Grapevine DMR6-1 Is a Candidate Gene for Susceptibility to Downy Mildew
Abstract
:1. Introduction
2. Materials and Methods
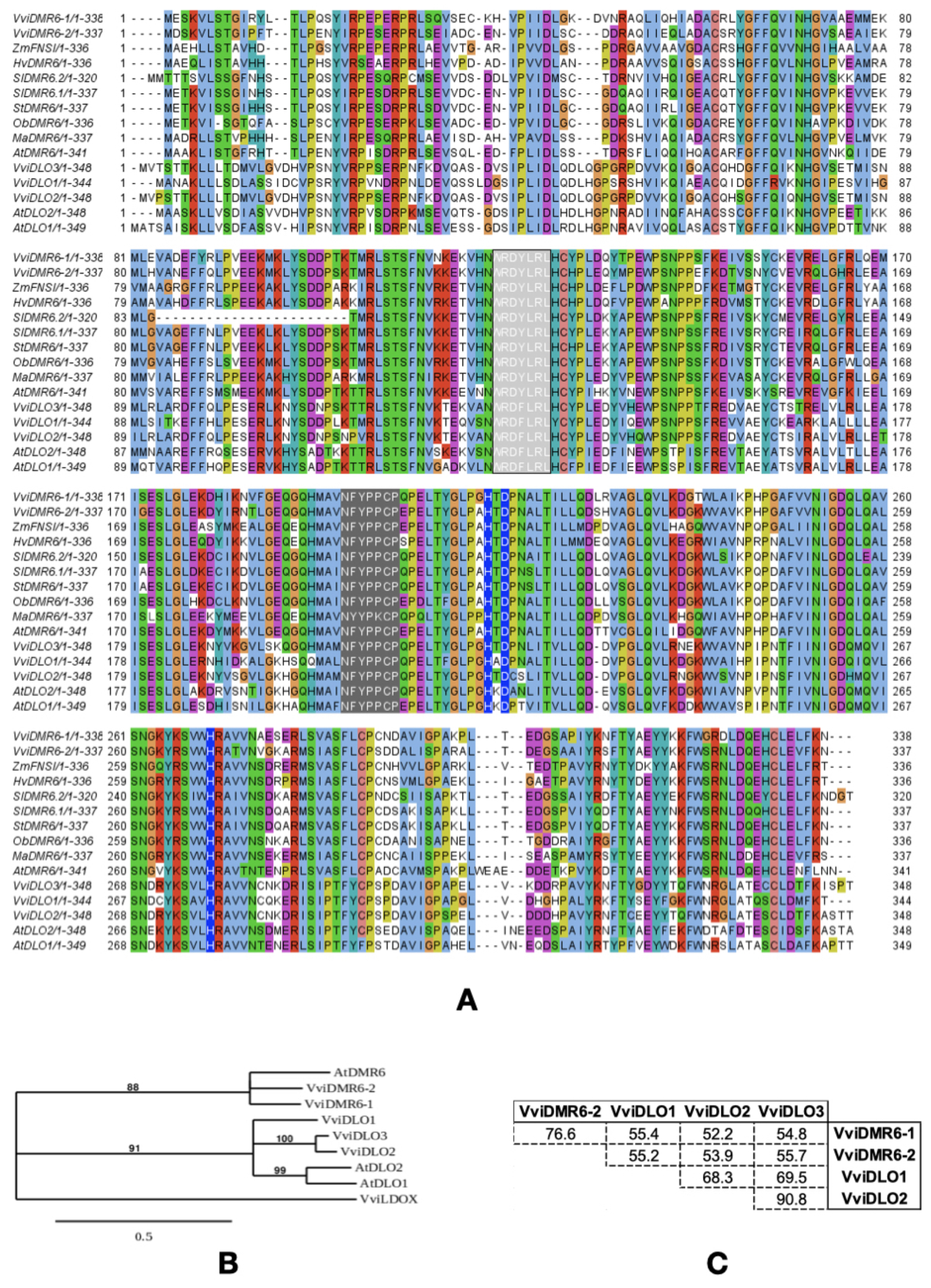
2.1. Multiple-Sequence Alignment and Phylogenetic Analysis
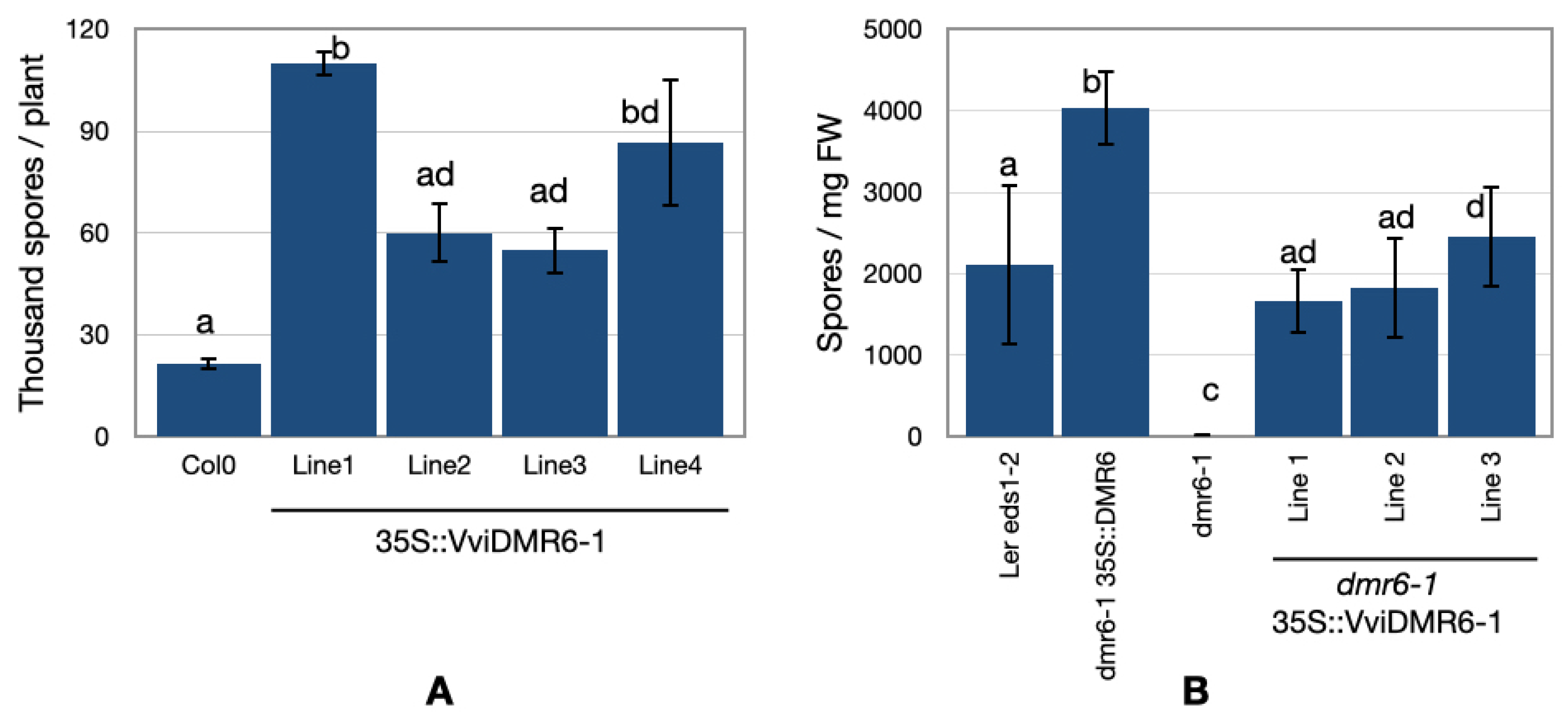
2.2. Overexpression of VviDMR6-1 in Arabidopsis thaliana and Complementation
2.3. cDNA Synthesis and qRT-PCR Analysis
2.4. Gene Expression Analysis in Grapevine Organs
2.5. BTH Treatment
2.6. Analysis in Senescent Leaves
2.7. Plasmopara viticola Inoculation Assays and Gene Expression Analysis
2.8. Local Expression of DMR6 and DLO Genes
2.9. Statistical Analyses
2.10. In Silico Analysis and Investigation Tools
3. Results
3.1. The Grapevine DMR6-DLO Gene Family
3.2. VviDMR6-1 Is an Ortholog of the A. thaliana DMR6 Gene
3.3. Expression of DMR6-DLO Gene Family in Grapevine Tissues
3.4. Induction of DMR6-DLO Genes by BTH and Senescence
3.5. Expression of DMR6-DLO Genes in Different Leaf Sectors under Pathogen Pressure
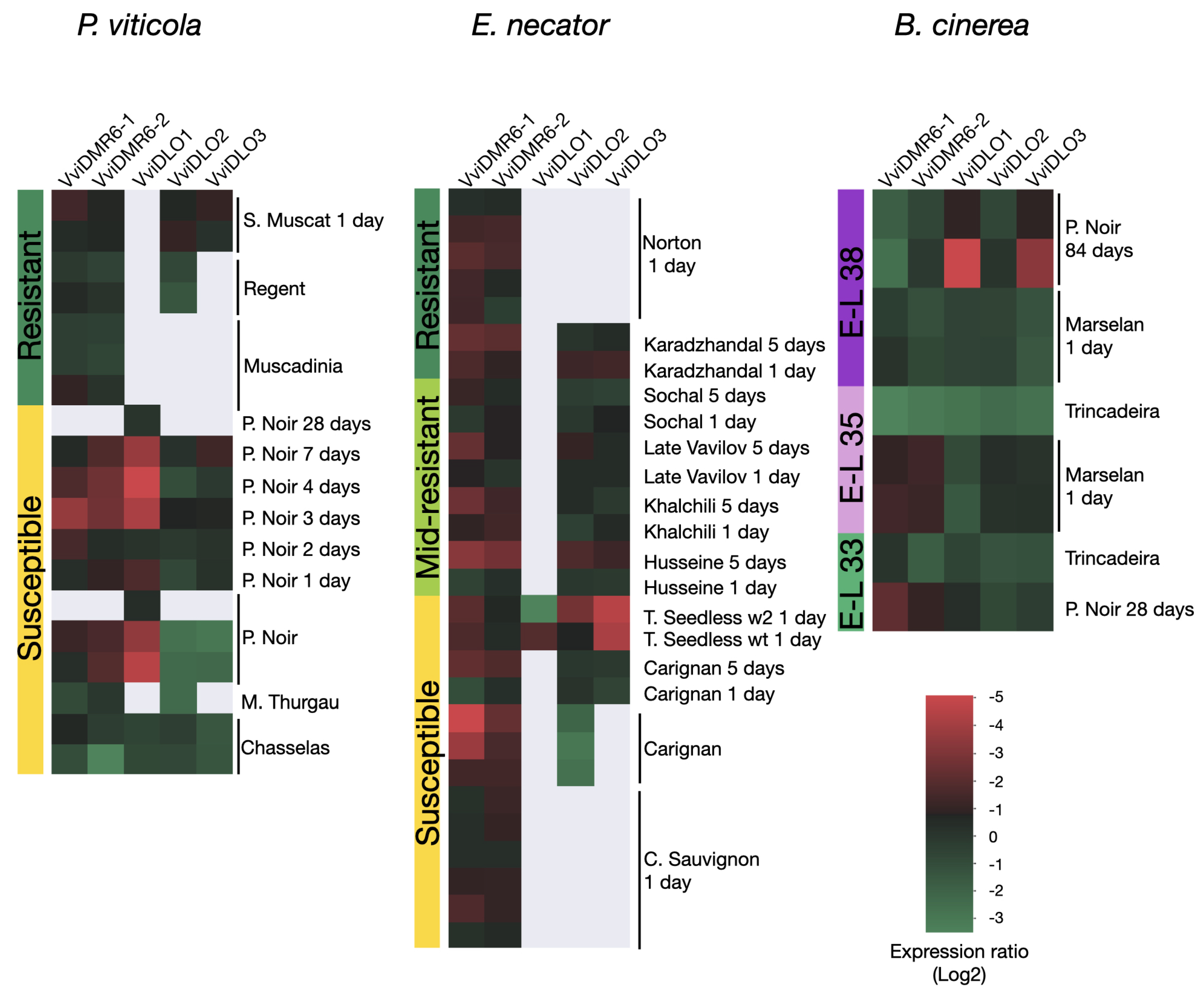
3.6. Gene Expression Meta-Analysis of the Grapevine DMR6 and DLO Genes in Response to Pathogens
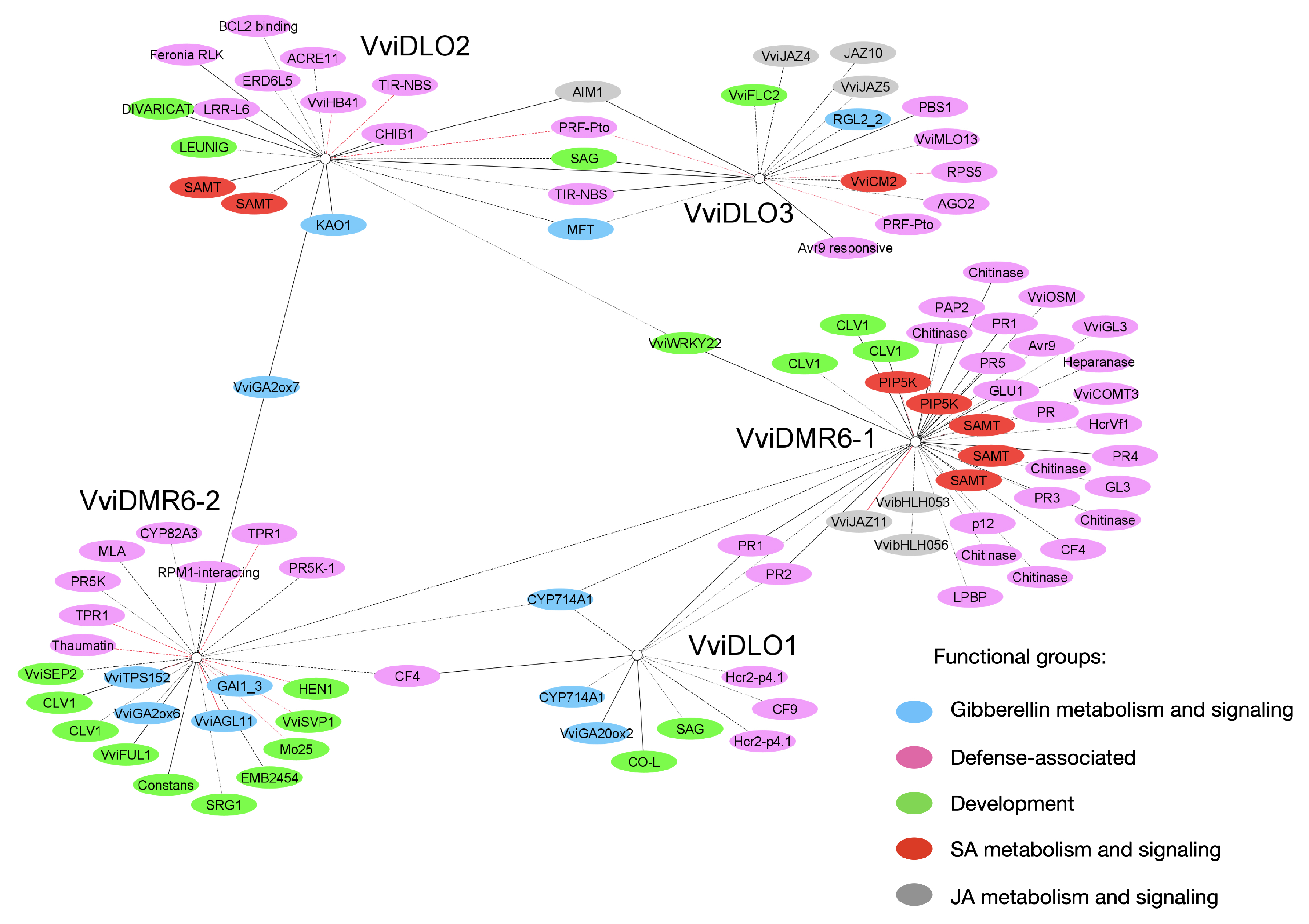
3.7. Gene Network Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Downy mildew |
| BTH | Benzothiadiazole |
| 2OG | 2-Oxoglutarate |
| RH | Relative Humidity |
| NRQ | Normalised Relative Quantity |
| GA | Gibberellin |
References
- Van Schie, C.C.; Takken, F.L. Susceptibility Genes 101: How to Be a Good Host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef]
- Jørgensen, I.H. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica 1992, 63, 141–152. [Google Scholar] [CrossRef]
- Büschges, R.; Hollricher, K.; Panstruga, R.; Simons, G.; Wolter, M.; Frijters, A.; van Daelen, R.; van der Lee, T.; Diergaarde, P.; Groenendijk, J.; et al. The Barley Mlo Gene: A Novel Control Element of Plant Pathogen Resistance. Cell 1997, 88, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Pavan, S.; Zheng, Z.; Zappel, N.F.; Reinstädler, A.; Lotti, C.; De Giovanni, C.; Ricciardi, L.; Lindhout, P.; Visser, R.; et al. Naturally occurring broad-spectrum powdery mildew resistance in a Central American tomato accession is caused by loss of Mlo function. Mol. Plant-Microbe Interact. 2008, 21, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Humphry, M.; Reinstädler, A.; Ivanov, S.; Bisseling, T.; Panstruga, R. Durable broad-spectrum powdery mildew resistance in pea er1 plants is conferred by natural loss-of-function mutations in PsMLO1. Mol. Plant Pathol. 