Bcl-2 Family Members and the Mitochondrial Import Machineries: The Roads to Death
Abstract
:1. Introduction
2. Protein Import across the Mitochondrial Outer Membrane
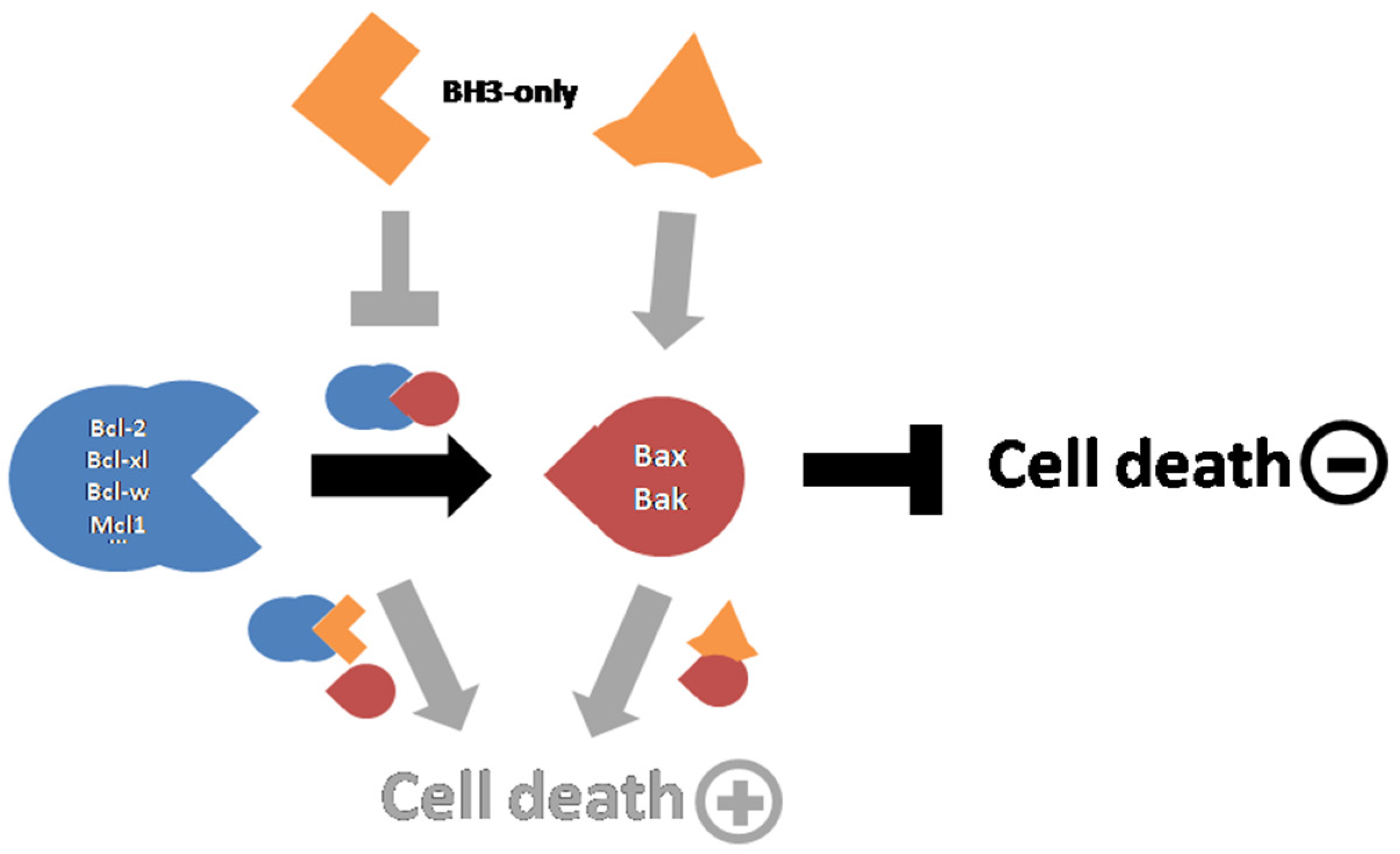
3. Apoptosis and Cell Death
4. Do Bcl-2 Family Proteins Actually Need Mitochondrial Receptors?
4.1. Bcl-2 Family Members vs. Bcl-2 Homologs
4.2. The Positive Charges in the C-Terminal End of Bcl-2 and Bcl-xL
4.3. Bax
4.4. Bak
4.5. Other Bcl-2 Homologs
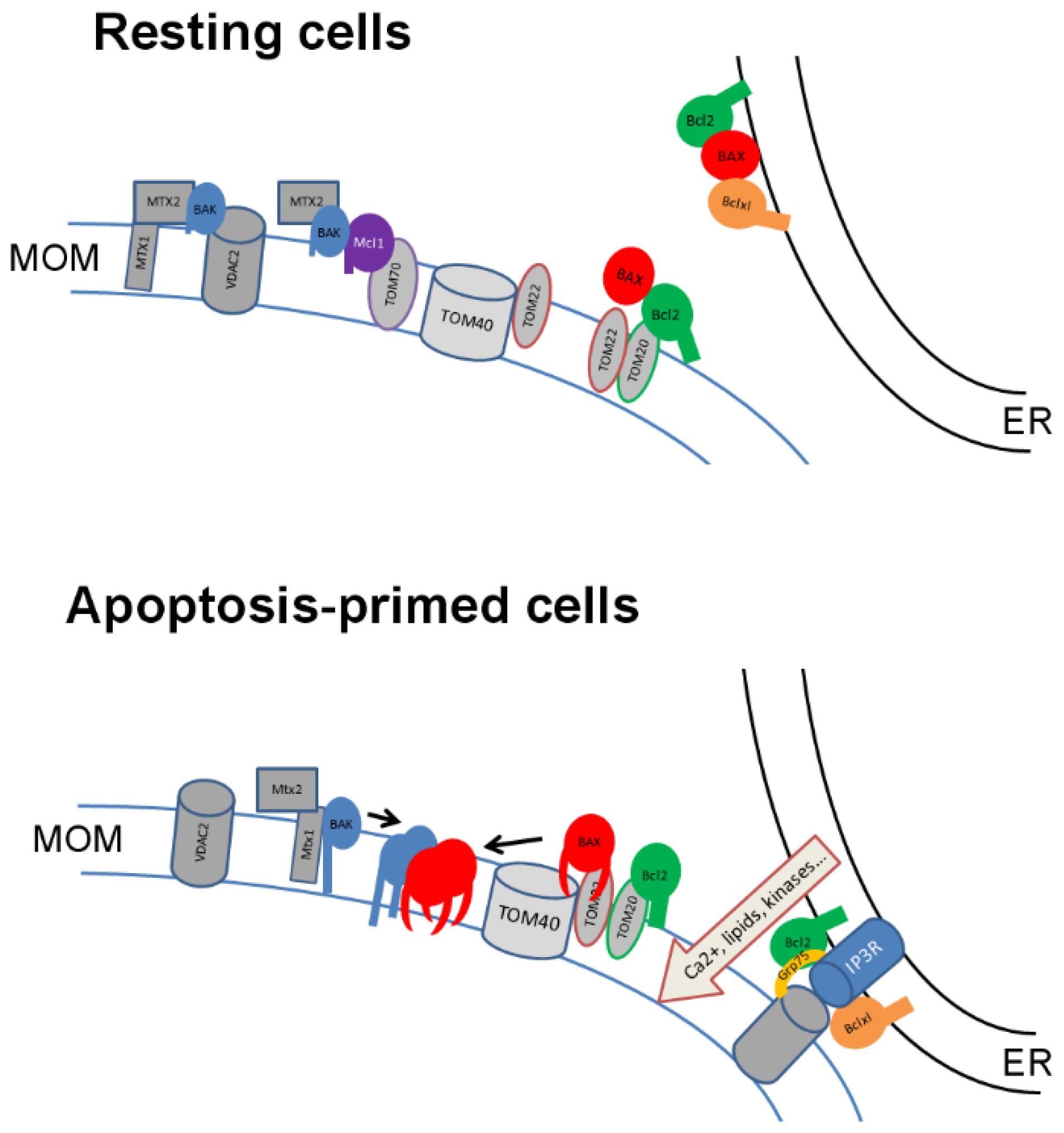
5. Bcl2 Family and Mitochondrial Import Proteins
6. Functional Consequences of Interactions between Import Proteins and Bcl-2 Family
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.W. Mitochondrial evolution. Cold Spring Harb. Perspect. Biol. 2012, 4, a011403. [Google Scholar] [CrossRef] [Green Version]
- Kurland, C.G.; Andersson, S.G. Origin and evolution of the mitochondrial proteome. Microbiol. Mol. Biol. Rev. 2000, 64, 786–820. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, R.; Huynen, M.A. Mosaic origin of the mitochondrial proteome. Proteomics 2010, 10, 4012–4024. [Google Scholar] [CrossRef]
- Zmasek, C.M.; Zhang, Q.; Ye, Y.; Godzik, A. Surprising complexity of the ancestral apoptosis network. Genome Biol. 2007, 8, R226. [Google Scholar] [CrossRef] [Green Version]
- Popgeorgiev, N.; Sa, J.D.; Jabbour, L.; Banjara, S.; Nguyen, T.T.M.; Akhavan-E-Sabet, A.; Gadet, R.; Ralchev, N.; Manon, S.; Hinds, M.G.; et al. Ancient and conserved functional interplay between Bcl-2 family proteins in the mitochondrial pathway of apoptosis. Sci. Adv. 2020, 30, eabc4149. [Google Scholar] [CrossRef] [PubMed]
- Guérin, J.; Buchanan, S.K. Protein import and export across the bacterial outer membrane. Curr. Opin. Struct. Biol. 2021, 69, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Ann. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Becker, T. Mechanisms and pathways of mitochondrial outer membrane protein biogenesis. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148323. [Google Scholar] [CrossRef]
- Mazur, M.; Kmita, H.; Wojtkowska, M. The Diversity of the Mitochondrial Outer Membrane Protein Import Channels: Emerging Targets for Modulation. Molecules 2021, 26, 4087. [Google Scholar] [CrossRef]
- Petit, E.; Oliver, L.; Vallette, F.M. The mitochondrial outer membrane protein import machinery: A new player in apoptosis? Front. Biosci. 2009, 14, 3563–3570. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, L.C.; Saenz, A.J.; Bornstein, P. Metaxin 1 interacts with metaxin 2, a novel related protein associated with the mammalian mitochondrial outer membrane. J. Cell. Biochem. 1999, 74, 11–22. [Google Scholar] [CrossRef]
- Palmer, C.S.; Anderson, A.J.; Stojanovski, D. Mitochondrial protein import dysfunction: Mitochondrial disease, neurodegenerative disease and cancer. FEBS Lett. 2021, 595, 1107–1131. [Google Scholar] [CrossRef]
- Kang, B.H.; Xia, F.; Pop, R.; Dohi, T.; Socolovsky, M.; Altieri, D.C. Developmental control of apoptosis by the immunophilin aryl hydrocarbon receptor-interacting protein (AIP) involves mitochondrial import of the survivin protein. J. Biol. Chem. 2011, 286, 16758–16767. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Cui, Y.; Huang, Y.; Liu, H.; Li, L.; Li, M.; Ruan, K.C.; Zhou, Q.; Wang, C. Tom70 mediates Sendai virus-induced apoptosis on mitochondria. J. Virol. 2015, 89, 3804–3818. [Google Scholar] [CrossRef] [Green Version]
- Chiusolo, V.; Jacquemin, G.; Yonca Bassoy, E.; Vinet, L.; Liguori, L.; Walch, M.; Kozjak-Pavlovic, V.; Martinvalet, D. Granzyme B enters the mitochondria in a Sam50-, Tim22- and mtHsp70-dependent manner to induce apoptosis. Cell Death Differ. 2017, 24, 747–758. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Strasser, A.; Vaux, D.L. Cell Death in the Origin and Treatment of Cancer. Mol. Cell 2020, 18, 1045–1054. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Cossman, J.; Jaffe, E.; Croce, C.M. Involvement of the bcl-2 gene in human follicular lymphoma. Science 1985, 228, 1440–1443. [Google Scholar] [CrossRef]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, M.; Dubyak, G.; Chen, L.; Nuñez, G.; Miesfeld, R.L.; Distelhorst, C.W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc. Natl. Acad. Sci. USA 1994, 91, 6569–6573. [Google Scholar] [CrossRef] [Green Version]
