Novel Genetic Prognostic Signature for Lung Adenocarcinoma Identified by Differences in Gene Expression Profiles of Low- and High-Grade Histological Subtypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Populations
2.2. Histopathological Analysis
2.3. Total RNA Sequencing Library Construction
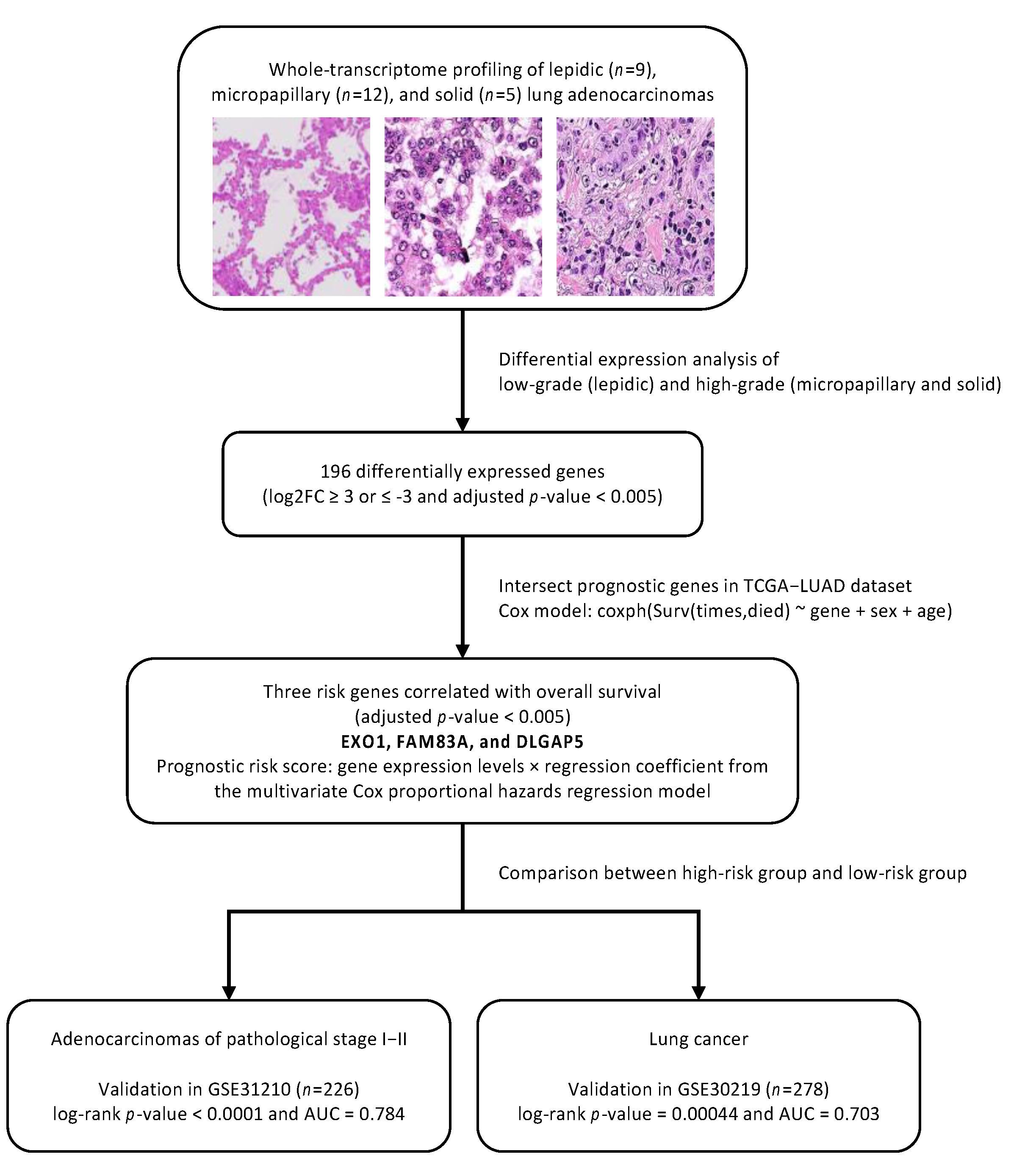
2.4. Differential Gene Expression Analysis
2.5. Construction and Validation of Prognostic Genes
3. Results
3.1. Patient Clinicopathological Characteristics
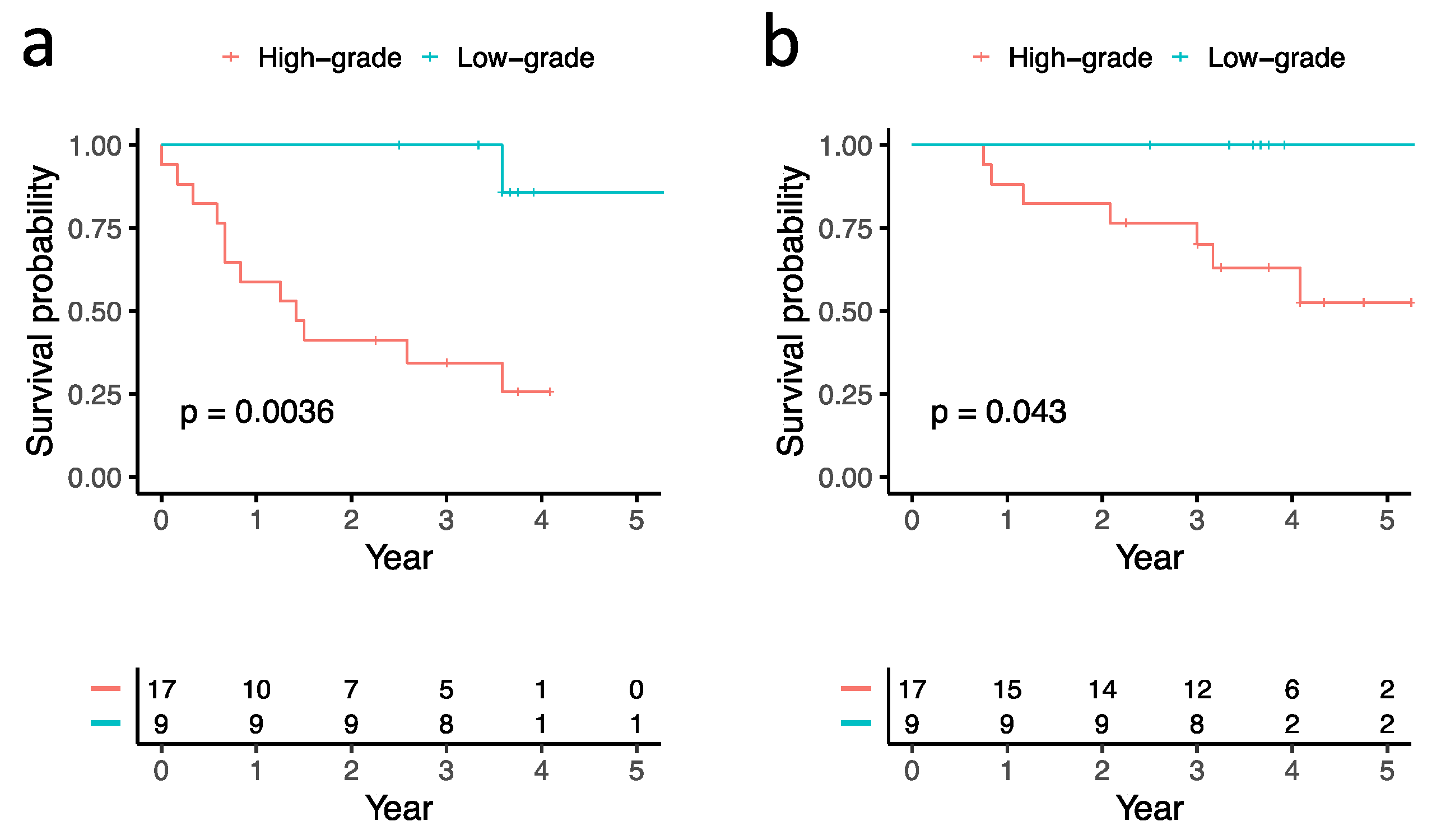
3.2. Correlations between Histological Subtypes and Clinical Outcomes
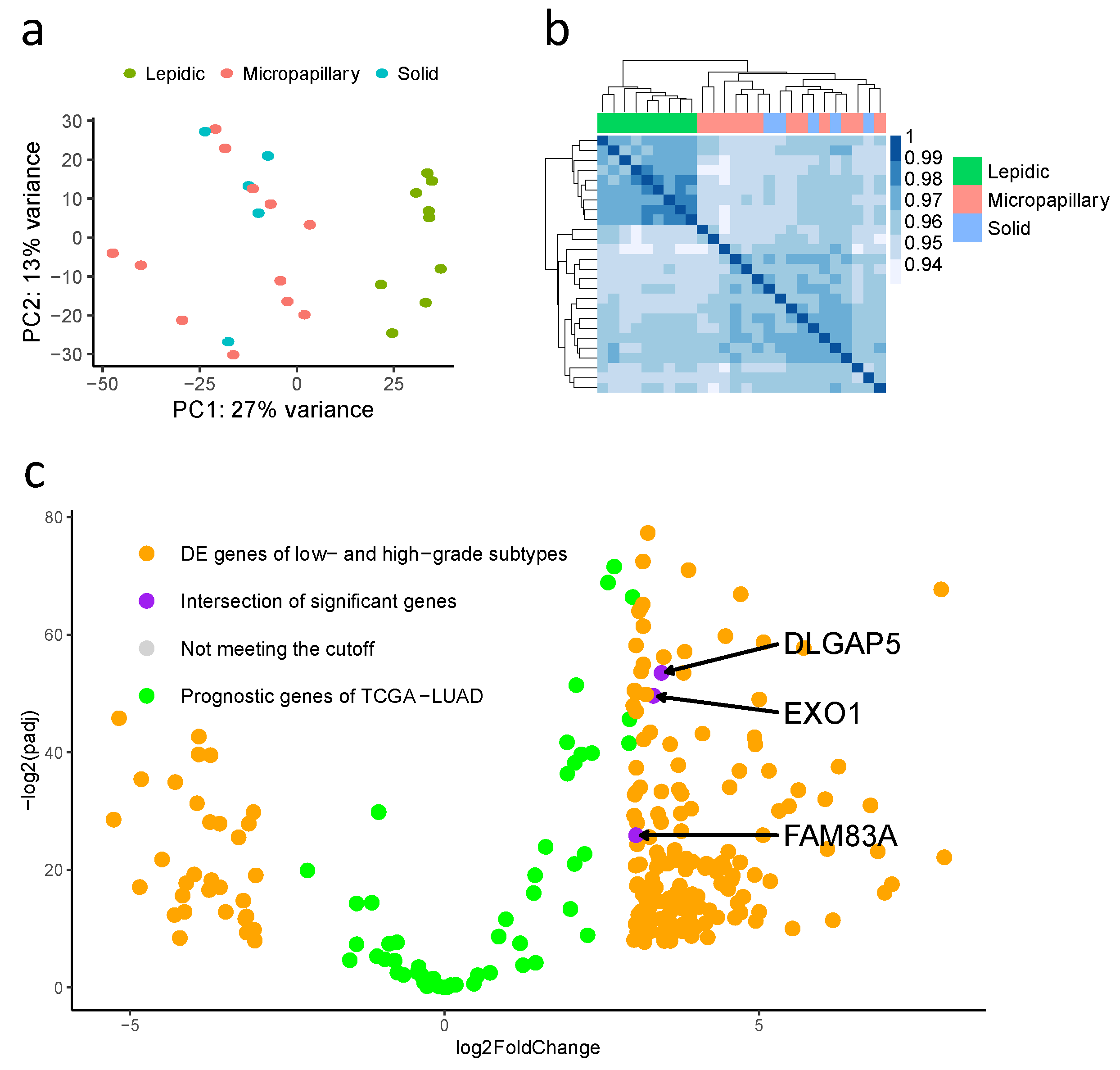
3.3. Differentially Expressed Genes in Low-Grade (Lepidic-Predominant) and High-Grade (Solid or Micropapillary-Predominant) Adenocarcinomas
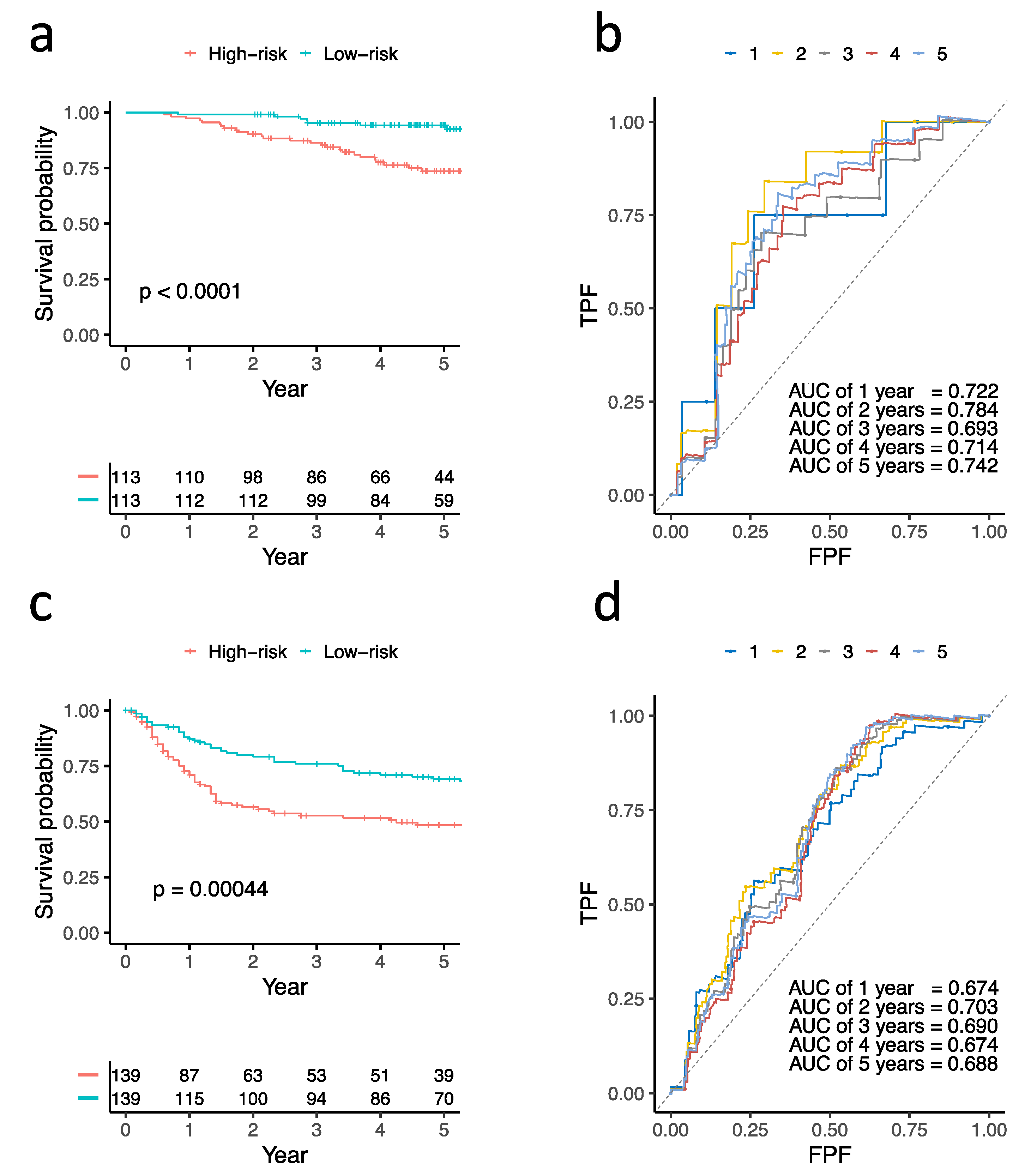
3.4. Construction and Validation of Three Prognostic Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.; Riely, G.J.; Van Schil, P.E.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, A.L.; Ocampo, P.S.; Xia, Y.; Zhong, H.; Russell, P.A.; Minami, Y.; Cooper, W.A.; Yoshida, A.; Bubendorf, L.; Papotti, M.; et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1599–1610. [Google Scholar] [CrossRef]
- Russell, P.A.; Wainer, Z.; Wright, G.M.; Daniels, M.; Conron, M.; Williams, R.A. Does Lung Adenocarcinoma Subtype Predict Patient Survival? A Clinicopathologic Study Based on the New International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Lung Adenocarcinoma Classification. J. Thorac. Oncol. 2011, 6, 1496–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, J.-J.; Jeng, W.-J.; Chou, T.-Y.; Hsu, W.-H.; Wu, K.-J.; Huang, B.-S.; Wu, Y.-C. Prognostic Value of the New International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Lung Adenocarcinoma Classification on Death and Recurrence in Completely Resected Stage I Lung Adenocarcinoma. Ann. Surg. 2013, 258, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Nitadori, J.; Bograd, A.J.; Kadota, K.; Sima, C.S.; Rizk, N.P.; Morales, E.A.; Rusch, V.W.; Travis, W.D.; Adusumilli, P.S. Impact