Benzaldehyde Attenuates the Fifth Stage Larval Excretory–Secretory Product of Angiostrongylus cantonensis-Induced Injury in Mouse Astrocytes via Regulation of Endoplasmic Reticulum Stress and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Parasite and Experimental Infection
2.3. A. cantonensis Excretory/Secretory Product Preparation
2.4. Astrocyte Culture
2.5. Extraction of Total RNA and cDNA Synthesis
2.6. RNA Sequencing (RNA-Seq) Library Preparation and Sequencing
2.7. Bioinformatics Analysis
2.8. Quantitative Real-Time PCR
2.9. Protein Extraction and Western Blotting
2.10. Catalase Activity Assay
2.11. Superoxide Dismutase (SOD) Activity Assay
2.12. Glutathione S-Transferase (GST) Activity Assay
2.13. ROS Detection
2.14. Antioxidant Capacity Assay
2.15. Statistical Analysis
3. Results
3.1. Transcriptome Profile of Mouse Astrocytes after A. cantonensis L5 ESPs or Benzaldehyde Treatment
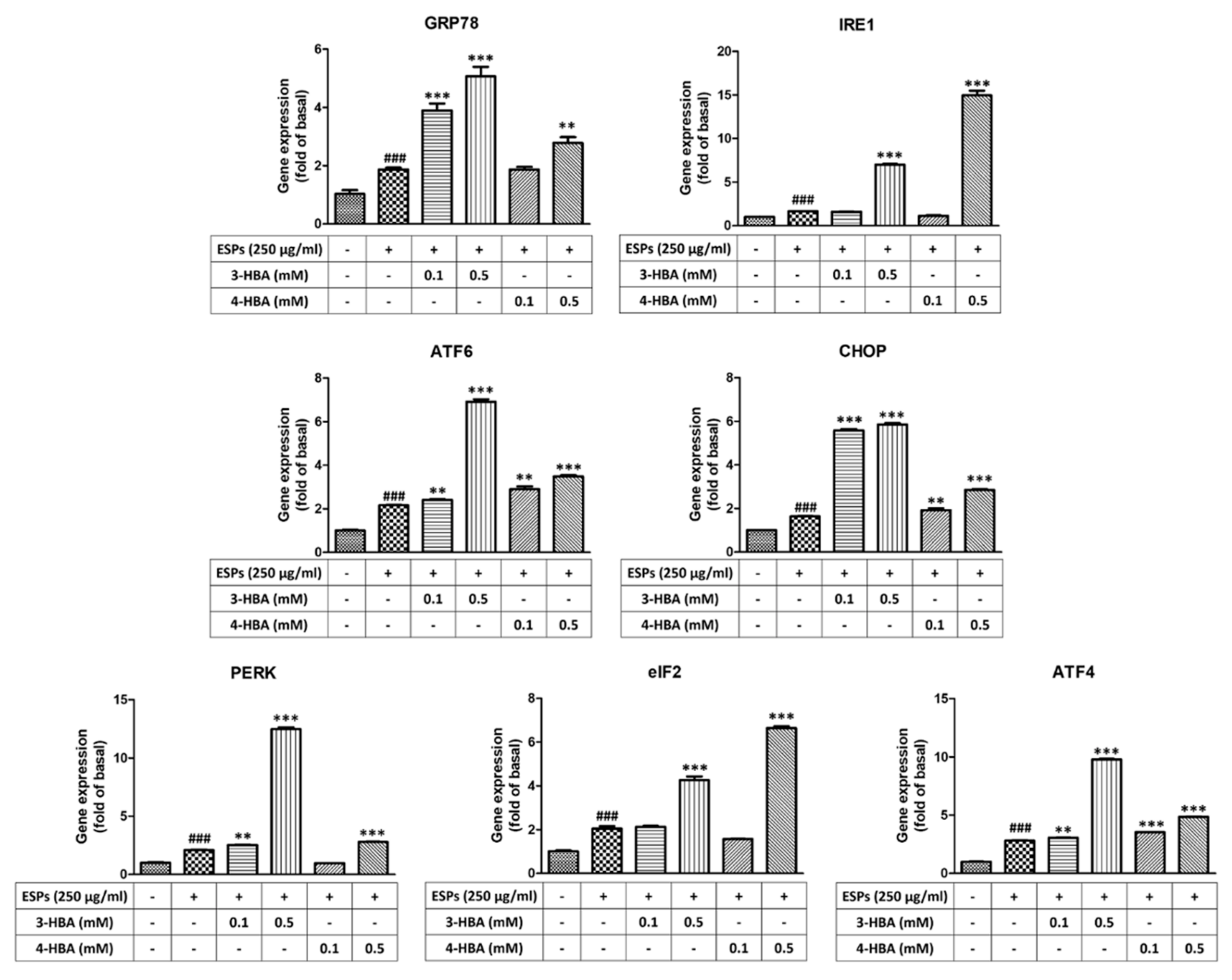
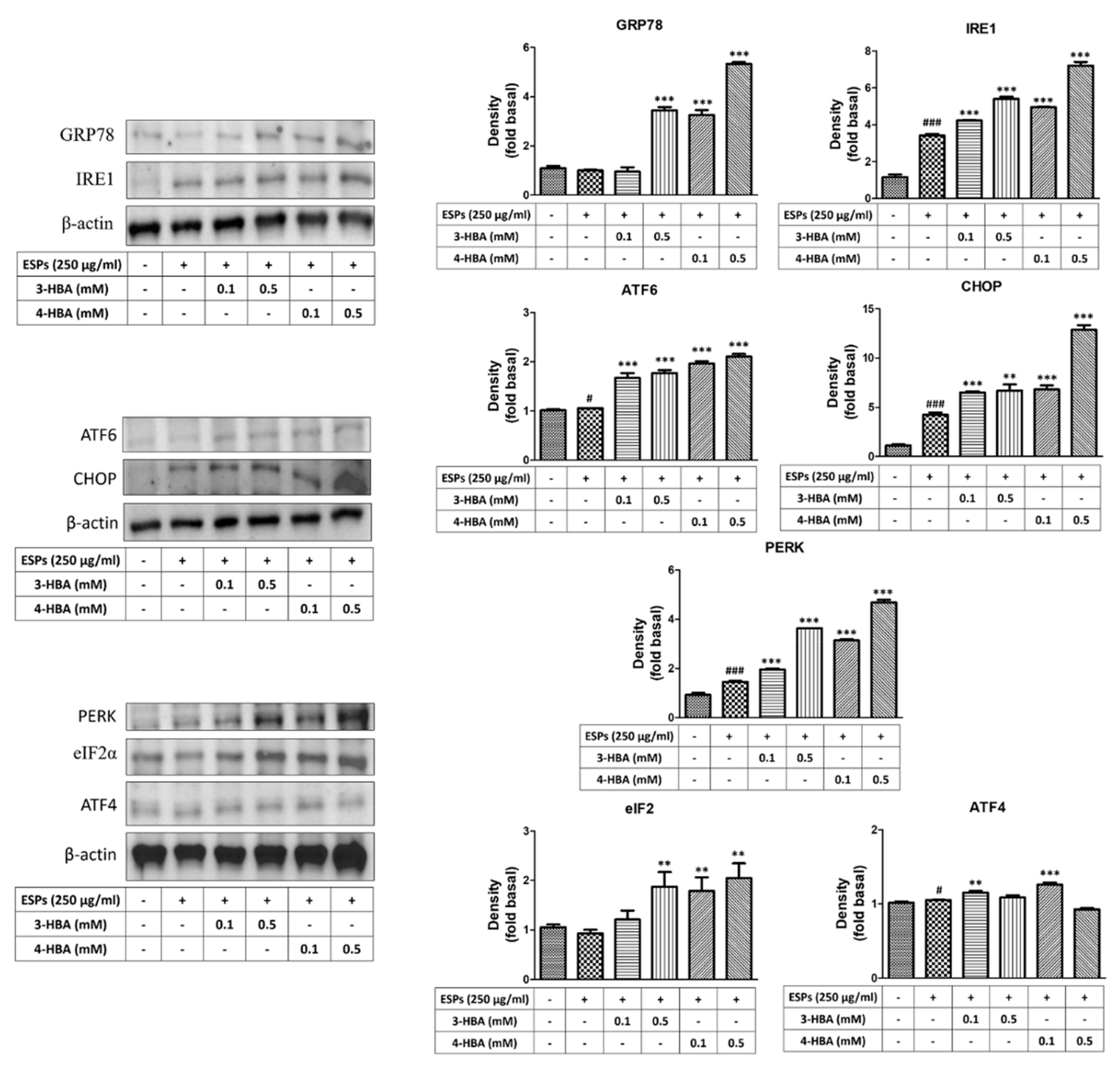
3.2. The Expression of ER Stress-Related Molecules after 3-HBA and 4-HBA Treatment
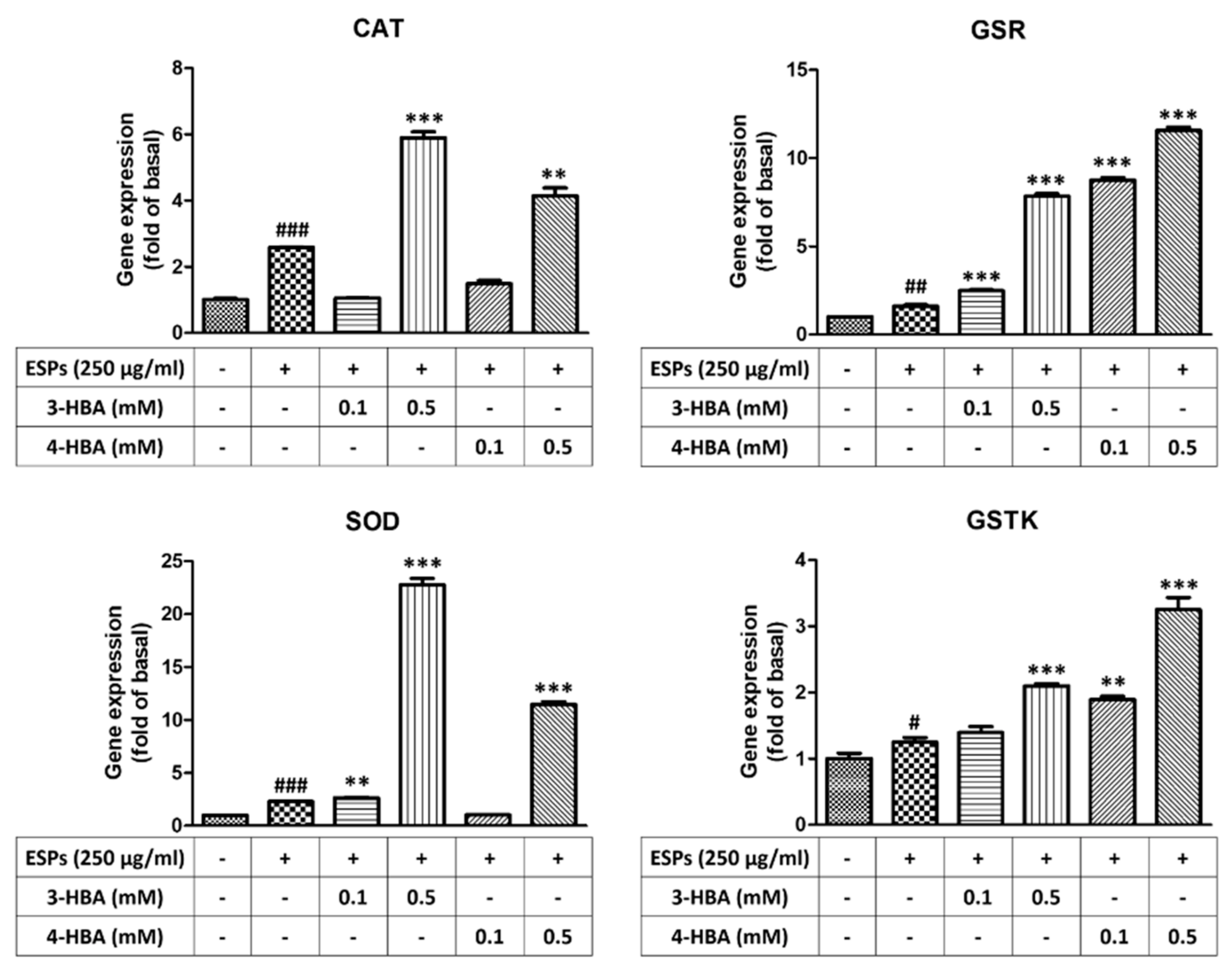
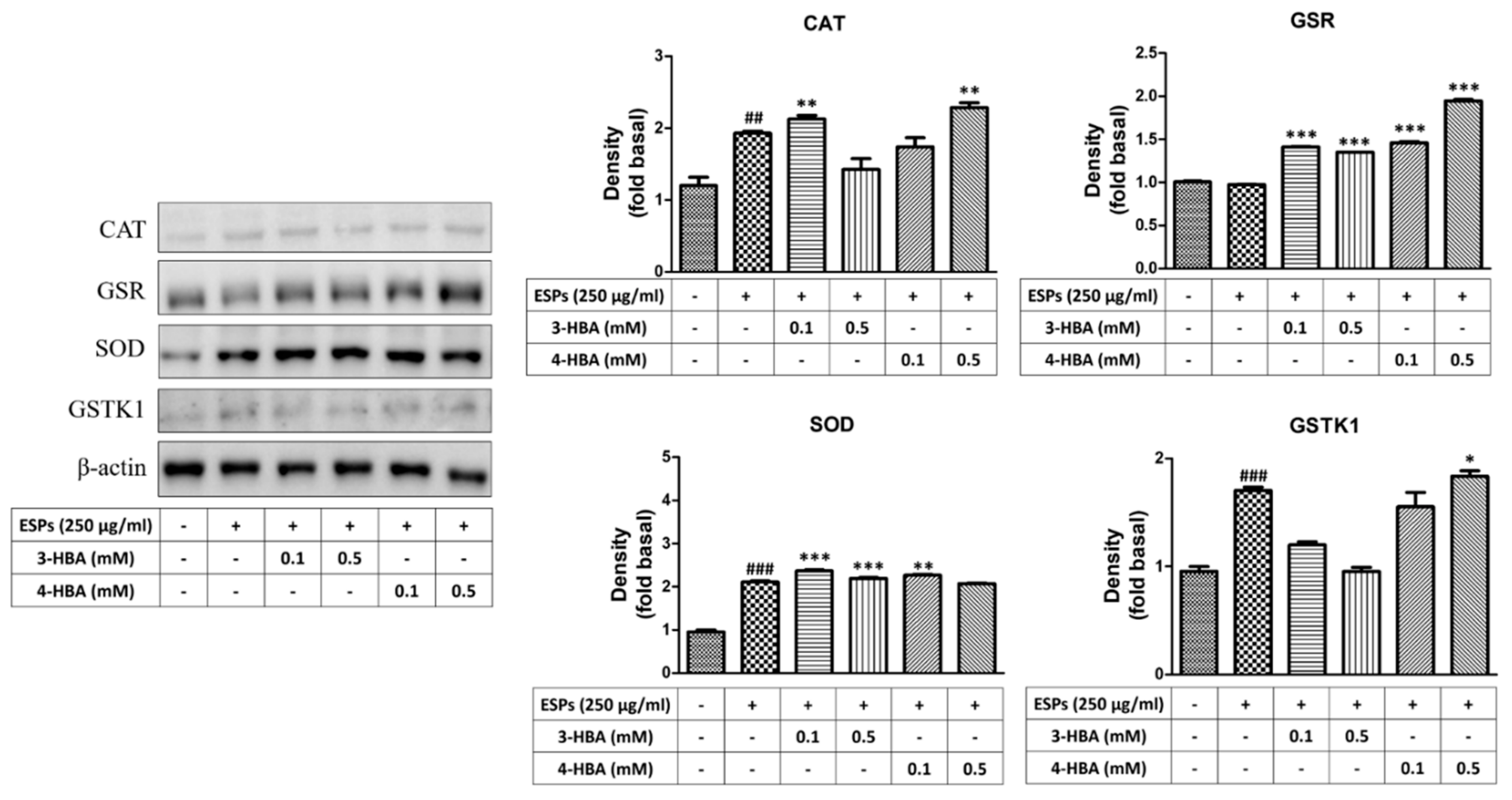
3.3. The Expression of Oxidative Stress-Related Molecules after 3-HBA and 4-HBA Treatment
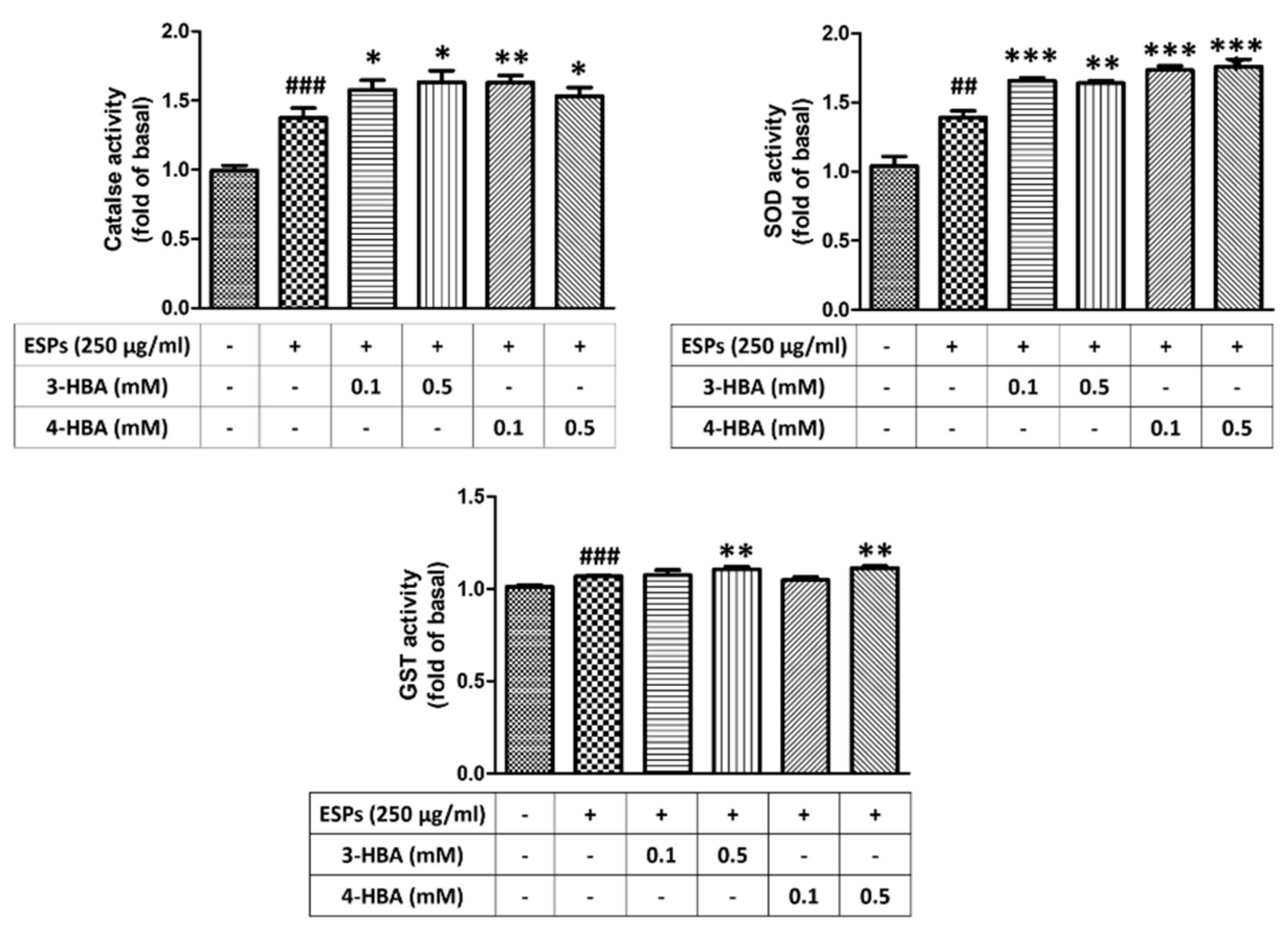
3.4. The Activity of Antioxidant after 3-HBA and 4-HBA Treatment
3.5. 3-HBA and 4-HBA Inhibited ROS Generation after ESPs Treatment
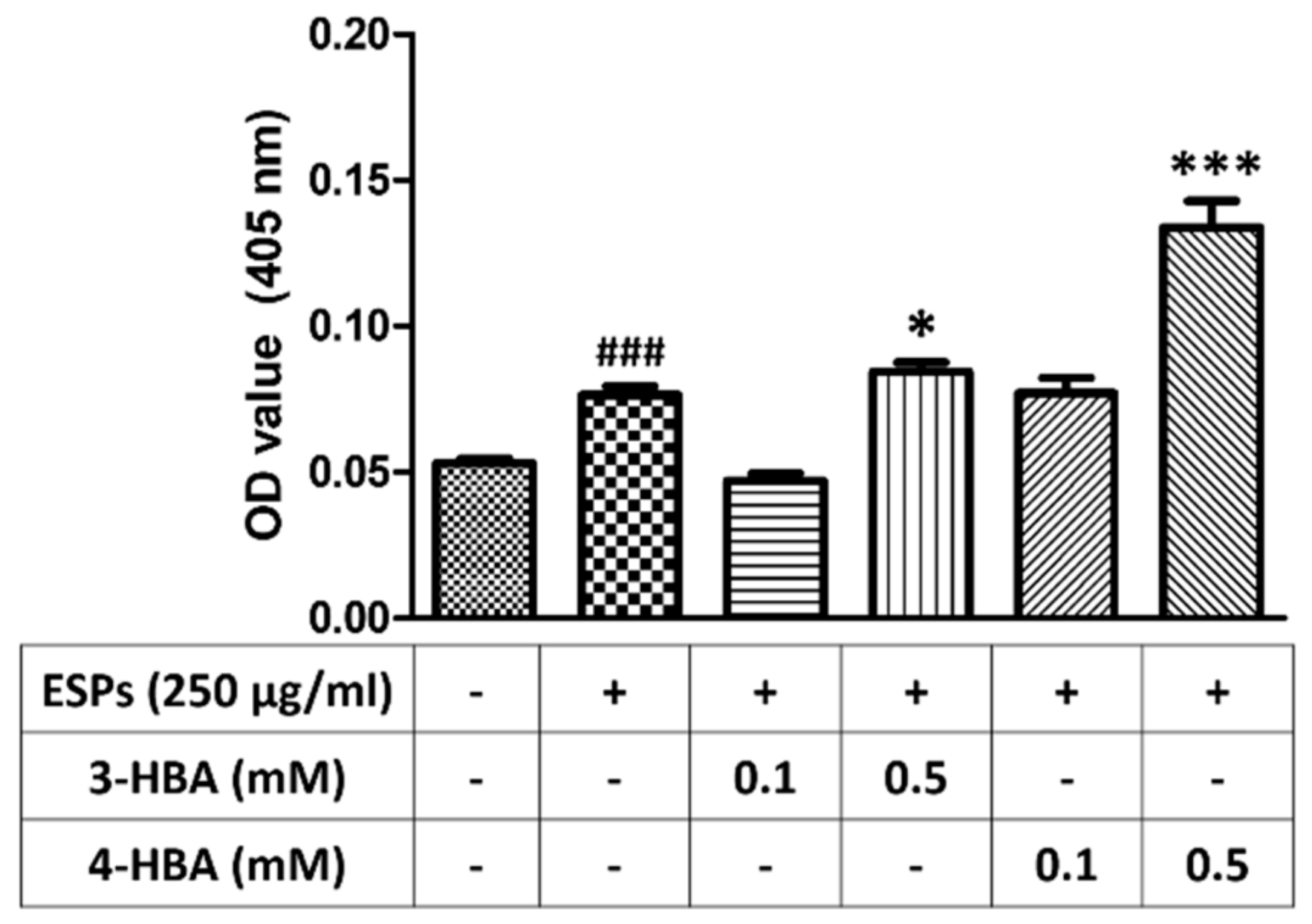
3.6. 3-HBA and 4-HBA Elevate the Antioxidant Capacity after ESPs Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.-C.; Chao, D.; Chen, E.-R. Experimental infection routes of Angiostrongylus cantonensis in mice. J. Helminthol. 1991, 65, 296–300. [Google Scholar] [CrossRef]
- Ash, L.R. The occurrence of Angiostrongylus cantonensis in frogs of New Caledonia with observations on paratenic hosts of metastrongyles. J. Parasitol. 1968, 54, 432–436. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, D.; Zhu, X.; Chen, X.; Lun, Z. Human angiostrongyliasis. Lancet Infect. Dis. 2008, 8, 621–630. [Google Scholar] [CrossRef]