- Susin, S.A.; Zamzami, N.; Castedo, M.; Hirsch, T.; Marchetti, P.; Macho, A.; Daugas, E.; Geuskens, M.; Kroemer, G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J. Exp. Med. 1996, 184, 1331–1341. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Imai, Y.; Nakayama, H.; Takahashi, K.; Takio, K.; Takahashi, R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 2001, 8, 613–621. [Google Scholar] [CrossRef]
- Li, L.Y.; Luo, X.; Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001, 412, 95–99. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, E.V.; Priault, M.; Pietkiewicz, D.; Cheng, E.H.; Antonsson, B.; Manon, S.; Korsmeyer, S.J.; Mannella, C.A.; Kinnally, K.W. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J. Cell Biol. 2001, 155, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Dejean, L.M.; Martinez-Caballero, S.; Guo, L.; Hughes, C.; Teijido, O.; Ducret, T.; Ichas, F.; Korsmeyer, S.J.; Antonsson, B.; Jonas, E.A.; et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol. Biol. Cell 2005, 16, 2424–2432. [Google Scholar] [CrossRef] [Green Version]
- Große, L.; Wurm, C.A.; Brüser, C.; Neumann, D.; Jans, D.C.; Jakobs, S. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016, 35, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Gallego, R.; Mund, M.; Cosentino, K.; Schneider, J.; Unsay, J.; Schraermeyer, U.; Engelhardt, J.; Ries, J.; García-Sáez, A.J. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 2016, 35, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giam, M.; Huang, D.C.; Bouillet, P. BH3-only proteins and their roles in programmed cell death. Oncogene 2008, 27, S128–S136. [Google Scholar] [CrossRef]
- Bonneau, B.; Prudent, J.; Popgeorgiev, N.; Gillet, G. Non-apoptotic roles of Bcl-2 family: The calcium connection. Biochim. Biophys. Acta 2013, 1833, 1755–1765. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.L.; Gillet, G.; Prudent, J.; Popgeorgiev, N. Bcl-2 Family of Proteins in the Control of Mitochondrial Calcium Signalling: An Old Chap with New Roles. Int. J. Mol. Sci. 2021, 22, 3730. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Ricci, J.E. How does metabolism affect cell death in cancer? FEBS J. 2016, 283, 2653–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiden, M.G.V.; Thompson, C.B. Bcl-2 proteins: Regulators of apoptosis or of mitochondrial homeostasis? Nat. Cell Biol. 1999, 1, E209–E216. [Google Scholar] [CrossRef]
- Gottlieb, E.; Vander Heiden, M.G.; Thompson, C.B. Bcl-x(L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol. Cell. Biol. 2000, 20, 5680–5689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquin, M.A.; Chiche, J.; Zunino, B.; Bénéteau, M.; Meynet, O.; Pradelli, L.A.; Marchetti, S.; Cornille, A.; Carles, M.; Ricci, J.E. GAPDH binds to active Akt, leading to Bcl-xL increase and escape from caspase-independent cell death. Cell Death Differ. 2013, 20, 1043–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, A.; Jockel, J.; Wei, M.C.; Korsmeyer, S.J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998, 17, 3878–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boise, L.H.; González-García, M.; Postema, C.E.; Ding, L.; Lindsten, T.; Turka, L.A.; Mao, X.; Nuñez, G.; Thompson, C.B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993, 74, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Kozopas, K.M.; Yang, T.; Buchan, H.L.; Zhou, P.; Craig, R.W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. USA 1993, 90, 3516–3520. [Google Scholar] [CrossRef] [Green Version]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Kiefer, M.C.; Brauer, M.J.; Powers, V.C.; Wu, J.J.; Umansky, S.R.; Tomei, L.D.; Barr, P.J. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature 1995, 374, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar] [PubMed]