2011, 12, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Nonomura, T.; Appiano, M.; Pavan, S.; Matsuda, Y.; Toyoda, H.; Wolters, A.M.A.; Visser, R.G.; Bai, Y. Loss of Function in Mlo Orthologs Reduces Susceptibility of Pepper and Tomato to Powdery Mildew Disease Caused by Leveillula taurica. PLoS ONE 2013, 8, e70723. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Pessina, S.; Angeli, D.; Martens, S.; Visser, R.G.; Bai, Y.; Salamini, F.; Velasco, R.; Schouten, H.J.; Malnoy, M. The knock-down of the expression of MdMLO19 reduces susceptibility to powdery mildew (Podosphaera leucotricha) in apple (Malus domestica). Plant Biotechnol. J. 2016, 14, 2033–2044. [Google Scholar] [CrossRef]
- Pessina, S.; Lenzi, L.; Perazzolli, M.; Campa, M.; Dalla Costa, L.; Urso, S.; Valè, G.; Salamini, F.; Velasco, R.; Malnoy, M. Knockdown of MLO genes reduces susceptibility to powdery mildew in grapevine. Hortic. Res. 2016, 3, 16016. [Google Scholar] [CrossRef] [Green Version]
- van Damme, M.; Huibers, R.P.; Elberse, J.; Van den Ackerveken, G. Arabidopsis DMR6 encodes a putative 2OG-Fe(II) oxygenase that is defence-associated but required for susceptibility to downy mildew. Plant J. 2008, 54, 785–793. [Google Scholar] [CrossRef]
- Zeilmaker, T.; Ludwig, N.R.; Elberse, J.; Seidl, M.F.; Berke, L.; Van Doorn, A.; Schuurink, R.C.; Snel, B.; Van den Ackerveken, G. DOWNY MILDEW RESISTANT 6 and DMR6-LIKE OXYGENASE 1 are partially redundant but distinct suppressors of immunity in Arabidopsis. Plant J. 2015, 81, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Zhao, L.; Zhao, J.Z.; Li, Y.J.; Wang, J.B.; Guo, R.; Gan, S.S.; Liu, C.J.; Zhanga, K.W. S5H/DMR6 encodes a salicylic acid 5-hydroxylase that fine-tunes salicylic acid homoeostasis. Plant Physiol. 2017, 175, 1082–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomazella, D.P.D.T.; Seong, K.; Mackelprang, R.; Dahlbeck, D.; Geng, Y.; Gill, U.S.; Qi, T.; Pham, J.; Giuseppe, P.; Lee, C.Y.; et al. Loss of function of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026152118. [Google Scholar] [CrossRef]
- Zhang, K.; Halitschke, R.; Yin, C.; Liu, C.J.; Gan, S.S. Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc. Natl. Acad. Sci. USA 2013, 110, 14807–14812. [Google Scholar] [CrossRef] [Green Version]
- Kieu, N.P.; Lenman, M.; Wang, E.S.; Petersen, B.L.; Andreasson, E. Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci. Rep. 2021, 11, 4487. [Google Scholar] [CrossRef]
- Tripathi, J.N.; Ntui, V.O.; Shah, T.; Tripathi, L. CRISPR/Cas9-mediated editing of DMR6 orthologue in banana ( Musa spp.) confers enhanced resistance to bacterial disease. Plant Biotechnol. J. 2021, 19, 1291–1293. [Google Scholar] [CrossRef]