- Wallace, G.D.; Rosen, L. Studies on eosinophilic meningitis. 2. Experimental infection of shrimp and crabs with Angiostrongylus cantonensis. Am. J. Epidemiol. 1966, 84, 120–131. [Google Scholar] [CrossRef]
- Wallace, G.D.; Rosen, L. Studies on eosinophilic meningitis: Experimental infection of fresh-water and marine fish with Angiostrongylus cantonensis. Am. J. Epidemiol. 1967, 85, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.A.; Thiengo, S.C.; Fernandez, M.A.; Graeff-Teixeira, C.; Morassutti, A.L.; Mourão, F.R.P.; Miranda, C.O.S.; Jorge, M.M.; Costa, L.F.; Gomes, S.R. Infection by Angiostrongylus cantonensis in both humans and the snail Achatina (Lissachatina) fulica in the city of Macapá, in the Amazon Region of Brazil. Memórias Inst. Oswaldo Cruz 2020, 115, e200115. [Google Scholar] [CrossRef] [PubMed]
- Federspiel, F.; Skovmand, S.; Skarphedinsson, S. Eosinophilic meningitis due to Angiostrongylus cantonensis in Europe. Int. J. Infect. Dis. 2020, 93, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, N.S.; Park, S.Y.; Blackburn, B.G.; Sejvar, J.J.; Gaynor, K.; Chung, H.; Leniek, K.; Herwaldt, B.L.; Effler, P.V. Distribution of eosinophilic meningitis cases attributable to Angiostrongylus cantonensis, Hawaii. Emerg. Infect. Dis. 2007, 13, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Guo, Y.-H.; Wei, F.-R.; Zhang, Y.; Xiao, N.; Zhou, X.-N. Control of eosinopilic meningitis caused by Angiostrongylus cantonensis in China. Adv. Parasitol. 2020, 110, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Morton, N.J.; Britton, P.; Palasanthiran, P.; Bye, A.; Sugo, E.; Kesson, A.; Ardern-Holmes, S.; Snelling, T.L. Severe hemorrhagic meningoencephalitis due to Angiostrongylus cantonensis among young children in Sydney, Australia. Clin. Infect. Dis. 2013, 57, 1158–1161. [Google Scholar] [CrossRef] [Green Version]
- Waugh, C.A.; Lindo, J.F.; Lorenzo-Morales, J.; Robinson, R.D. An epidemiological study of A. cantonensis in Jamaica subsequent to an outbreak of human cases of eosinophilic meningitis in 2000. Parasitology 2016, 143, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.; Chen, X.; Yin, C.; Wang, J.; Qi, H.; Ji, A. Angiostrongylus cantonensis: Effect of combination therapy with albendazole and dexamethasone on Th cytokine gene expression in PBMC from patients with eosinophilic meningitis. Exp. Parasitol. 2009, 123, 1–5. [Google Scholar] [CrossRef]