- Hengartner, M.O.; Horvitz, H.R.C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 1994, 76, 665–676. [Google Scholar] [CrossRef]
- Wang, K.; Yin, X.M.; Chao, D.T.; Milliman, C.L.; Korsmeyer, S.J. BID: A novel BH3 domain-only death agonist. Genes Dev. 1996, 10, 2859–2869. [Google Scholar] [CrossRef] [Green Version]
- Chou, J.J.; Li, H.; Salvesen, G.S.; Yuan, J.; Wagner, G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 1999, 96, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Muchmore, S.W.; Sattler, M.; Liang, H.; Meadows, R.P.; Harlan, J.E.; Yoon, H.S.; Nettesheim, D.; Chang, B.S.; Thompson, C.B.; Wong, S.L.; et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 1996, 381, 335–341. [Google Scholar] [CrossRef]
- Suzuki, M.; Youle, R.J.; Tjandra, N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell 2000, 103, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Petros, A.M.; Medek, A.; Nettesheim, D.G.; Kim, D.H.; Yoon, H.S.; Swift, K.; Matayoshi, E.D.; Oltersdorf, T.; Fesik, S.W. Solution structure of the antiapoptotic protein bcl-2. Proc. Natl. Acad. Sci. USA 2001, 98, 3012–3017. [Google Scholar] [CrossRef] [Green Version]
- Willis, S.N.; Fletcher, J.I.; Kaufmann, T.; van Delft, M.F.; Chen, L.; Czabotar, P.E.; Ierino, H.; Lee, E.F.; Fairlie, W.D.; Bouillet, P.; et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 2007, 315, 856–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouacheria, A.; Rech de Laval, V.; Combet, C.; Hardwick, J.M. Evolution of Bcl-2 homology motifs: Homology versus homoplasy. Trends Cell Biol. 2013, 23, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Schweers, R.L.; Zhang, J.; Randall, M.S.; Loyd, M.R.; Li, W.; Dorsey, F.C.; Kundu, M.; Opferman, J.T.; Cleveland, J.L.; Miller, J.L.; et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19500–19505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, T.; Schlipf, S.; Sanz, J.; Neubert, K.; Stein, R.; Borner, C. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J. Cell Biol. 2003, 160, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Maity, A.; Sinha, S.; Dastidar, S.G. Dissecting the thermodynamic contributions of the charged residues in the membrane anchoring of Bcl-xl C-terminal domain. Chem. Phys. Lipids 2019, 218, 112–124. [Google Scholar] [CrossRef]
- Nechushtan, A.; Smith, C.L.; Hsu, Y.T.; Youle, R.J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999, 18, 2330–2341. [Google Scholar] [CrossRef] [Green Version]
- Birkinshaw, R.W.; Czabotar, P.E. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin. Cell Dev. Biol. 2017, 72, 152–162. [Google Scholar] [CrossRef]
- Tremblais, K.; Oliver, L.; Juin, P.; Le Cabellec, T.M.; Meflah, K.; Vallette, F.M. The C-terminus of bax is not a membrane addressing/anchoring signal. Biochem. Biophys. Res. Commun. 1999, 260, 582–591. [Google Scholar] [CrossRef]
- Gardai, S.J.; Hildeman, D.A.; Frankel, S.K.; Whitlock, B.B.; Frasch, S.C.; Borregaard, N.; Marrack, P.; Bratton, D.L.; Henson, P.M. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J. Biol. Chem. 2004, 279, 21085–21095. [Google Scholar] [CrossRef] [Green Version]
- Xin, M.; Gao, F.; May, W.S.; Flagg, T.; Deng, X. Protein kinase Czeta abrogates the proapoptotic function of Bax through phosphorylation. J. Biol. Chem. 2007, 282, 21268–21277. [Google Scholar] [CrossRef] [Green Version]
- Simonyan, L.; Renault, T.T.; Novais, M.J.; Sousa, M.J.; Côrte-Real, M.; Camougrand, N.; Gonzalez, C.; Manon, S. Regulation of Bax/mitochondria interaction by AKT. FEBS Lett. 2016, 590, 13–21. [Google Scholar] [CrossRef]