of Micropapillary Histologic Subtype in Selecting Limited Resection vs Lobectomy for Lung Adenocarcinoma of 2cm or Smaller. J. Natl. Cancer Inst. 2013, 105, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Okada, M. Subtyping lung adenocarcinoma according to the novel 2011 IASLC/ATS/ERS classification: Correlation with patient prognosis. Thorac. Surg. Clin. 2013, 23, 179–186. [Google Scholar] [CrossRef]
- Thunnissen, E.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Chirieac, L.R.; Dacic, S.; Flieder, D.; Gazdar, A.; Geisinger, K.; Hasleton, P.; et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod. Pathol. 2012, 25, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chung, J.-H.; Jheon, S.; Sung, S.W.; Lee, C.-T.; Lee, J.H.; Choe, G. The Accuracy of Frozen Section Diagnosis of Pulmonary Nodules: Evaluation of Inflation Method during Intraoperative Pathology Consultation with Cryosection. J. Thorac. Oncol. 2010, 5, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Warth, A.; Stenzinger, A.; von Brünneck, A.-C.; Goeppert, B.; Cortis, J.; Petersen, I.; Hoffmann, H.; Schnabel, P.A.; Weichert, W. Interobserver variability in the application of the novel IASLC/ATS/ERS classification for pulmonary adenocarcinomas. Eur. Respir. J. 2012, 40, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-M.; Chen, L.-W.; Wang, H.-J.; Chen, L.-R.; Lor, K.-L.; Chen, Y.-C.; Lin, M.-W.; Hsieh, M.-S.; Chen, J.-S.; Chang, Y.-C.; et al. Extraction of radiomic values from lung adenocarcinoma with near-pure subtypes in the International Association for the Study of Lung Cancer/the American Thoracic Society/the European Respiratory Society (IASLC/ATS/ERS) classification. Lung Cancer 2018, 119, 56–63. [Google Scholar] [CrossRef]
- Cooper, W.A.; Bubendorf, L.; Kadota, K.; Landanyi, M.; MacMahon, H.; Matsubara, D.; Yoshizawa, A. Invasive non-mucinous adenocarcinoma of the lung. In WHO Classification of Tumours: Thoracic Tumours/WHO Classification of Tumours Editorial Board; IARC: Lyon, France, 2021; pp. 64–74. [Google Scholar]