- Hasley, J.A.R.; Navet, N.; Tian, M. CRISPR/Cas9-mediated mutagenesis of sweet basil candidate susceptibility gene ObDMR6 enhances downy mildew resistance. PLoS ONE 2021, 16, e0253245. [Google Scholar] [CrossRef]
- Pirrello, C.; Zeilmaker, T.; Bianco, L.; Giacomelli, L.; Moser, C.; Vezzulli, S. Mining grapevine downy mildew susceptibility genes: A resource for genomics-based breeding and tailored gene editing. Biomolecules 2021, 11, 181. [Google Scholar] [CrossRef]
- Canaguier, A.; Grimplet, J.; Di Gaspero, G.; Scalabrin, S.; Duchêne, E.; Choisne, N.; Mohellibi, N.; Guichard, C.; Rombauts, S.; Le Clainche, I.; et al. A new version of the grapevine reference genome assembly (12X.v2) and of its annotation (VCost.v3). Genom. Data 2017, 14, 56–62. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [Green Version]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.; Karlen, Y.; Bakker, O.; Van den hoff, M.J.; Moorman, A.F. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Coombe, B. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Lenzi, L.; Caruso, C.; Bianchedi, P.L.; Pertot, I.; Perazzolli, M. Laser Microdissection of Grapevine Leaves Reveals Site-Specific Regulation of Transcriptional Response to Plasmopara viticola. Plant Cell Physiol. 2016, 57, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Fasoli, M.; Dal Santo, S.; Zenoni, S.; Tornielli, G.B.; Farina, L.; Zamboni, A.; Porceddu, A.; Venturini, L.; Bicego, M.; Murino, V.; et al. The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 2012, 24, 3489–3505. [Google Scholar] [CrossRef] [Green Version]
- Moretto, M.; Sonego, P.; Pilati, S.; Malacarne, G.; Costantini, L.; Grzeskowiak, L.; Bagagli, G.; Grando, M.S.; Moser, C.; Engelen, K. VESPUCCI: Exploring patterns of gene expression in grapevine. Front. Plant Sci. 2016, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Mckinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 51–56. [Google Scholar]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Pilati, S.; Malacarne, G.; Navarro-Payá, D.; Tomè, G.; Riscica, L.; Cavecchia, V.; Matus, J.T.; Moser, C.; Blanzieri, E. Vitis OneGenE: A Causality-Based Approach to Generate Gene Networks in Vitis vinifera Sheds Light on the Laccase and Dirigent Gene Families. Biomolecules 2021, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Grimplet, J.; Cramer, G.R.; Dickerson, J.A.; Mathiason, K.; van Hemert, J.; Fennell, A.Y. VitisNet: “Omics” Integration through Grapevine Molecular Networks. PLoS ONE 2009, 4, e8365. [Google Scholar] [CrossRef] [Green Version]
- Zeilmaker, T. Functional and Applied Aspects of the DOWNY MILDEW RESISTANT 1 and 6 Genes in Arabidopsis. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2012. [Google Scholar]