- Bellot, G.; Cartron, P.F.; Er, E.; Oliver, L.; Juin, P.; Armstrong, L.C.; Bornstein, P.; Mihara, K.; Manon, S.; Vallette, F.M. TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax. Cell Death Differ. 2007, 14, 785–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czabotar, P.E.; Westphal, D.; Dewson, G.; Ma, S.; Hockings, C.; Fairlie, W.D.; Lee, E.F.; Yao, S.; Robin, A.Y.; Smith, B.J.; et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 2013, 152, 519–531. [Google Scholar] [CrossRef] [Green Version]
- García-Sáez, A.J.; Mingarro, I.; Pérez-Payá, E.; Salgado, J. Membrane-insertion fragments of Bcl-xL, Bax, and Bid. Biochemistry 2004, 43, 10930–10943. [Google Scholar] [CrossRef] [PubMed]
- Cramer, W.A.; Heymann, J.B.; Schendel, S.L.; Deriy, B.N.; Cohen, F.S.; Elkins, P.A.; Stauffacher, C.V. Structure-function of the channel-forming colicins. Ann. Rev. Biophys. Biomol. Struct. 1995, 24, 611–641. [Google Scholar] [CrossRef]
- Heimlich, G.; McKinnon, A.D.; Bernardo, K.; Brdiczka, D.; Reed, J.C.; Kain, R.; Krönke, M.; Jürgensmeier, J.M. Bax-induced cytochrome c release from mitochondria depends on alpha-helices-5 and -6. Biochem. J. 2004, 378, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Cartron, P.F.; Bellot, G.; Oliver, L.; Grandier-Vazeille, X.; Manon, S.; Vallette, F.M. Bax inserts into the mitochondrial outer membrane by different mechanisms. FEBS Lett. 2008, 582, 3045–3051. [Google Scholar] [CrossRef] [Green Version]
- Oliver, L.; Priault, M.; Tremblais, K.; LeCabellec, M.; Meflah, K.; Manon, S.; Vallette, F.M. The substitution of the C-terminus of bax by that of bcl-xL does not affect its subcellular localization but abrogates its pro-apoptotic properties. FEBS Lett. 2000, 487, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Priault, M.; Cartron, P.F.; Camougrand, N.; Antonsson, B.; Vallette, F.M.; Manon, S. Investigation of the role of the C-terminus of Bax and of tc-Bid on Bax interaction with yeast mitochondria. Cell Death Differ. 2003, 10, 1068–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartron, P.F.; Arokium, H.; Oliver, L.; Meflah, K.; Manon, S.; Vallette, F.M. Distinct domains control the addressing and the insertion of Bax into mitochondria. J. Biol. Chem. 2005, 280, 10587–10598. [Google Scholar] [CrossRef] [Green Version]
- Arokium, H.; Camougrand, N.; Vallette, F.M.; Manon, S. Studies of the interaction of substituted mutants of BAX with yeast mitochondria reveal that the C-terminal hydrophobic alpha-helix is a second ART sequence and plays a role in the interaction with anti-apoptotic BCL-xL. J. Biol. Chem. 2004, 279, 52566–52573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schinzel, A.; Kaufmann, T.; Schuler, M.; Martinalbo, J.; Grubb, D.; Borner, C. Conformational control of Bax localization and apoptotic activity by Pro168. J. Cell Biol. 2004, 164, 1021–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, T.P.; Reyna, D.E.; Priyadarshi, A.; Chen, H.C.; Li, S.; Wu, Y.; Ganesan, Y.T.; Malashkevich, V.N.; Cheng, E.H.; Gavathiotis, E. An Autoinhibited Dimeric Form of BAX Regulates the BAX Activation Pathway. Mol. Cell 2016, 63, 485–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonyan, L.; Légiot, A.; Lascu, I.; Durand, G.; Giraud, M.F.; Gonzalez, C.; Manon, S. The substitution of Proline 168 favors Bax oligomerization and stimulates its interaction with LUVs and mitochondria. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1144–1155. [Google Scholar] [CrossRef]
- Shen, Z.J.; Esnault, S.; Schinzel, A.; Borner, C.; Malter, J.S. The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation. Nat. Immunol. 2009, 10, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Dewson, G.; Kratina, T.; Czabotar, P.; Day, C.L.; Adams, J.M.; Kluck, R.M. Bak activation for apoptosis involves oligomerization of dimers via their alpha6 helices. Mol. Cell 2009, 36, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Sandow, J.J.; Tan, I.K.; Huang, A.S.; Masaldan, S.; Bernardini, J.P.; Wardak, A.Z.; Birkinshaw, R.W.; Ninnis, R.L.; Liu, Z.; Dalseno, D.; et al. Dynamic reconfiguration of pro-apoptotic BAK on membranes. EMBO J. 2021, 40, e107237. [Google Scholar] [CrossRef]