- Hu, S.-Y.; Hsieh, M.-S.; Hsu, H.-H.; Tsai, T.-M.; Chiang, X.-H.; Tsou, K.-C.; Liao, H.-C.; Lin, M.-W.; Chen, J.-S. Correlation of tumor spread through air spaces and clinicopathological characteristics in surgically resected lung adenocarcinomas. Lung Cancer 2018, 126, 189–193. [Google Scholar] [CrossRef]
- Didion, J.P.; Martin, M.; Collins, F.S. Atropos: Specific, sensitive, and speedy trimming of sequencing reads. PeerJ 2017, 5, e3720. [Google Scholar] [CrossRef] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Picard Toolkit. Broad Institute, GitHub Repository. 2019. Available online: https://broadinstitute.github.io/picard/ (accessed on 19 April 2019).
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Res. 2012, 22, 1760–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, R.L.; Heath, A.P.; Ferretti, V.; Varmus, H.E.; Lowy, D.R.; Kibbe, W.A.; Staudt, L.M. Toward a Shared Vision for Cancer Genomic Data. N. Engl. J. Med. 2016, 375, 1109–1112. [Google Scholar] [CrossRef]
- Anaya, J. OncoLnc: Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput. Sci. 2016, 2, e67. [Google Scholar] [CrossRef] [Green Version]
- Okayama, H.; Kohno, T.; Ishii, Y.; Shimada, Y.; Shiraishi, K.; Iwakawa, R.; Furuta, K.; Tsuta, K.; Shibata, T.; Yamamoto, S.; et al. Identification of Genes Upregulated in ALK-Positive and EGFR/KRAS/ALK-Negative Lung Adenocarcinomas. Cancer Res. 2012, 72, 100–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseaux, S.; Debernardi, A.; Jacquiau, B.; Vitte, A.-L.; Vesin, A.; Nagy-Mignotte, H.; Moro-Sibilot, D.; Brichon, P.-Y.; Lantuejoul, S.; Hainaut, P.; et al. Ectopic Activation of Germline and Placental Genes Identifies Aggressive Metastasis-Prone Lung Cancers. Sci. Transl. Med. 2013, 5, 186ra66. [Google Scholar] [CrossRef] [Green Version]