- Jhan, K.-Y.; Cheng, C.-J.; Jung, S.-M.; Lai, Y.-J.; Chen, K.-Y.; Wang, L.-C. Co-Therapy of Albendazole and Dexamethasone Reduces Pathological Changes in the Cerebral Parenchyma of Th-1 and Th-2 Dominant Mice Heavily Infected with Angiostrongylus cantonensis: Histopathological and RNA-seq Analyses. Biomolecules 2021, 11, 536. [Google Scholar] [CrossRef]
- Huang, N.-K.; Chern, Y.; Fang, J.-M.; Lin, C.-I.; Chen, W.-P.; Lin, Y.-L. Neuroprotective principles from Gastrodia elata. J. Nat. Prod. 2007, 70, 571–574. [Google Scholar] [CrossRef]
- Moon, C.Y.; Ku, C.R.; Cho, Y.H.; Lee, E.J. Protocatechuic aldehyde inhibits migration and proliferation of vascular smooth muscle cells and intravascular thrombosis. Biochem. Biophys. Res. Commun. 2012, 423, 116–121. [Google Scholar] [CrossRef]
- Kong, B.S.; Im, S.J.; Lee, Y.J.; Cho, Y.H.; Do, Y.R.; Byun, J.W.; Ku, C.R.; Lee, E.J. Vasculoprotective Effects of 3-Hydroxybenzaldehyde against VSMCs Proliferation and ECs Inflammation. PLoS ONE 2016, 11, e0149394. [Google Scholar] [CrossRef]
- Wang, R.-S.; Nakajima, T.; Kawamoto, T.; Honma, T. Effects of aldehyde dehydrogenase-2 genetic polymorphisms on metabolism of structurally different aldehydes in human liver. Drug Metab. Dispos. 2002, 30, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, J.-H.; Lee, D.-U.; Lee, J.-T.; Kim, J.-S.; Yong, C.-S.; Kim, J.-A.; Ha, J.-S.; Huh, K. 4-Hydroxybenzaldehyde from Gastrodia elata B1. is active in the antioxidation and GABAergic neuromodulation of the rat brain. J. Ethnopharmacol. 2000, 73, 329–333. [Google Scholar] [CrossRef]
- Kang, C.W.; Han, Y.E.; Kim, J.; Oh, J.H.; Cho, Y.H.; Lee, E.J. 4-Hydroxybenzaldehyde accelerates acute wound healing through activation of focal adhesion signalling in keratinocytes. Sci. Rep. 2017, 7, 14192. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-H.; Ryu, B.; Ngo, D.-H.; Kim, W.-S.; Kim, D.G.; Kim, S.