- Cartron, P.F.; Oliver, L.; Martin, S.; Moreau, C.; LeCabellec, M.T.; Jezequel, P.; Meflah, K.; Vallette, F.M. The expression of a new variant of the pro-apoptotic molecule Bax, Baxpsi, is correlated with an increased survival of glioblastoma multiforme patients. Hum. Mol. Genet. 2002, 11, 675–687. [Google Scholar] [CrossRef] [Green Version]
- Cartron, P.F.; Priault, M.; Oliver, L.; Meflah, K.; Manon, S.; Vallette, F.M. The N-terminal end of Bax contains a mitochondrial-targeting signal. J. Biol. Chem. 2003, 278, 11633–11641. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Kozopas, K.M.; Craig, R.W. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J. Cell Biol. 1995, 128, 1173–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akgul, C.; Moulding, D.A.; White, M.R.; Edwards, S.W. In vivo localisation and stability of human Mcl-1 using green fluorescent protein (GFP) fusion proteins. FEBS Lett. 2000, 478, 72–76. [Google Scholar] [CrossRef]
- Germain, M.; Duronio, V. The N terminus of the anti-apoptotic BCL-2 homologue MCL-1 regulates its localization and function. J. Biol. Chem. 2007, 282, 32233–32242. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, T.; Holler, N.; Micheau, O.; Martinon, F.; Tinel, A.; Hofmann, K.; Tschopp, J. Bcl-rambo, a novel Bcl-2 homologue that induces apoptosis via its unique C-terminal extension. J. Biol. Chem. 2001, 276, 19548–19554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazawa, M.; Matsubara, H.; Matsushita, Y.; Watanabe, M.; Vo, N.; Yoshida, H.; Yamaguchi, M.; Kataoka, T. The Human Bcl-2 Family Member Bcl-rambo Localizes to Mitochondria and Induces Apoptosis and Morphological Aberrations in Drosophila. PLoS ONE 2016, 11, e0157823. [Google Scholar]
- Fujiwara, M.; Tian, L.; Le, P.T.; DeMambro, V.E.; Becker, K.A.; Rosen, C.J.; Guntur, A.R. The mitophagy receptor Bcl-2-like protein 13 stimulates adipogenesis by regulating mitochondrial oxidative phosphorylation and apoptosis in mice. J. Biol. Chem. 2019, 294, 12683–12694. [Google Scholar] [CrossRef]
- Murakawa, T.; Yamaguchi, O.; Hashimoto, A.; Hikoso, S.; Takeda, T.; Oka, T.; Yasui, H.; Ueda, H.; Akazawa, Y.; Nakayama, H.; et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 2015, 6, 7527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brien, G.; Debaud, A.L.; Robert, X.; Oliver, L.; Trescol-Biemont, M.C.; Cauquil, N.; Geneste, O.; Aghajari, N.; Vallette, F.M.; Haser, R.; et al. C-terminal residues regulate localization and function of the antiapoptotic protein Bfl-1. J. Biol. Chem. 2009, 284, 30257–30263. [Google Scholar] [CrossRef] [Green Version]
- Echeverry, N.; Bachmann, D.; Ke, F.; Strasser, A.; Simon, H.U.; Kaufmann, T. Intracellular localization of the BCL-2 family member BOK and functional implications. Cell Death Differ. 2013, 20, 785–799. [Google Scholar] [CrossRef] [Green Version]
- Motz, C.; Martin, H.; Krimmer, T.; Rassow, J. Bcl-2 and porin follow different pathways of TOM-dependent insertion into the mitochondrial outer membrane. J. Mol. Biol. 2002, 323, 729–738. [Google Scholar] [CrossRef]
- Lalier, L.; Mignard, V.; Joalland, M.P.; Lanoé, D.; Cartron, P.F.; Manon, S.; Vallette, F.M. TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis. Cell Death Dis. 2021, 12, 182. [Google Scholar] [CrossRef]
- Chou, C.H.; Lee, R.S.; Yang-Yen, H.F. An internal EELD domain facilitates mitochondrial targeting of Mcl-1 via a Tom70-dependent pathway. Mol. Biol Cell 2006, 17, 3952–3963. [Google Scholar] [CrossRef] [Green Version]
- Cartron, P.F.; Petit, E.; Bellot, G.; Oliver, L.; Vallette, F.M. Metaxins 1 and 2, two proteins of the mitochondrial protein sorting and assembly machinery, are essential for Bak activation during TNF alpha triggered apoptosis. Cell. Signal. 2014, 26, 1928–1934. [Google Scholar] [CrossRef]