- Kratz, J.R.; He, J.; Eeden, S.K.V.D.; Zhu, Z.-H.; Gao, W.; Pham, P.T.; Mulvihill, M.; Ziaei, F.; Zhang, H.; Su, B.; et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: Development and international validation studies. Lancet 2012, 379, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Woodard, G.A.; Gubens, M.A.; Jahan, T.M.; Jones, K.D.; Kukreja, J.; Theodore, P.R.; Cardozo, S.; Jew, G.; Clary-Macy, C.; Jablons, D.M.; et al. Prognostic Molecular Assay Might Improve Identification of Patients At Risk for Recurrence in Early-Stage Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2014, 15, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Woodard, G.A.; Wang, S.X.; Kratz, J.R.; Zoon-Besselink, C.T.; Chiang, C.-Y.; Gubens, M.A.; Jahan, T.M.; Blakely, C.M.; Jones, K.D.; Mann, M.J.; et al. Adjuvant Chemotherapy Guided by Molecular Profiling and Improved Outcomes in Early Stage, Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Der, S.D.; Sykes, J.; Pintilie, M.; Zhu, C.; Strumpf, D.; Liu, N.; Jurisica, I.; Shepherd, F.A.; Tsao, M. Validation of a Histology-Independent Prognostic Gene Signature for Early-Stage, Non–Small-Cell Lung Cancer Including Stage IA Patients. J. Thorac. Oncol. 2014, 9, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.-S.; Feng, M.-T.; Chen, X.; Gao, Y.; Chen, L.; Li, L.-D.; Li, D.-H.; Cao, Y.-Q. Exonuclease 1 (EXO1) is a Potential Prognostic Biomarker and Correlates with Immune Infiltrates in Lung Adenocarcinoma. OncoTargets Ther. 2021, 14, 1033–1048. [Google Scholar] [CrossRef]
- Keijzers, G.; Liu, D.; Rasmussen, L.J. Exonuclease 1 and its versatile roles in DNA repair. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 440–451. [Google Scholar] [CrossRef]
- Keijzers, G.; Bakula, D.; Petr, M.A.; Madsen, N.G.K.; Teklu, A.; Mkrtchyan, G.; Osborne, B.; Scheibye-Knudsen, M. Human Exonuclease 1 (EXO1) Regulatory Functions in DNA Replication with Putative Roles in Cancer. Int. J. Mol. Sci. 2018, 20, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goellner, E.M.; Putnam, C.D.; Graham, W.J.; Rahal, C.M.; Li, B.-Z.; Kolodner, R.D. Identification of Exo1-Msh2 interaction motifs in DNA mismatch repair and new Msh2-binding partners. Nat. Struct. Mol. Biol. 2018, 25, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Schmutte, C.; Marinescu, R.C.; Sadoff, M.M.; Guerrette, S.; Overhauser, J.; Fishel, R. Human exonuclease I interacts with the mismatch repair protein hMSH2. Cancer Res. 1998, 58, 4537–4542. [Google Scholar]
