Ozonation of Whole Blood Results in an Increased Release of Microparticles from Blood Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Blood Collection
2.3. Blood Ozonation/Aeration
2.4. Microparticle Isolation
2.5. Quantification and Characterization of MPs
2.6. Coagulation Tests
2.7. Statistical Analysis
3. Results
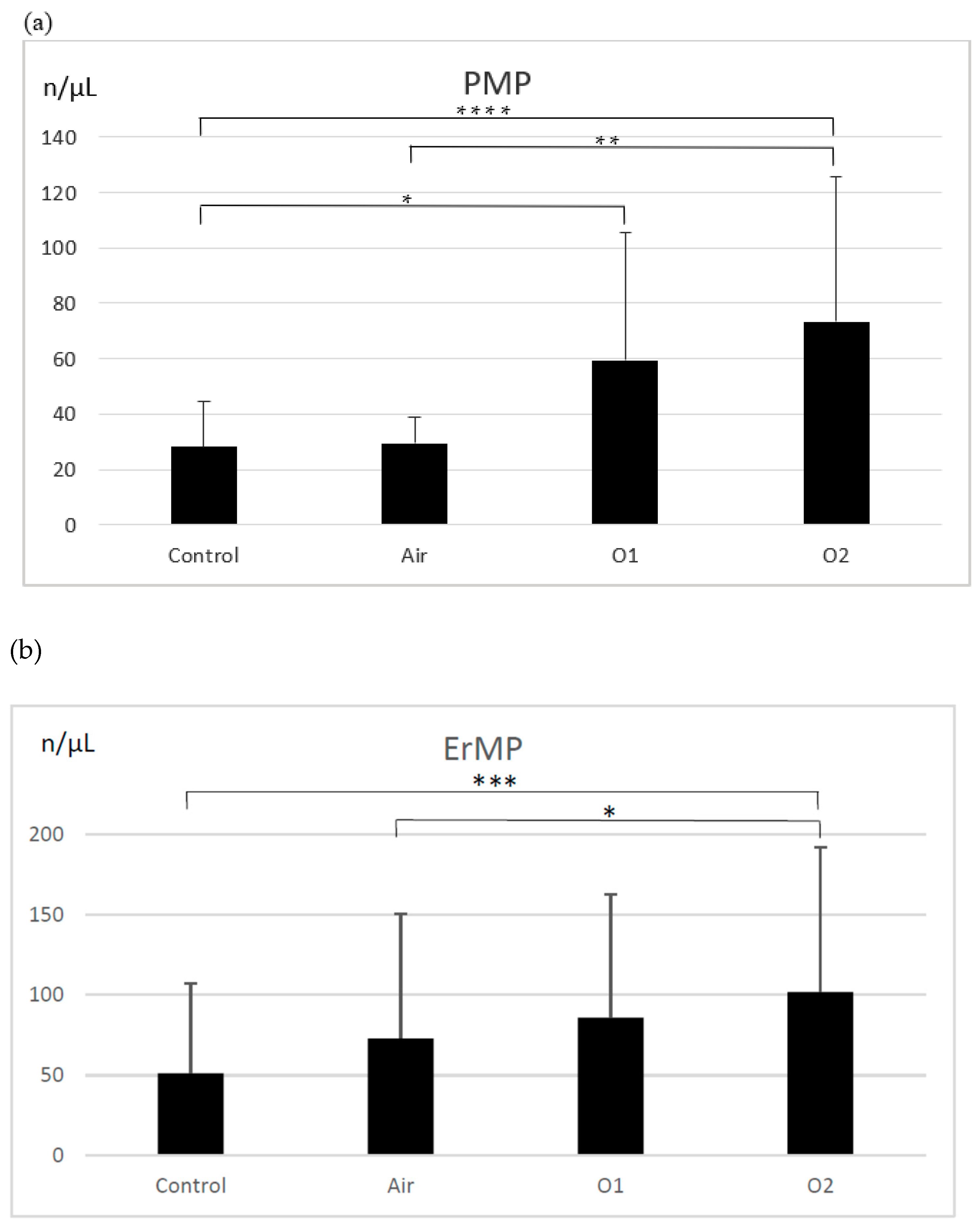
3.1. Microparticles
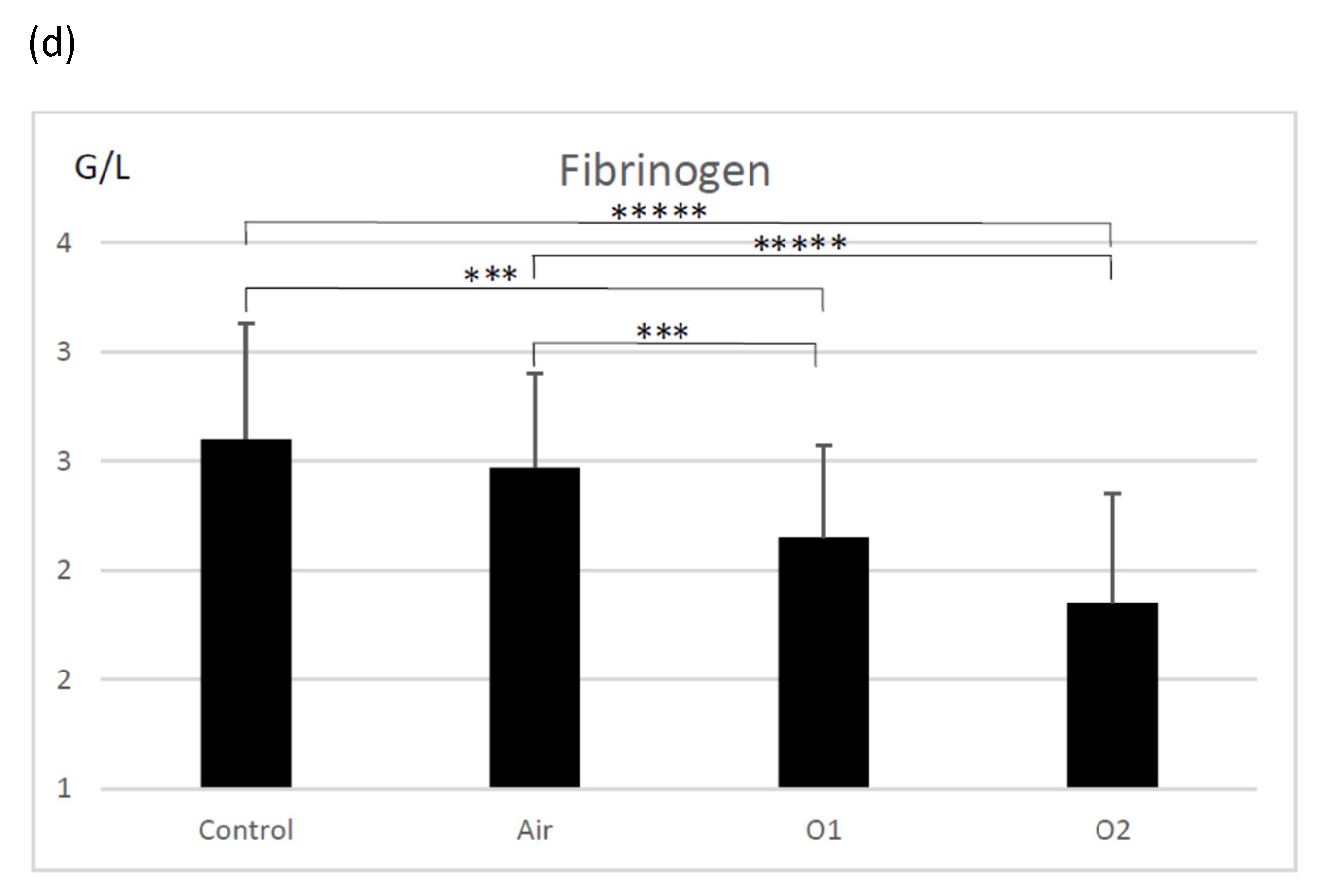
3.2. Coagulation Tests
3.3. Correlations between Generated Microparticles of Different Origin
3.4. Correlations between the Generated Microparticles and Markers of Hemostasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinkman, R.; Lamberts, H.B. Ozone as a possible radiomimetic gas. Nature 1958, 181, 1202–1203. [Google Scholar] [CrossRef]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, A.S. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas Res. 2017, 7, 212–219. [Google Scholar]
- Bocci, V.; Larini, A.; Micheli, V. Restoration of normoxia by ozone therapy may control neoplastic growth: A review and a working hypothesis. J. Altern. Complement. Med. 2005, 11, 257–265. [Google Scholar] [CrossRef]
- Inal, M.; Dokumacioglu, A.; Ozcelik, E.; Ucar, O. The effects of ozone therapy and coenzyme Q(1)(0) combination on oxidative stress markers in healthy subjects. Ir. J. Med. Sci. 2011, 180, 703–707. [Google Scholar] [CrossRef]
- Bocci, V.A.; Zanardi, I.; Travagli, V. Ozone acting on human blood yields a hormetic dose-response relationship. J. Transl. Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zheng, J.; Liu, Q.; Yang, Y.; Zhang, Y. The effect and safety of ozone autohemotherapy combined with pharmacological therapy in postherpetic neuralgia. J. Pain Res. 2018, 11, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Izadi, M.; Sureda, A.; Jonaidi-Jafari, N.; Banki, A.; Nabavi, S.F.; Nabavi, S.M. Therapeutic relevance of ozone therapy in degenerative diseases: Focus on diabetes and spinal pain. J. Cell Physiol. 2018, 233, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Travagli, V.; Zanardi, I. May oxygen-ozone therapy improves cardiovascular disorders? Cardiovasc. Hematol. Disord. Drug Targets 2009, 9, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Viebahn-Hänsler, R.; Fernández, O.S.L.; Fahmy, Z. Ozone in medicine: Clinical evaluation and evidence classification of the systemic ozone applications, major autohemotherapy and rectal insufflation, according to the requirements for evidence-based medicine. Ozone Sci. Eng. 2016, 38, 322–345. [Google Scholar] [CrossRef]