- Grimplet, J.; Adam-Blondon, A.F.; Bert, P.F.; Bitz, O.; Cantu, D.; Davies, C.; Delrot, S.; Pezzotti, M.; Rombauts, S.; Cramer, G.R. The grapevine gene nomenclature system. BMC Genom. 2014, 15, 1077. [Google Scholar] [CrossRef] [Green Version]
- Falcone Ferreyra, M.L.; Emiliani, J.; Rodriguez, E.J.; Campos-Bermudez, V.A.; Grotewold, E.; Casati, P. The Identification of Maize and Arabidopsis Type I FLAVONE SYNTHASEs Links Flavones with Hormones and Biotic Interactions. Plant Physiol. 2015, 169, 1090–1107. [Google Scholar] [CrossRef] [Green Version]
- Low, Y.C.; Lawton, M.A.; Di, R. Validation of barley 2OGO gene as a functional orthologue of Arabidopsis DMR6 gene in Fusarium head blight susceptibility. Sci. Rep. 2020, 10, 9935. [Google Scholar] [CrossRef]
- Van Damme, M.; Andel, A.; Huibers, R.P.; Panstruga, R.; Weisbeek, P.J.; Van Den Ackerveken, G. Identification of Arabidopsis loci required for susceptibility to the downy mildew pathogen Hyaloperonospora parasitica. Mol. Plant-Microbe Interact. 2005, 18, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Clemens, M.; Faralli, M.; Varotto, C.; Malnoy, M.; Oechel, W.; Dalla Costa, L. A COMPASS for VESPUCCI: A FAIR way to explore the grapevine transcriptomic landscape. Front. Plant Sci. 2021. manuscript submitted for publication. [Google Scholar]
- Rowland, O.; Ludwig, A.A.; Merrick, C.J.; Baillieul, F.; Tracy, F.E.; Durrant, W.E.; Fritz-Laylin, L.; Nekrasov, V.; Sjölander, K.; Yoshioka, H.; et al. Functional Analysis of Avr9/Cf-9 Rapidly Elicited Genes Identifies a Protein Kinase, ACIK1, That Is Essential for Full Cf-9—Dependent Disease Resistance in Tomato. Plant Cell 2005, 17, 295–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Ackerveken, G.F.; Vossen, P.; De Wit, P.J. The AVR9 Race-Specific Elicitor of Cladosporium fulvum Is Processed by Endogenous and Plant Proteases. Plant Physiol. 1993, 103, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabbage, M.; Kessens, R.; Dickman, M.B. A plant Bcl-2-associated athanogene is proteolytically activated to confer fungal resistance. Microb. Cell 2016, 3, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.M.; Jones, D.A.; Parniske, M.; Harrison, K.; Balint-Kurti, P.J.; Hatzixanthis, K.; Jones, J.D. Characterization of the tomato Cf-4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf-4 and Cf-9. Plant Cell 1997, 9, 2209–2224. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.A.; Thomas, C.M.; Hammond-Kosack, K.E.; Balint-Kurti, P.J.; Jones, J.D. Isolation of the Tomato Cf-9 Gene for Resistance to Cladosporium fulvum by Transposon Tagging. Science 1994, 266, 789–793. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, K.; Zhu, X.; Jiu, S.; Dong, T.; Liu, Z.; Le, G.; Jia, H.; Fang, J. Genome-Wide Identification and Functional Analysis of Chitinase Gene Family in Grape. Res. Sq. 2020. [Google Scholar] [CrossRef] [Green Version]