- Tsubouchi, H.; Ogawa, H. Exo1 Roles for Repair of DNA Double-Strand Breaks and Meiotic Crossing Over inSaccharomyces cerevisiae. Mol. Biol. Cell 2000, 11, 2221–2233. [Google Scholar] [CrossRef] [Green Version]
- Hsu, N.-Y.; Wang, H.-C.; Wang, C.-H.; Chiu, C.-F.; Tseng, H.-C.; Liang, S.-Y.; Tsai, C.-W.; Lin, C.-C.; Bau, D.-T. Lung cancer susceptibility and genetic polymorphisms of Exo1 gene in Taiwan. Anticancer Res. 2009, 29, 725–730. [Google Scholar] [PubMed]
- Jin, G.; Wang, H.; Hu, Z.; Liu, H.; Sun, W.; Ma, H.; Chen, D.; Miao, R.; Tian, T.; Jin, L.; et al. Potentially functional polymorphisms of EXO1 and risk of lung cancer in a Chinese population: A case-control analysis. Lung Cancer 2008, 60, 340–346. [Google Scholar] [CrossRef]
- Luo, F.; Wang, Y.; Lin, D.; Li, J.; Yang, K. Exonuclease 1 expression is associated with clinical progression, metastasis, and survival prognosis of prostate cancer. J. Cell. Biochem. 2019, 120, 11383–11389. [Google Scholar] [CrossRef]
- Qi, L.; Zhou, B.; Chen, J.; Hu, W.; Bai, R.; Ye, C.; Weng, X.; Zheng, S. Significant prognostic values of differentially expressed-aberrantly methylated hub genes in breast cancer. J. Cancer 2019, 10, 6618–6634. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-W.; Li, Z.-H.; Lei, L.; Liu, C.-C.; Wang, Z.; Fei, L.-R.; Yang, M.-Q.; Huang, W.-J.; Xu, H.-T. FAM83A Promotes Lung Cancer Progression by Regulating the Wnt and Hippo Signaling Pathways and Indicates Poor Prognosis. Front. Oncol. 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Dong, X.; Yin, Y.; Su, Y.; Xu, Q.; Zhang, Y.; Pang, X.; Zhang, Y.; Chen, W. BJ-TSA-9, a Novel Human Tumor-Specific Gene, Has Potential as a Biomarker of Lung Cancer. Neoplasia 2005, 7, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-Y.; Meier, R.; Furuta, S.; Lenburg, M.E.; Kenny, P.A.; Xu, R.; Bissell, M.J. FAM83A confers EGFR-TKI resistance in breast cancer cells and in mice. J. Clin. Investig. 