-K. 4-hydroxybenzaldehyde-chitooligomers suppresses H2O2-induced oxidative damage in microglia BV-2 cells. Carbohydr. Res. 2017, 440, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Choi, J.-W.; Han, H.Y.; Kim, W.S.; Song, H.-Y.; Byun, E.-B.; Byun, E.-H.; Lee, Y.-H.; Yuk, J.-M. 4-Hydroxybenzaldehyde Restricts the Intracellular Growth of Toxoplasma gondii by Inducing SIRT1-Mediated Autophagy in Macrophages. Korean J. Parasitol. 2020, 58, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.-Y.; Chen, Y.-J.; Cheng, C.-J.; Jhan, K.-Y.; Chiu, C.-H.; Wang, L.-C. 3-Hydroxybenzaldehyde and 4-Hydroxybenzaldehyde enhance survival of mouse astrocytes treated with Angiostrongylus cantonensis young adults excretory/secretory products. Biomed. J. 2020, 43. [Google Scholar] [CrossRef]
- Wang, L.-C.; Jung, S.-M.; Chen, K.-Y.; Wang, T.-Y.; Li, C.-H. Temporal-spatial pathological changes in the brains of permissive and non-permissive hosts experimentally infected with Angiostrongylus cantonensis. Exp. Parasitol. 2015, 157, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.G.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Guasconi, L.; Serradell, M.C.; Masih, D.T.; Chiapello, L.S. Immunomodulatory Effect of Fasciola hepatica Excretory-Secretory Products on Macrophages. Methods Mol. Biol. 2020, 2137, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, C.; Navarro, S.; Wangchuk, P.; Wilson, D.; Daly, N.L.; Loukas, A. Identifying the immunomodulatory components of helminthes. Parasite Immunol. 2015, 37, 293–303. [Google Scholar] [CrossRef]
- Crowe, J.; Lumb, F.E.; Harnett, M.M.; Harnett, W. Parasite excretory-secretory products and their effects on metabolic syndrome. Parasite Immunol. 2017, 39, e12410. [Google Scholar] [CrossRef] [PubMed]
- Mehrdana, F.; Buchmann, K. Excretory/secretory products of anisakid nematodes: Biological and pathological roles. Acta Vet. Scand. 2017, 59, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Hao, C.; Zhuang, Q.; Zhan, B.; Sun, X.; Huang, J.; Cheng, Y.; Zhu, X. Excretory/Secretory Products From Trichinella spiralis Adult Worms Attenuated DSS-Induced Colitis in Mice by Driving PD-1-Mediated M2 Macrophage Polarization. Front. Immunol. 2020, 11, 563784. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Chiu, C.