- Watabane, A.; Nong, V.H.; Zhang, D.; Arahira, M.; Yeboah, N.A.; Udaka, K.; Fukazawa, C. Molecular Cloning and Ethylene-Inducible Expression of Chib1 Chitinase from Soybean (Glycine max (L.) Merr.). Biosci. Biotechnol. Biochem. 1999, 63, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Cui, X.; Lin, S.; Gan, S.; Xing, H.; Dou, D. GmCYP82A3, a Soybean Cytochrome P450 Family Gene Involved in the Jasmonic Acid and Ethylene Signaling Pathway, Enhances Plant Resistance to Biotic and Abiotic Stresses. PLoS ONE 2016, 11, e0162253. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Bidney, D.L.; Yalpani, N.; Duvick, J.P.; Crasta, O.; Folkerts, O.; Lu, G. Overexpression of a Gene Encoding Hydrogen Peroxide-Generating Oxalate Oxidase Evokes Defense Responses in Sunflower. Plant Physiol. 2003, 133. [Google Scholar] [CrossRef] [Green Version]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin Elicits Defense Responses in Grapevine and Induces Protection Against Botrytis cinerea and Plasmopara viticola. Mol. Plant-Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.G.; Schaart, J.G.; Groenwold, R.; Jacobsen, E.; Schouten, H.J.; Krens, F.A. Functional analysis and expression profiling of HcrVf1 and HcrVf2 for development of scab resistant cisgenic and intragenic apples. Plant Mol. Biol. 2011, 75, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckey, C.; Korell, M.; Leib, K.; Biedenkopf, D.; Jansen, C.; Langen, G.; Kogel, K.H. Identification of powdery mildew-induced barley genes by cDNA-AFLP: Functional assessment of an early expressed MAP kinase. Plant Mol. Biol. 2004, 55, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ravensdale, M.; Bernoux, M.; Ve, T.; Kobe, B.; Thrall, P.H.; Ellis, J.G.; Dodds, P.N. Intramolecular Interaction Influences Binding of the Flax L5 and L6 Resistance Proteins to their AvrL567 Ligands. PLoS Pathog. 2012, 8, e1003004. [Google Scholar] [CrossRef]
- Wei, F.; Wing, R.A.; Wise, R.P. Genome Dynamics and Evolution of the Mla (Powdery Mildew) Resistance Locus in Barley. Plant Cell 2002, 14, 1903–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccardi, T.L.; Barthe, G.A.; Derrick, K.S. A novel protein associated with citrus blight has sequence similarities to expansin. Plant Mol. Biol. 1998, 38, 775–783. [Google Scholar] [CrossRef]