2012, 122, 3211–3220. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Song, H.; Wang, Z.; Jiao, P.; Xu, J.; Li, X.; Du, H.; Wu, H.; Zhong, Y. FAM83A promotes proliferation and metastasis via Wnt/beta-catenin signaling in head neck squamous cell carcinoma. J. Transl. Med. 2021, 19, 423. [Google Scholar] [CrossRef]
- Lan, C.; Liu, C.-C.; Nie, X.-C.; Lei, L.; Xiao, Z.-X.; Li, M.-X.; Tang, X.-N.; Jia, M.-Y.; Xu, H.-T. FAM83A Promotes the Proliferative and Invasive Abilities of Cervical Cancer Cells via Epithelial-Mesenchymal Transition and the Wnt Signaling Pathway. J. Cancer 2021, 12, 6320–6329. [Google Scholar] [CrossRef]
- Richtmann, S.; Wilkens, D.; Warth, A.; Lasitschka, F.; Winter, H.; Christopoulos, P.; Herth, F.J.F.; Muley, T.; Meister, M.; Schneider, M.A. FAM83A and FAM83B as Prognostic Biomarkers and Potential New Therapeutic Targets in NSCLC. Cancers 2019, 11, 652. [Google Scholar] [CrossRef] [Green Version]
- Bassal, S.; Nomura, N.; Venter, D.; Brand, K.; McKay, M.J.; van der Spek, P.J. Characterization of a Novel Human Cell-Cycle-Regulated Homologue of Drosophila dlg1. Genomics 2001, 77, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A. “HURP on” we’re off to the kinetochore! J. Cell Biol. 2006, 173, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H.S.; Clarke, P.R. Cell Biology: Ran, Mitosis and the Cancer Connection. Curr. Biol. 2006, 16, R466–R468. [Google Scholar] [CrossRef] [Green Version]
- Koffa, M.D.; Casanova, C.M.; Santarella, R.; Koecher, T.; Wilm, M.; Mattaj, I. HURP Is Part of a Ran-Dependent Complex Involved in Spindle Formation. Curr. Biol. 2006, 16, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Tsou, A.-P.; Yang, C.-W.; Huang, C.-Y.F.; Yu, R.C.-T.; Lee, Y.-C.G.; Chang, C.-W.; Chen, B.-R.; Chung, Y.-F.; Fann, M.-J.; Chi, C.