- Ang, W.-J.; Jiang, L.; Wang, Y.; Kuang, Z.-M. Ozone therapy induced sinus arrest in a hypertensive patient with chronic kidney disease. A case report. Medicine 2017, 96, e9265. [Google Scholar]
- Üreyen, Ç.M.; Baş, C.Y.; Arslan, Ş. Myocardial infarction after ozone therapy: Is ozone therapy dr. Jekyll or Mr. Hyde? Cardiology 2015, 132, 101–104. [Google Scholar] [CrossRef]
- Giunta, R.; Coppola, A.; Luongo, C.; Sammartino, A.; Guastafierro, S.; Grassia, A.; Giunta, L.; Mascolo, L.; Tirelli, A.; Coppola, L. Ozonized autohemotransfusion improves hemorheological parameters and oxygen delivery to tissues in patients with peripheral occlusive arterial disease. Ann. Hematol. 2001, 80, 745–748. [Google Scholar] [CrossRef]
- Volkhovskaya, N.B.; Tkachenko, S.B.; Belopolsky, A.A. Modulation of phagocytic activity of blood polynuclear leukocytes with ozonized physiological saline. Bull. Exp. Biol. Med. 2008, 146, 559–561. [Google Scholar] [CrossRef]
- Bocci, V.; Paulesu, L. Studies on the biological effects of ozone 1. Induction of interferon gamma on human leucocytes. Haematologica 1990, 75, 510–515. [Google Scholar]
- Sen, R.; Baltimore, D. Inducibility of kappa immunoglobulin enhance binding protein Nf-kappa B by a posttranslational mechanism. Cell 1986, 47, 921–928. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Yogev, Y. A humoral solution: Autologous blood products and tissue repair. Cell. Immunol. 2020, 356, 104178. [Google Scholar] [CrossRef]
- Burgassi, S.; Zanardi, I.; Travagli, V.; Montomoli, E.; Bocci, V. How much ozone bactericidal activity is compromised by plasma components? J. Appl. Microbiol. 2009, 106, 1715–1721. [Google Scholar] [CrossRef]
- Valacchi, G.; Bocci, V. Studies on the biological effects of ozone: 10. Release of factors from ozonated human platelets. Mediat. Inflamm. 1999, 8, 205–209. [Google Scholar] [CrossRef]
- Orakdogen, M.; Uslu, S.; Emon, S.T.; Somay, H.; Meric, Z.C.; Hakan, T. The effect of ozone therapy on experimental vasospasm in the rat femoral artery. Turk. Neurosurg. 2016, 26, 860–865. [Google Scholar] [CrossRef][Green Version]
