | Escherichia coli and Staphylococcus aureus cultures and their biofilm formation were inhibited by all hybrid coatings. Antibacterial effects increased significantly for AgNPs-loaded coatings and appeared to improve with CS content in biofilm assays. | Dental and orthopedic implants | [96] |
| ternary HAp/chitosan/GO | electrophoretic deposition method (EPD) | There was no Staphylococcus aureus and Escherichia coli bacteria growth in broth medium after 1 day and OD600 results at 1 day post inoculation for the 2 wt.% GO addition in coating. | Dental and orthopedic implants | [97] |
| Poly (lactic acid) (PLA) scaffold surface-modified with chitosan and HAp (PLA/CS/HAp composite) | 3D printing | In vitro cell seeding results indicated that bone cells could attach and proliferate at a higher rate on the surface of the CS/HAp modified composite scaffolds compared to the PLA. All the samples were non-toxic to cells and composite scaffolds having CS and HAp on the surface offer better substrate to the cells to adhere, proliferate and migrate. | scaffolds | [98] |
| chitosan/gelatin layer with silica-gentamicin nanoparticles | electrophoretic deposition, EPD | The good mechanical properties and adherence of the generated coatings on both substrates show the ability for forming a potentially superior bone-to-implant interface for enhancing prosthetic devices performance. | orthopedic implants | [99] |
| Chitosan/ZnO nanoparticle | deposition | CS/ZnO-coated Ti can be an appropriate material resisting E. coli biofilm formation and was compatible with MG-63 cells. Therefore, the coating can be used for orthopedic and dental implant applications. | Dental and orthopedic implants | [100] |
| Nanocomposite of chitosan-g-poly (acrylamide)/Zn (CPA-Zn) | microwave radiations | In-vitro studies indicated the multifunctional nanocarriers advantage and feasibility for remote-controlled drug release systems | Drug Delivery | [101] |
| Chitosan Composites | Fabrication Technique | Implant | In-Vitro/In-Vivo Achievement | Ref. |
|---|---|---|---|---|
| HAp-Chitosan Composite Coating | Electrodeposition | Ti6Al4V alloy | The in-vitro antibacterial and cell viability capabilities of the HNT-CS-MHA composite coating on Ti6Al4V were outstanding; thus, it will serve as an indispensable implant material for orthopedic applications because of its increased corrosion resistance and bioactivity. | [128] |
| Cellulose nanofiber-reinforced chitosan hydrogel composites | - | Ex vivo research using pig vertebral unit models indicated that implanting CNF-reinforced CHI hydrogels into AF disc lesions aids to disc biomechanics rehabilitation by reaching the functions of a healthy disc. | [129] | |
| Composite of chitosan-gelatin/silica (Si)-antibiotic (gentamicin, Ge) | Spray deposition Electrophoretic deposition. | Commercially pure titanium (cpTi grade 2) | Regarding antibacterial inhibition capabilities, antibacterial activity against both strains (S. aureus and E. coli) was established using chitosan/gelatin/Si-Ge nanoparticle coatings on titanium substrates, indicating a broad inhibition region surrounding the samples. Both the bare Ti and the coated samples failed to prevent bacterial growth effectively. The presence of silica-based glasses and amorphous silica-based coatings increased cell survival. | [130] |
| CNT-reinforced chitosan-based ceramic composite | A flexible chemical conversion approach | Pure magnesium | Both biphasic and triphasic composite coatings exhibit improved antibacterial activity when compared to the standard ampicillin. The presence of a greater zone of inhibition shows that CNT-reinforced chitosan-based composite coatings have the potential to limit bacterial growth significantly. | [131] |
| Hybrid ZnO/chitosan caoting | dip-coating | Surface-modified porous titanium | Cytocompatibility testing revealed that the chitosan/ZnO coating is more compatible with MG-63 cells than pure Ti. | [100] |
| A chitosan and calcium phosphate-based composite | - | - | At any time, no substantial new bone growth was found in the implants themselves. However, significant new bone growth was detected further away from the drill hole in the rat mandible. The findings indicate that chitosan polymers containing between 50% and 70% DDA boost the normal bone rebuilding mechanism. | [132] |
| Chitosan/Ce-doped nanobioactive glass (NBG) composite | Electrophoretic deposition | 316L stainless steel | When immersed in SBF, the coatings had no cytotoxic impact and formed apatite-like crystals. Additionally, gentamicin was released in a sustained manner by Fickian diffusion. Additionally, drugs/coatings containing NBG demonstrated a greater antibacterial impact than chitosan coatings. | [133] |
| Polyvinyl alcohol/chitosan/bioactive glass composite | Electrophoretic deposition | 316L stainless steel | The disintegration rate of the coats demonstrates that the composite coating with 20% PVA coating has the highest bioactivity and hydroxyapatite formation ability when compared to the 15% and 25% PVA coatings. Similarly, the adhesive test showed that the composite containing 20% PVA is more adhesive than the others. | [134] |
| Chitosan/gelatin/silica-gentamicin nanocomposite | Electrophoretic deposition | Stainless steel AISI 316L and commercially pure titanium (cp Ti grade 2) | The excellent adhesion and mechanical capabilities of the produced coatings on both substrates reveal their potential for generating a possibly superior bone-to-implant interface, hence improving the function of prosthetic devices. | [99] |
| Iron oxide-hydroxyapatite-chitosan composite | Electrophoretic deposition | AZ91 Mg alloy | The large increase in iron oxide particles inhibited the growth of microorganisms. The composite coatings also enhanced the apatite mineralization. The hemolysis ratio was less than 5%, indicating that the coatings were naturally compatible with blood. Hydroxyapatite-iron oxide-chitosan composite coatings have a wide range of potential applications in biomedical implant applications. | [135] |
| The composite of mesoporous bioactive glass nanoparticle (Ag–Sr MBGN) doped with Ag–Sr, and loaded with chitosan/gelatin | Electrophoretic deposition | 316L stainless steel | After immersion in SBF, C/G/Ag–Sr MBGN coatings produced a thick HA crystal. Additionally, the coatings demonstrated antimicrobial activity against gram-negative bacteria. The inclusion of Sr to MBGNs decreased Ag’s toxicity. | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifianjazi, F.; Khaksar, S.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Salahshour, P.; Sadeghi, F.; Rostamnia, S.; Vahdat, S.M. Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review. Biomolecules 2022, 12, 155. https://doi.org/10.3390/biom12020155
Sharifianjazi F, Khaksar S, Esmaeilkhanian A, Bazli L, Eskandarinezhad S, Salahshour P, Sadeghi F, Rostamnia S, Vahdat SM. Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review. Biomolecules. 2022; 12(2):155. https://doi.org/10.3390/biom12020155
Chicago/Turabian StyleSharifianjazi, Fariborz, Samad Khaksar, Amirhossein Esmaeilkhanian, Leila Bazli, Sara Eskandarinezhad, Peyman Salahshour, Farnaz Sadeghi, Sadegh Rostamnia, and Seyed Mohammad Vahdat. 2022. "Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review" Biomolecules 12, no. 2: 155. https://doi.org/10.3390/biom12020155
APA StyleSharifianjazi, F., Khaksar, S., Esmaeilkhanian, A., Bazli, L., Eskandarinezhad, S., Salahshour, P., Sadeghi, F., Rostamnia, S., & Vahdat, S. M. (2022). Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review. Biomolecules, 12(2), 155. https://doi.org/10.3390/biom12020155