-H.; Wang, L.-C. Anti-apoptotic effects of Sonic hedgehog signalling through oxidative stress reduction in astrocytes co-cultured with excretory-secretory products of larval Angiostrongylus cantonensis. Sci. Rep. 2017, 7, 41574. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-Y.; Cheng, C.-J.; Cheng, C.-C.; Jhan, K.-Y.; Chen, Y.-J.; Wang, L.-C. The excretory/secretory products of fifth-stage larval Angiostrongylus cantonensis induces autophagy via the Sonic hedgehog pathway in mouse brain astrocytes. PLoS Negl. Trop. Dis. 2020, 14, e0008290. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Chen, Y.-J.; Cheng, C.-J.; Jhan, K.-Y.; Wang, L.-C. Excretory/secretory products of Angiostrongylus cantonensis fifth-stage larvae induce endoplasmic reticulum stress via the Sonic hedgehog pathway in mouse astrocytes. Parasites Vectors 2020, 13, 317. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Lu, P.-J.; Cheng, C.-J.; Jhan, K.-Y.; Yeh, S.-C.; Wang, L.-C. Proteomic analysis of excretory-secretory products from young adults of Angiostrongylus cantonensis. Memórias Inst. Oswaldo Cruz 2019, 114, e180556. [Google Scholar] [CrossRef]
- Choi, H.-H.; Shin, D.-M.; Kang, G.; Kim, K.-H.; Park, J.B.; Hur, G.M.; Lee, H.-M.; Lim, Y.-J.; Park, J.-K.; Jo, E.-K.; et al. Endoplasmic reticulum stress response is involved in Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis. FEBS Lett. 2010, 584, 2445–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samali, A.; Fitzgerald, U.; Deegan, S.; Gupta, S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int. J. Cell Biol. 2010, 2010, 170215. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.S. Calcium Signaling: From Basic to Bedside. Adv. Exp. Med. Biol. 2020, 1131, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Fribley, A.; Zhang, K.; Kaufman, R.J. Regulation of apoptosis by the unfolded protein response. Methods Mol. Biol. 2009, 559, 191–204. [Google Scholar] [CrossRef] [Green Version]
- Oakes, S.A. Endoplasmic Reticulum Stress Signaling in Cancer Cells. Am. J. Pathol. 2020, 90, 934–946. [Google Scholar] [CrossRef] [Green Version]