- Nakano, M.; Nishihara, M.; Yoshioka, H.; Takahashi, H.; Sawasaki, T.; Ohnishi, K.; Hikichi, Y.; Kiba, A. Suppression of DS1 Phosphatidic Acid Phosphatase Confirms Resistance to Ralstonia solanacearum in Nicotiana benthamiana. PLoS ONE 2013, 8, e75124. [Google Scholar] [CrossRef] [Green Version]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef]
- Kortekamp, A. Expression analysis of defence-related genes in grapevine leaves after inoculation with a host and a non-host pathogen. Plant Physiol. Biochem. 2006, 44, 58–67. [Google Scholar] [CrossRef]
- Jacobs, A.K.; Dry, I.B.; Robinson, S.P. Induction of different pathogenesis-related cDNAs in grapevine infected with powdery mildew and treated with ethephon. Plant Pathol. 1999, 48, 325–336. [Google Scholar] [CrossRef]
- Monteiro, S.; Barakat, M.; Piçarra-Pereira, M.A.; Teixeira, A.R.; Ferreira, R.B. Osmotin and Thaumatin from Grape: A Putative General Defense Mechanism Against Pathogenic Fungi. Phytopathology 2003, 93, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Wu, J.; Zhang, Y.; Agüero, C.B.; Li, X.; Liu, S.; Wang, C.; Walker, M.A.; Lu, J. Overexpression of a thaumatin-like protein gene from Vitis amurensis improves downy mildew resistance in Vitis vinifera grapevine. Protoplasma 2017, 254, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.L.; Girard, I.J.; Giesbrecht, S.; Whyard, S.; Fernando, W.G.D.; de Kievit, T.R.; Belmonte, M.F. Tissue-specific mRNA profiling of the Brassica napus-Sclerotinia sclerotiorum interaction uncovers novel regulators of plant immunity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mucyn, T.S.; Clemente, A.; Andriotis, V.M.; Balmuth, A.L.; Oldroyd, G.E.; Staskawicz, B.J.; Rathjen, J.P. The Tomato NBARC-LRR Protein Prf Interacts with Pto Kinase In Vivo to Regulate Specific Plant Immunity. Plant Cell 2006, 18, 2792–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Guo, D.; Wang, L.; Li, H.; Wang, C.; Guo, X. Functions of RPM1-interacting protein 4 in plant immunity. Planta 2021, 253, 11. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, F.; Zhang, Y.; Cheng, Y.T.; Wiermer, M.; Li, X.; Zhang, Y. Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc. Natl. Acad. Sci. USA 2010, 107, 13960–13965. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhu, X.; Wang, K.; Lu, C.; Luo, M.; Shan, T.; Zhang, Z. A wheat caffeic acid 3-O-methyltransferase TaCOMT-3D positively contributes to both resistance to sharp eyespot disease and stem mechanical strength. Sci. Rep. 2018, 8, 6543. [Google Scholar] [CrossRef]