- Di Paolo, N.; Bocci, V.; Garosi, G.; Borrelli, E.; Bravi, A.; Bruci, A.; Aldinucci, C.; Capotondo, L. Extracorporeal blood oxygenation and ozonation (EBOO) in man. preliminary report. Int. J. Artif. Organs. 2000, 23, 131–141. [Google Scholar] [CrossRef]
- Borrelli, E.; Bocci, V. Visual improvement following ozone therapy in dry age related macular degeneration; a review. Med. Hypothesis Discov. Innov. Ophthalmol. 2013, 2, 47–51. [Google Scholar]
- Re, L.; Martínez-Sánchez, G.; Bordicchia, M.; Malcangi, G.; Pocognoli, A.; Morales-Segura, M.A.; Rothchild, J.; Rojas, A. Is ozone pre-conditioning effect linked to Nrf2/EpRE activation pathway in vivo? A preliminary result. Eur. J. Pharmacol. 2014, 742, 158–162. [Google Scholar] [CrossRef]
- Cisterna, B.; Costanzo, M.; Nodari, A.; Galiè, M.; Zanzoni, S.; Bernardi, P.; Covi, V.; Tabaracci, G.; Malatesta, M. Ozone activates the Nrf2 pathway and improves preservation of explanted adipose tissue in vitro. Antioxidants 2020, 9, 989. [Google Scholar] [CrossRef]
- Galiè, M.; Covi, V.; Tabaracci, G.; Malatesta, M. The role of Nrf2 in the antioxidant cellular response to medical ozone exposure. Int. J. Mol. Sci. 2019, 20, 4009. [Google Scholar] [CrossRef]
- Helbing, T.; Olivier, C.; Bode, C.; Moser, M.; Diehl, P. Role of microparticles in endothelial dysfunction and arterial hypertension. World J. Cadiol. 2014, 6, 1135–1139. [Google Scholar] [CrossRef]
- Date, K.; Ettelaie, C.; Maraveyas, A. Tissue factor-bearing microparticles and inflammation: A potential mechanism for the development of venous thromboembolism in cancer. J. Thromb. Haemost. 2017, 15, 2289–2299. [Google Scholar] [CrossRef]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef]
- Kogure, T.; Yan, I.K.; Lin, W.-L.; Patel, T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: A mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer 2013, 4, 261–272. [Google Scholar] [CrossRef]
- Boon, R.A.; Vickers, K.C. Intercellular transport of microRNAs. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 186–192. [Google Scholar] [CrossRef]
- Schouten, M.; Wiersinga, W.J.; Levi, M.; van der Poll, T. Inflammation, endothelium, and coagulation in sepsis. J. Leukoc. Biol. 2008, 83, 536–545. [Google Scholar] [CrossRef]