- Petit, E.; Cartron, P.F.; Oliver, L.; Vallette, F.M. The phosphorylation of Metaxin 1 controls Bak activation during TNFα induced cell death. Cell. Signal. 2017, 30, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, H.S.; Li, M.X.; Tan, I.K.L.; Ninnis, R.L.; Reljic, B.; Scicluna, K.; Dagley, L.F.; Sandow, J.J.; Kelly, G.L.; Samson, A.L.; et al. VDAC2 enables BAX to mediate apoptosis and limit tumor development. Nat. Commun. 2018, 26, 4976. [Google Scholar] [CrossRef] [Green Version]
- Colin, J.; Garibal, J.; Mignotte, B.; Guénal, I. The mitochondrial TOM complex modulates bax-induced apoptosis in Drosophila. Biochem. Biophys. Res. Commun. 2009, 379, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Norberg, E.; Walter, K.M.; Schreiner, P.; Kemper, C.; Rapaport, D.; Zhivotovsky, B.; Orrenius, S. The mitochondrial TOM complex is required for tBid/Bax-induced cytochrome c release. J. Biol. Chem. 2007, 282, 27633–27639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renault, T.T.; Grandier-Vazeille, X.; Arokium, H.; Velours, G.; Camougrand, N.; Priault, M.; Teijido, O.; Dejean, L.M.; Manon, S. The cytosolic domain of human Tom22 modulates human Bax mitochondrial translocation and conformation in yeast. FEBS Lett. 2012, 586, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Szklarz, L.K.S.; Kozjak-Pavlovic, V.; Vögtle, F.N.; Chacinska, A.; Milenkovic, D.; Vogel, S.; Dürr, M.; Westermann, B.; Guiard, B.; Martinou, J.C.; et al. Preprotein transport machineries of yeast mitochondrial outer membrane are not required for Bax-induced release of intermembrane space proteins. J. Mol. Biol. 2007, 368, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Ross, K.; Rudel, T.; Kozjak-Pavlovic, V. TOM-independent complex formation of Bax and Bak in mammalian mitochondria during TNFalpha-induced apoptosis. Cell Death Differ. 2009, 16, 697–707. [Google Scholar] [CrossRef]
- Kim, M.; Jung, S.O.; Park, K.; Jeong, E.J.; Joung, H.A.; Kim, T.H.; Seol, D.W.; Chung, B.H. Detection of Bax protein conformational change using a surface plasmon resonance imaging-based antibody chip. Biochem. Biophys. Res. Commun. 2005, 338, 1834–1838. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Youle, R.J. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 1997, 272, 13829–13834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyerl, F.W.; Dai, S.; Murphy, G.A.; Crawford, F.; White, J.; Marrack, P.; Kappler, J.W. Elucidation of some Bax conformational changes through crystallization of an antibody-peptide complex. Cell Death Differ. 2007, 14, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Lalier, L.; Cartron, P.F.; Juin, P.; Nedelkina, S.; Manon, S.; Bechinger, B.; Vallette, F.M. Bax activation and mitochondrial insertion during apoptosis. Apoptosis 2007, 12, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Cheng, E.H.; Sheiko, T.V.; Fisher, J.K.; Craigen, W.J.; Korsmeyer, S.J. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 2003, 301, 513–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hockings, C.; Alsop, A.E.; Fennel, S.C.; Lee, E.F.; Fairlie, W.D.; Dewson, G.; Kluck, R.M. Mcl-1 and Bcl-xL sequestration of Bak confers differential resistance to BH3-only proteins. Cell Death Differ. 2018, 25, 721–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaltsman, Y.; Shachnai, L.; Yivgi-Ohana, N.; Schwarz, M.; Maryanovich, M.; Houtkooper, R.H.; Vaz, F.M.; De Leonardis, F.; Fiermonte, G.; Palmieri, F.; et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat. Cell Biol. 2010, 12, 553–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raemy, E.; Montessuit, S.; Pierredon, S.; van Kampen, A.H.; Vaz, F.M.; Martinou, J.C. Cardiolipin or MTCH2 can serve as tBID receptors during apoptosis. Cell Death Differ. 2016, 23, 1165–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwana, T.; Mackey, M.R.; Perkins, G.; Ellisman, M.H.; Latterich, M.; Schneiter, R.; Green, D.R.; Newmeyer, D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 2002, 111, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Lutter, M.; Fang, M.; Luo, X.; Nishijima, M.; Xie, X.; Wang, X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000, 2, 754–761. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Pariselli, F.; Dupaigne, P.; Budihardjo, I.; Lutter, M.; Antonsson, B.; Diolez, P.; Manon, S.; Martinou, J.C.; Goubern, M.; et al. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 2005, 12, 614–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipuk, J.E.; McStay, G.P.; Bharti, A.; Kuwana, T.; Clarke, C.J.; Siskind, L.J.; Obeid, L.M.; Green, D.R. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 2012, 148, 988–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalier, L.; Cartron, P.F.; Olivier, C.; Logé, C.; Bougras, G.; Robert, J.M.; Oliver, L.; Vallette, F.M. Prostaglandins antagonistically control Bax activation during apoptosis. Cell Death Differ. 2011, 18, 528–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christenson, E.; Merlin, S.; Saito, M.; Schlesinger, P. Cholesterol effects on BAX pore activation. J. Mol. Biol. 2008, 381, 1168–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, M.N.; Lin, S.H.; Peterson, Y.K.; Bielawska, A.; Szulc, Z.M.; Bittman, R.; Colombini, M. Bax and Bcl-xL exert their regulation on different sites of the ceramide channel. Biochem. J. 2012, 445, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, D.O.; Dengjel, J.; Wilfling, F.; Kozjak-Pavlovic, V.; Häcker, G.; Weber, A. The pro-apoptotic BH3-only protein Bim interacts with components of the translocase of the outer mitochondrial membrane (TOM). PLoS ONE 2015, 10, e0123341. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.; Harper, N.; Schwabe, J.; Cohen, G.M. BIM-mediated membrane insertion of the BAK pore domain is an essential requirement for apoptosis. Cell Rep. 2013, 5, 409–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwana, T.; King, L.E.; Cosentino, K.; Suess, J.; Garcia-Saez, A.J.; Gilmore, A.P.; Newmeyer, D.D. Mitochondrial residence of the apoptosis inducer BAX is more important than BAX oligomerization in promoting membrane permeabilization. J. Biol. Chem. 2020, 295, 1623–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautier, F.; Guillemin, Y.; Cartron, P.F.; Gallenne, T.; Cauquil, N.; Le Diguarher, T.; Casara, P.; Vallette, F.M.; Manon, S.; Hickman, J.A.; et al. Bax activation by engagement with, then release from, the BH3 binding site of Bcl-xL. Mol. Cell. Biol. 2011, 31, 832–844. [Google Scholar] [CrossRef] [Green Version]
- Renault, T.T.; Teijido, O.; Missire, F.; Ganesan, Y.T.; Velours, G.; Arokium, H.; Beaumatin, F.; Llanos, R.; Athané, A.; Camougrand, N.; et al. Bcl-xL stimulates Bax relocation to mitochondria and primes cells to ABT-737. Int. J. Biochem. Cell Biol. 2015, 64, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F.; Banerjee, S.; Suzuki, M.; Cleland, M.M.; Arnoult, D.; Wang, C.; Neutzner, A.; Tjandra, N.; Youle, R.J. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 2011, 145, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todt, F.; Cakir, Z.; Reichenbach, F.; Emschermann, F.; Lauterwasser, J.; Kaiser, A.; Ichim, G.; Tait, S.W.; Frank, S.; Langer, H.F.; et al. Differential retrotranslocation of mitochondrial Bax and Bak. EMBO J. 2015, 34, 67–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renault, T.T.; Teijido, O.; Antonsson, B.; Dejean, L.M.; Manon, S. Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): Keep your friends close but your enemies closer. Int. J. Biochem. Cell Biol. 2013, 45, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Shiota, T.; Mabuchi, H.; Tanaka-Yamano, S.; Yamano, K.; Endo, T. In vivo protein-interaction mapping of a mitochondrial translocator protein Tom22 at work. Proc. Natl. Acad. Sci. USA 2011, 108, 15179–15183. [Google Scholar] [CrossRef] [Green Version]
- Er, E.; Lalier, L.; Cartron, P.F.; Oliver, L.; Vallette, F.M. Control of Bax homodimerization by its carboxyl terminus. J. Biol. Chem. 2007, 282, 24938–24947. [Google Scholar] [CrossRef] [Green Version]