- Castilla, J.; Hetz, C.; Soto, C. Molecular mechanisms of neurotoxicity of pathological prion protein. Curr. Mol. Med. 2004, 4, 397–403. [Google Scholar] [CrossRef]
- Siwecka, N.; Rozpędek, W.; Pytel, D.; Wawrzynkiewicz, A.; Dziki, A.; Dziki, Ł.; Diehl, J.A.; Majsterek, I. Dual role of Endoplasmic Reticulum Stress-Mediated Unfolded Protein Response Signaling Pathway in Carcinogenesis. Int. J. Mol. Sci. 2019, 20, 4354. [Google Scholar] [CrossRef] [Green Version]
- Ghemrawi, R.; Khair, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6127. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Tilley, L.; Vennerstrom, J.L.; Roberts, D.; Rogerson, S.; Ginsburg, H. Oxidative stress in malaria parasite-infected erythrocytes: Host-parasite interactions. Int. J. Parasitol. 2004, 34, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Dogruman-Al, F.; Ercin, U.; Celebi, B.; Babur, C.; Bukan, N. Oxidative stress and tryptophan degradation pattern of acute Toxoplasma gondii infection in mice. Parasitol. Res. 2012, 111, 1725–1730. [Google Scholar] [CrossRef]
- Götz, A.; Ty, M.C.; Rodriguez, A. Oxidative Stress Enhances Dendritic Cell Responses to Plasmodium falciparum. Immunohorizons 2019, 3, 511–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado-Silva, A.; Cerqueira, P.G.; Grazielle-Silva, V.; Gadelha, F.R.; de Figueiredo Peloso, E.; Teixeira, S.M.R.; Machado, C.R. How Trypanosoma cruzi deals with oxidative stress: Antioxidant defence and DNA repair pathways. Mutat. Res. Rev. Mutat. Res. 2016, 767, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Feijó, D.F.; Dutra, F.F.; Carneiro, V.C.; Freitas, G.B.; Alves, L.S.; Mesquita, J.; Fortes, G.B.; Figueiredo, R.T.; Souza, H.S.; et al. Oxidative stress fuels Trypanosoma cruzi infection in mice. J. Clin. Investig. 2012, 122, 2531–2542. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Ghosh, E.; Mukherjee, A.K.; Nozaki, T.; Ganguly, S. Differential gene expression in Giardia lamblia under oxidative stress: Significance in eukaryotic evolution. Gene 2014, 535, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Schwertz, C.I.; Gabriel, M.E.; Henker, L.C.; Bottari, N.B.; do Carmo, G.; Guarda, N.d.S.; Moresco, R.N.; Machado, G.; Morsch, V.M.; Schetinger, M.R.; et al. Oxidative stress associated with pathological changes in the pancreas of cattle naturally infected by Eurytrema coelomaticum. Vet. Parasitol. 