- Rautou, P.-E.; Vion, A.-C.; Amabile, N.; Chironi, G.; Simon, A.; Tedgui, A.; Boulanger, C.M. Microparticles, vascular function, and atherothrombosis. Circ. Res. 2011, 109, 593–606. [Google Scholar] [CrossRef]
- Van Der Meijden, P.E.; Van, S.M.; Van, O.R.; Van, T.; Renne, A.J. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 2012, 10, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Lipets, E.; Vlasova, O.; Urnova, E.; Margolin, O.; Soloveva, A.; Ostapushchenko, O.; Andersen, J.; Ataullakhanov, F.; Panteleev, M. Circulating contact-pathway-activating microparticles together with factors IXa and XIa induce spontaneous clotting in plasma of hematology and cardiologic Patients. PLoS ONE 2014, 9, e87692. [Google Scholar] [CrossRef] [PubMed]
- Vallier, L.; Cointe, S.; Lacroix, R.; Bonifay, A.; Judicone, C.; Dignant-George, F.; Kwaan, H.C. Microparticles and fibrinolysis. Semin. Thromb. Hemost. 2017, 43, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Myśliwiec, M.; Sierko, E.; Sobierska, M.; Kruszewska, J.; Lipska, A.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Elevated microparticles, thrombin-antithrombin and VEGF levels in colorectal cancer patients undergoing chemotherapy. Pathol. Oncol. Res. 2020, 26, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Ciborowski, M.; Lipska, A.; Godzien, J.; Ferrarini, A.; Korsak, J.; Radziwon, P.; Tomasiak, M.; Barbas, C. Combination of LC-MS- and GC-MS-based metabolomics to study the effect of ozonated autohemotherapy on human blood. J. Proteome Res. 2012, 11, 6231–6241. [Google Scholar] [CrossRef] [PubMed]
- Travagli, V.; Zanardi, I.; Silvietti, A.; Nepi, S.; Tenori, L.; Bocci, V. Effects of ozone blood treatment on the metabolite profile of human blood. Int. J. Toxicol. 2010, 29, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Chou, M.L.; Goubran, H.; Cognasse, F.; Garraud, O.; Seghatchian, J. An overview of the role of microparticles/microvesicles in blood components: Are they clinically beneficial or harmful? Transfus. Apher. Sci. 2015, 53, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Almizraq, R.J.; Seghatchian, J.; Acker, J.P. Extracellular vesicles in transfusion-related immunomodulation and the role of blood component manufacturing. Transfus. Apher. Sci. 2016, 255, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ayersa, L.; Kohler, M.; Harrison, P.; Sargent, I.; Dragovic, R.; Schaap, M.; Nieuwland, R.; Brooks, S.A.; Ferry, B. Measurement of circulating cell-derived microparticles by flow cytometry: Sources of variability within the assay. Thromb. Res. 2011, 127, 370–377. [Google Scholar] [CrossRef]