-W.; et al. Identification of a novel cell cycle regulated gene, HURP, overexpressed in human hepatocellular carcinoma. Oncogene 2003, 22, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Dou, X.; You, X.; Shi, Q.; Ke, M.; Liu, G. Pan-cancer analysis of the oncogenic role of discs large homolog associated protein 5 (DLGAP5) in human tumors. Cancer Cell Int. 2021, 21, 457. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-X.; Yin, J.-Y.; Shen, Y.; Zhang, W.; Zhou, H.-H.; Liu, Z. Genome-scale analysis identifies NEK2, DLGAP5 and ECT2 as promising diagnostic and prognostic biomarkers in human lung cancer. Sci. Rep. 2017, 7, 8072. [Google Scholar] [CrossRef] [Green Version]
| Variables | All (n = 26) | Low-Grade Subtype (n = 9) | High-Grade Subtype (n = 17) | p-Value |
|---|---|---|---|---|
| age, years; mean (range) | 64.7 (41–85) | 67.8 (55–85) | 62.9 (41–85) | <0.001 |
| sex (female), n (%) | 14 (53.8%) | 5 (55.6%) | 9 (52.9%) | 0.899 |
| smoker, n (%) | 7 (26.9%) | 2 (22.2%) | 5 (29.4%) | 0.694 |
| lung cancer family history, n (%) | 6 (23.1%) | 2 (22.2%) | 4 (23.5%) | 0.940 |
| abnormal serum CEA level a | 5 (19.2%) | 0 (0%) | 5 (29.4%) | 0.070 |
| visceral pleural invasion, n (%) | 6 (23.1%) | 1 (11.1%) | 5 (29.4%) | 0.669 |
| lymphovascular invasion, n (%) | 9 (34.6%) | 0 (0%) | 9 (52.9%) | 0.007 |
| differentiation | 0.001 | |||
| well/moderate | 13 (50%) | 8 (88.9%) | 5 (29.4%) | |
| poor | 12 (46.2%) | 0 (0%) | 12 (70.6%) | |
| STAS positive, n (%) | 11 (42.3%) | 0 (0%) | 11 (64.7%) | 0.001 |
| predominant subtype, n (%) | <0.001 | |||
| lepidic | 9 (34.6%) | 9 (100%) | 0 (0%) | |
| micropapillary | 12 (46.2%) | 0 (0%) | 12 (70.6%) | |
| solid | 5 (19.2%) | 0 (0%) | 5 (29.4%) | |
| tumor size (cm) | 2.6 ± 1.2 | 1.9 ± 0.6 | 3.0 ± 1.2 | <0.001 |
| pN stage b | 0.047 | |||
| N0 | 18 (69.2%) | 9 (100%) | 9 (52.9%) | |
| N1 | 2 (7.7%) | 0 (0%) | 2 (11.8%) | |
| N2 | 6 (23.1%) | 0 (0%) | 6 (35.3%) | |
| TNM stage b, n (%) | 0.030 | |||
| IA | 12 (46.1%) | 8 (88.9%) | 4 (23.5%) | |
| IB | 5 (19.2%) | 1 (11.1%) | 4 (23.5%) | |