2016, 223, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Stumbo, A.D.; Goater, C.P.; Hontela, A. Parasite-induced oxidative stress in liver tissue of fathead minnows exposed to trematode cercariae. Parasitology 2012, 139, 1666–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Bhatia, S.; Sardana, S.; Senwar, K.R.; Dhillon, A.; Sharma, A.; Naved, T. In vitro antioxidant and antinociceptive properties of Porphyra vietnamensis. Biomedicine 2019, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Chung, L.-Y.; Chen, C.-H.; Wang, L.-C.; Chang, S.-J.; Yen, C.-M. Oxidative stress in mice infected with Angiostrongylus cantonensis coincides with enhanced glutathione-dependent enzymes activity. Exp. Parasitol. 2010, 126, 178–183. [Google Scholar] [CrossRef]
- Chung, L.-Y.; Wang, L.-C.; Chen, C.-H.; Lin, H.-Y.; Yen, C.-M. Kinetic change of oxidative stress in cerebrospinal fluid of mice infected with Angiostrongylus cantonensis. Redox Rep. 2010, 15, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, Z.; Wang, J.; Qi, H.; Li, X.; Zheng, X.; Yin, C. Treatment of angiostrongyliasis using a combination of albendazole and dexamethasone: The results of a retrospective and comparative study. Ann. Trop. Med. Parasitol. 2011, 105, 65–69. [Google Scholar] [CrossRef] [PubMed]

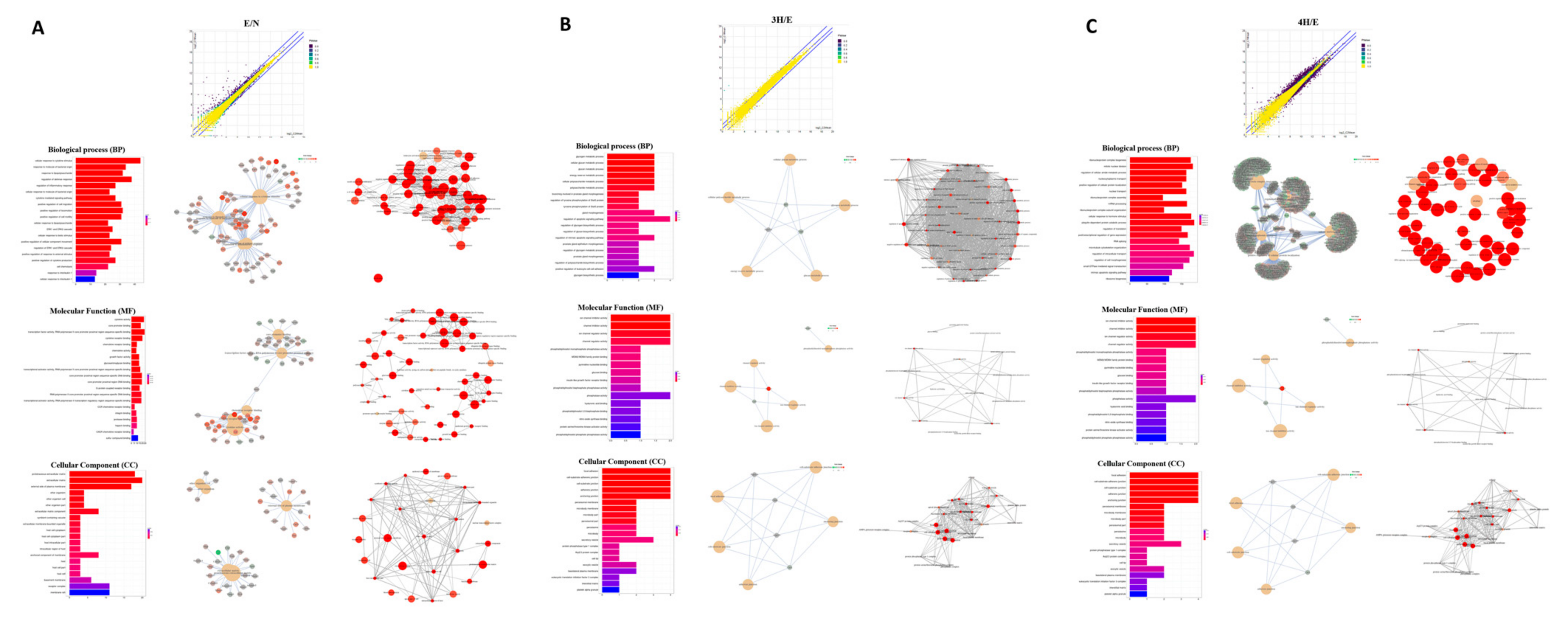

| Sample_Name | GC. | Length | Length_Mean | Phred | Q20_Ratio | Q30_Ratio | Qual_Mean | Read_Counts | Total_Bases |
|---|---|---|---|---|---|---|---|---|---|
| N_R1 | 48.65% | 20–151 | 144.32 | 33 | 99.04% | 96.29% | 36.42 | 25,920,999 | 3,740,844,537 |
| N_R2 | 48.78% | 20–151 | 143.69 | 33 | 98.43% | 94.35% | 36.10 | 25,920,999 | 3,724,577,535 |
| E_R1 | 48.00% | 20–151 | 144.66 | 33 | 98.97% | 96.05% | 36.38 | 23,617,944 | 3,416,535,104 |
| E_R2 | 48.22% | 20–151 | 144.22 | 33 | 98.61% | 94.87% | 36.19 | 23,617,944 | 3,406,183,730 |
| 3H1_R1 | 48.48% | 20–151 | 144.94 | 33 | 99.00% | 96.18% | 36.4 | 23,470,406 | 3,401,781,446 |