- Noulsri, E.; Palasuwan, A. Effects of donor age, donor sex, blood-component processing, and storage on cell-derived microparticle concentrations in routine blood-component preparation. Transfus. Apher. Sci. 2018, 57, 587–592. [Google Scholar] [CrossRef]
- Almizraq, R.J.; Seghatchian, J.; Holovati, J.L.; Acker, J.P. Extracellular vesicle characteristics in stored red blood cell concentrates are influenced by the method of detection. Transfus. Apher. Sci. 2017, 56, 254–260. [Google Scholar] [CrossRef]
- Gao, Y.; Lv, L.; Liu, S.; Ma, G.; Su, Y. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sang. 2013, 105, 11–17. [Google Scholar] [CrossRef]
- Rubin, O.; Canellini, G.; Delobel, J.; Lion, N.; Tissot, J.D. Red blood cell microparticles: Clinical relevance. Transfus. Med. Hemother. 2012, 39, 342–347. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Schifferli, J.A. Ectosomes as modulators of inflammation and immunity. Clin. Exp. Immunol. 2011, 163, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Murphy, W.G.; Smith, O.P. Circulating microparticles: Pathophysiology and clinical implications. Blood Rev. 2007, 21, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.A.; Shchegolikhin, A.N.; Bychkova, A.V.; Leonova, V.B.; Biryukova, M.I.; Kostanova, E.A. Ozone-induced oxidative modification of fibrinogen: Role of the D regions. Free Radic. Biol. Med. 2014, 77, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Olivier, R.; Delobel, J.; Prudent, M.; Lion, N.; Kohl, K.; Tucker, E.I.; Tissot, J.-D.; Angelillo-Scherrer, A. Red blood celliderived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion 2013, 53, 1744–1754. [Google Scholar]
- Lacroix, R.; Plawinski, L.; Robert, S.; Doeuvre, L.; Sabatier, F.; Martinez de Lizarrondo, S.; Mezzapesa, A.; Anfosso, F.; Leroyer, A.S.; Poullin, P.; et al. Leukocyte- and endothelial-derived microparticles: A circulating source for fibrinolysis. Haematologica 2012, 97, 1864–1872. [Google Scholar] [CrossRef]