- Vasquez-Montes, V.; Rodnin, M.V.; Kyrychenko, A.; Ladokhin, A.S. Lipids modulate the BH3-independent membrane targeting and activation of BAX and Bcl-xL. Proc. Natl. Acad. Sci. USA 2021, 118, e2025834118. [Google Scholar] [CrossRef] [PubMed]
- Mignard, V.; Dubois, N.; Lanoé, D.; Joalland, M.P.; Oliver, L.; Pecqueur, C.; Heymann, D.; Paris, F.; Vallette, F.M.; Lalier, L. Sphingolipid distribution at mitochondria-associated membranes (MAMs) upon induction of apoptosis. J. Lipid Res. 2020, 61, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Légiot, A.; Céré, C.; Dupoiron, T.; Kaabouni, M.; Camougrand, N.; Manon, S. Mitochondria-Associated Membranes (MAMs) are involved in Bax mitochondrial localization and cytochrome c release. Microb. Cell 2019, 6, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Pervaiz, S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 2010, 17, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Vance, J.E. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta 2014, 1841, 595–609. [Google Scholar] [CrossRef]
- Roberts, D.J.; Miyamoto, S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015, 22, 248–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, K.; Sato, M.; Umezawa, Y. Fluorescent indicators for Akt/protein kinase B and dynamics of Akt activity visualized in living cells. J. Biol. Chem. 2003, 278, 30945–30951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santi, S.A.; Lee, H. The Akt isoforms are present at distinct subcellular locations. Am. J. Physiol. Cell Physiol. 2010, 298, C580–C591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Means, R.E.; Katz, S.G. Balancing life and death: BCL-2 family members at diverse ER-mitochondrial contact sites. FEBS J. 2021; in press. [Google Scholar] [CrossRef]
- Trécesson, S.C.; Souazé, F.; Basseville, A.; Bernard, A.C.; Pécot, J.; Lopez, J.; Bessou, M.; Sarosiek, K.A.; Letai, A.; Barillé-Nion, S.; et al. BCL-XL directly modulates RAS signalling to favour cancer cell stemness. Nat. Commun. 2017, 8, 1123. [Google Scholar] [CrossRef] [PubMed]
- Moyzis, A.G.; Lally, N.S.; Liang, W.; Leon, L.J.; Najor, R.H.; Orogo, A.M.; Gustafsson, Å.B. Mcl-1-mediated mitochondrial fission protects against stress but impairs cardiac adaptation to exercise. J. Mol. Cell. Cardiol. 2020, 146, 109–120. [Google Scholar] [CrossRef] [PubMed]

| Proteins | 32 C-Terminal Residues | |
|---|---|---|
| Proteins having a C-terminal hydrophobic α-helix and an identified “X-domain” | Bcl-2 |  |
Bcl-xL | 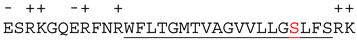 | |
Bcl-w |  | |
| Proteins having a C-terminal hydrophobic α-helix but not yet identified “X-domain” | Bax |  |
| Bak |  | |
| Mcl-1 | 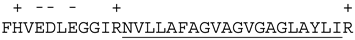 | |
Bcl-2L13 (rambo) |  | |
| Proteins that do not have a predicted C-terminal hydrophobic α-helix | Bid | 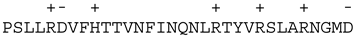 |
| Bim | 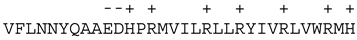 | |
| Bad | 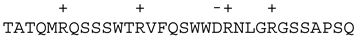 | |
| Puma |  | |
Bcl-2A1 (Bfl-1) | 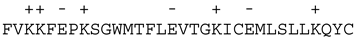 | |
| Bok | 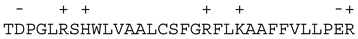 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalier, L.; Vallette, F.; Manon, S. Bcl-2 Family Members and the Mitochondrial Import Machineries: The Roads to Death. Biomolecules 2022, 12, 162. https://doi.org/10.3390/biom12020162
Lalier L, Vallette F, Manon S. Bcl-2 Family Members and the Mitochondrial Import Machineries: The Roads to Death. Biomolecules. 2022; 12(2):162. https://doi.org/10.3390/biom12020162
Chicago/Turabian StyleLalier, Lisenn, François Vallette, and Stéphen Manon. 2022. "Bcl-2 Family Members and the Mitochondrial Import Machineries: The Roads to Death" Biomolecules 12, no. 2: 162. https://doi.org/10.3390/biom12020162
APA StyleLalier, L., Vallette, F., & Manon, S. (2022). Bcl-2 Family Members and the Mitochondrial Import Machineries: The Roads to Death. Biomolecules, 12(2), 162. https://doi.org/10.3390/biom12020162