| 3H1_R2 | 48.68% | 20–151 | 143.85 | 33 | 98.28% | 94.04% | 36.04 | 23,470,406 | 3,376,330,842 |
| 3H5_R1 | 49.22% | 20–151 | 145.23 | 33 | 99.03% | 96.28% | 36.42 | 23,669,860 | 3,437,686,443 |
| 3H5_R2 | 49.38% | 20–151 | 144.55 | 33 | 98.42% | 94.38% | 36.1 | 23,669,860 | 3,421,432,553 |
| 4H1_R1 | 48.82% | 20–151 | 144.87 | 33 | 98.99% | 96.18% | 36.4 | 27,305,448 | 3,955,655,684 |
| 4H1_R2 | 48.97% | 20–151 | 143.86 | 33 | 98.31% | 94.10% | 36.06 | 27,305,448 | 3,928,240,745 |
| 4H5_R1 | 49.20% | 20–151 | 145.02 | 33 | 99.02% | 96.25% | 36.41 | 25,074,561 | 3,636,192,527 |
| 4H5_R2 | 49.37% | 20–151 | 144.24 | 33 | 98.32% | 94.09% | 36.06 | 25,074,561 | 3,616,740,196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.-Y.; Chen, Y.-J.; Cheng, C.-J.; Jhan, K.-Y.; Wang, L.-C. Benzaldehyde Attenuates the Fifth Stage Larval Excretory–Secretory Product of Angiostrongylus cantonensis-Induced Injury in Mouse Astrocytes via Regulation of Endoplasmic Reticulum Stress and Oxidative Stress. Biomolecules 2022, 12, 177. https://doi.org/10.3390/biom12020177
Chen K-Y, Chen Y-J, Cheng C-J, Jhan K-Y, Wang L-C. Benzaldehyde Attenuates the Fifth Stage Larval Excretory–Secretory Product of Angiostrongylus cantonensis-Induced Injury in Mouse Astrocytes via Regulation of Endoplasmic Reticulum Stress and Oxidative Stress. Biomolecules. 2022; 12(2):177. https://doi.org/10.3390/biom12020177
Chicago/Turabian StyleChen, Kuang-Yao, Yi-Ju Chen, Chien-Ju Cheng, Kai-Yuan Jhan, and Lian-Chen Wang. 2022. "Benzaldehyde Attenuates the Fifth Stage Larval Excretory–Secretory Product of Angiostrongylus cantonensis-Induced Injury in Mouse Astrocytes via Regulation of Endoplasmic Reticulum Stress and Oxidative Stress" Biomolecules 12, no. 2: 177. https://doi.org/10.3390/biom12020177
APA StyleChen, K.-Y., Chen, Y.-J., Cheng, C.-J., Jhan, K.-Y., & Wang, L.-C. (2022). Benzaldehyde Attenuates the Fifth Stage Larval Excretory–Secretory Product of Angiostrongylus cantonensis-Induced Injury in Mouse Astrocytes via Regulation of Endoplasmic Reticulum Stress and Oxidative Stress. Biomolecules, 12(2), 177. https://doi.org/10.3390/biom12020177