- Re, L.; Rowen, R.; Travagli, V. Ozone therapy and its use in medicine. Cardiology 2016, 134, 99–100. [Google Scholar] [CrossRef]
- Biedunkiewicz, B.; Lizakowski, S.; Tylicki, L.; Skiboeska, A.; Nieweglowski, T.; Chamienia, A.; Dębska-Slizien, A.; Rutkowski, B. Blood coagulation unaffected by ozonated autohemotherapy in patients on maintenance hemodialysis. Arch. Med. Res. 2006, 37, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; García, M.; Pina, C.; Menéndez, S. Efecto del ozono sobre la actividad plaquetaria en paciente diabéticos tratados con ozonoterapia: Informe preliminar. Rev. Cuba. Investig. Biomed. 2001, 20, 45–47. [Google Scholar]
- Martínez, Y.; Zamora, Z.; González, R.; Guanche, D. Effect of ozone oxidative preconditioning on bleeding time and venous thrombosis in a septic shock model in rats. Cienc. Biol. 2010, 41, 41–51. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boczkowska-Radziwon, B.; Olbromski, P.J.; Rogowska, A.; Bujno, M.; Myśliwiec, M.; Żebrowska, A.; Średziński, D.; Polityńska, B.; Wojtukiewicz, M.Z.; Radziwon, P. Ozonation of Whole Blood Results in an Increased Release of Microparticles from Blood Cells. Biomolecules 2022, 12, 164. https://doi.org/10.3390/biom12020164
Boczkowska-Radziwon B, Olbromski PJ, Rogowska A, Bujno M, Myśliwiec M, Żebrowska A, Średziński D, Polityńska B, Wojtukiewicz MZ, Radziwon P. Ozonation of Whole Blood Results in an Increased Release of Microparticles from Blood Cells. Biomolecules. 2022; 12(2):164. https://doi.org/10.3390/biom12020164
Chicago/Turabian StyleBoczkowska-Radziwon, Barbara, Piotr Józef Olbromski, Anna Rogowska, Magdalena Bujno, Marta Myśliwiec, Agnieszka Żebrowska, Dariusz Średziński, Barbara Polityńska, Marek Z. Wojtukiewicz, and Piotr Radziwon. 2022. "Ozonation of Whole Blood Results in an Increased Release of Microparticles from Blood Cells" Biomolecules 12, no. 2: 164. https://doi.org/10.3390/biom12020164
APA StyleBoczkowska-Radziwon, B., Olbromski, P. J., Rogowska, A., Bujno, M., Myśliwiec, M., Żebrowska, A., Średziński, D., Polityńska, B., Wojtukiewicz, M. Z., & Radziwon, P. (2022). Ozonation of Whole Blood Results in an Increased Release of Microparticles from Blood Cells. Biomolecules, 12(2), 164. https://doi.org/10.3390/biom12020164