- Godfrey, D.; Able, A.J.; Dry, I.B. Induction of a Grapevine Germin-Like Protein (VvGLP3) Gene Is Closely Linked to the Site of Erysiphe necator Infection: A Possible Role in Defense? Mol. Plant-Microbe Interact. 2007, 20, 1112–1125. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Nolan, T.M.; Song, G.; Liu, S.; Xie, Z.; Chen, J.; Schnable, P.S.; Walley, J.W.; Yin, Y. FERONIA Receptor Kinase Contributes to Plant Immunity by Suppressing Jasmonic Acid Signaling in Arabidopsis thaliana. Curr. Biol. 2018, 28, 3316–3324. [Google Scholar] [CrossRef] [Green Version]
- Hazak, O.; Hardtke, C.S. CLAVATA 1-type receptors in plant development. J. Exp. Bot. 2016, 67, 4827–4833. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T.; Magome, H.; Hanada, A.; Takeda-Kamiya, N.; Mander, L.N.; Kamiya, Y.; Yamaguchi, S. Functional analysis of arabidopsis CYP714A1 and CYP714A2 reveals that they are distinct gibberellin modification enzymes. Plant Cell Physiol. 2013, 54, 1837–1851. [Google Scholar] [CrossRef] [Green Version]
- Helliwell, C.A.; Chandler, P.M.; Poole, A.; Dennis, E.S.; Peacock, W.J. The CYP88A cytochrome P450, ent-kaurenoic acid oxidase, catalyzes three steps of the gibberellin biosynthesis pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, L.; Rota-Stabelli, O.; Masuero, D.; Acheampong, A.K.; Moretto, M.; Caputi, L.; Vrhovsek, U.; Moser, C. Gibberellin metabolism in Vitis vinifera L. during bloom and fruit-set: Functional characterization and evolution of grapevine gibberellin oxidases. J. Exp. Bot. 2013, 64, 4403–4419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delker, C.; Zolman, B.K.; Miersch, O.; Wasternack, C. Jasmonate biosynthesis in Arabidopsis thaliana requires peroxisomal β-oxidation enzymes—Additional proof by properties of pex6 and aim1. Phytochemistry 2007, 68, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Krinke, O.; Ruelland, E.; Valentovà, O.; Vergnolle, C.; Renou, J.P.; Taconnat, L.; Flemr, M.; Burketovà, L.; Zachowski, A. Phosphatidylinositol 4-Kinase Activation Is an Early Response to Salicylic Acid in Arabidopsis Suspension Cells. Plant Physiol. 2007, 144, 1347–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callard, D.; Axelos, M.; Mazzolini, L. Novel Molecular Markers for Late Phases of the Growth Cycle of Arabidopsis thaliana Cell-Suspension Cultures Are Expressed during Organ Senescence. Plant Physiol. 1996, 112, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Vannozzi, A.; Wang, G.; Liang, Y.H.; Tornielli, G.B.; Zenoni, S.; Cavallini, E.; Pezzotti, M.; Cheng, Z.M. Genome and transcriptome analysis of the grapevine (Vitis vinifera L.) WRKY gene family. Hortic. Res. 2014, 1, 14016. [Google Scholar] [CrossRef] [Green Version]