| IIA | 2 (7.7%) | 0 (0%) | 2 (11.8%) | |
| IIB | 1 (3.8%) | 0 (0%) | 1 (5.9%) | |
| IIIA | 6 (23.1%) | 0 (0%) | 6 (35.3%) | |
| surgical method | 0.056 | |||
| lobectomy | 18 (69.2%) | 4 (44.4%) | 14 (82.4%) | |
| segmentectomy | 3 (11.5%) | 1 (11.1%) | 2 (11.8%) | |
| wedge resection | 5 (19.2%) | 4 (44.4%) | 1 (5.9%) | |
| clinical outcomes | ||||
| follow-up period (months) | 44.2 (9–117) | 51.6 (30–117) | 40.9 (9–79) | |
| tumor recurrence | 13 (50.0%) | 1 (11.1%) | 12 (70.6%) | |
| 5-y DFS (%) | 85.7% | 25.7% | 0.004 | |
| death | 8 (30.8%) | 1 (11.1%) | 7 (41.2%) | |
| 5-y OS (%) | 100% | 52.6% | 0.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Hsieh, M.-S.; Lin, M.-W.; Lee, Y.-H.; Hsiao, Y.-J.; Su, K.-Y.; Su, T.-J.; Yu, S.-L.; Chen, J.-S. Novel Genetic Prognostic Signature for Lung Adenocarcinoma Identified by Differences in Gene Expression Profiles of Low- and High-Grade Histological Subtypes. Biomolecules 2022, 12, 160. https://doi.org/10.3390/biom12020160
Chang C-C, Hsieh M-S, Lin M-W, Lee Y-H, Hsiao Y-J, Su K-Y, Su T-J, Yu S-L, Chen J-S. Novel Genetic Prognostic Signature for Lung Adenocarcinoma Identified by Differences in Gene Expression Profiles of Low- and High-Grade Histological Subtypes. Biomolecules. 2022; 12(2):160. https://doi.org/10.3390/biom12020160
Chicago/Turabian StyleChang, Chia-Ching, Min-Shu Hsieh, Mong-Wei Lin, Yi-Hsuan Lee, Yi-Jing Hsiao, Kang-Yi Su, Te-Jen Su, Sung-Liang Yu, and Jin-Shing Chen. 2022. "Novel Genetic Prognostic Signature for Lung Adenocarcinoma Identified by Differences in Gene Expression Profiles of Low- and High-Grade Histological Subtypes" Biomolecules 12, no. 2: 160. https://doi.org/10.3390/biom12020160
APA StyleChang, C.-C., Hsieh, M.-S., Lin, M.-W., Lee, Y.-H., Hsiao, Y.-J., Su, K.-Y., Su, T.-J., Yu, S.-L., & Chen, J.-S. (2022). Novel Genetic Prognostic Signature for Lung Adenocarcinoma Identified by Differences in Gene Expression Profiles of Low- and High-Grade Histological Subtypes. Biomolecules, 12(2), 160. https://doi.org/10.3390/biom12020160