- Toth, Z.; Winterhagen, P.; Kalapos, B.; Su, Y.; Kovacs, L.; Kiss, E. Expression of a Grapevine NAC Transcription Factor Gene Is Induced in Response to Powdery Mildew Colonization in Salicylic Acid-Independent Manner. Sci. Rep. 2016, 6, 30825. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Brosché, M.; Sipari, N.; Tang, S.; Overmyer, K. Regulation of ABA dependent wound induced spreading cell death by MYB108. New Phytol. 2013, 200, 634–640. [Google Scholar] [CrossRef]
- Alonso-Ramírez, A.; Rodríguez, D.; Reyes, D.; Jiménez, J.A.; Nicolás, G.; López-Climent, M.; Gómez-Cadenas, A.; Nicolás, C. Evidence for a Role of Gibberellins in Salicylic Acid-Modulated Early Plant Responses to Abiotic Stress in Arabidopsis Seeds. Plant Physiol. 2009, 150, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Križnik, M.; Petek, M.; Dobnik, D.; Ramšak, Ž.; Baebler, Š.; Pollmann, S.; Kreuze, J.F.; Žel, J.; Gruden, K. Salicylic Acid Perturbs sRNA-Gibberellin Regulatory Network in Immune Response of Potato to Potato virus Y Infection. Front. Plant Sci. 2017, 8, 2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Gene ID | Name | Functional Annotation | Citation | Subnetwork(s) |
|---|---|---|---|---|
| VIT_11s0016g04840 | ACRE11 (Avr9-Cf9 Rapidly Elicited) | DEF | [44] | DLO2 |
| VIT_10s0042g01180 | AGO2 (ARGONAUTE 2) | DEF | DLO3 | |
| VIT_07s0031g03090 | Avr9 (Elicitor Protein) | DEF | [45] | DMR6-1 |
| VIT_18s0001g04150 | Avr9 Responsive | DEF | DLO3 | |
| VIT_10s0003g04270 | BCL2 Binding Anthogene | DEF | [46] | DLO2 |
| VIT_18s0089g00650 | CF4 | DEF | [47] | DMR6-1 |
| VIT_19s0027g01230 | CF4 | DEF | [47] | DMR6-2, DLO1 |
| VIT_19s0085g00160 | CF9 | DEF | [48] | DLO1 |
| VIT_04s0008g00140 | CHI28 (Chitinase) | DEF | DMR6-1 | |
| VIT_05s0094g00300 | Chitinase | DEF | DMR6-1 | |
| VIT_05s0094g00320 | Chitinase | DEF | DMR6-1 | |
| VIT_05s0094g00330 | Chitinase | DEF | DMR6-1 | |
| VIT_05s0094g00340 | Chitinase | DEF | [49] | DMR6-1 |
| VIT_16s0050g02230 | Chitinase | DEF | DMR6-1 | |
| VIT_15s0046g01600 | CHIB1 (Chitinase) | DEF | [50] | DLO2 |
| VIT_18s0001g11470 | CYP82A3 | DEF | [51] | DMR6-2 |
| VIT_14s0030g00220 | ERD6L5 (Sugar Transporter) | DEF | DLO2 | |
| VIT_14s0060g02760 | GL3 (Germin-Like 3) | DEF | [52] | DMR6-1 |
| VIT_05s0077g01150 | GLU1 (Beta-1;3-Glucanase) | DEF | [53] | DMR6-1 |
| VIT_06s0004g00310 | Hcr2-p4.1 | DEF | DLO1 | |
| VIT_06s0004g00330 | Hcr2-p4.1 | DEF | DLO1 | |
| VIT_01s0010g03210 | HcrVf1 | DEF | [54] | DMR6-1 |
| VIT_11s0118g00420 | Heparanase | DEF | [55] | DMR6-1 |
| VIT_16s0100g00270 | LPBP (Peptidoglycan Binding) | DEF | DMR6-1 | |
| VIT_18s0041g00190 | LRR-L6 | DEF | [56] | DLO2 |
| VIT_18s0001g11250 | MLA | DEF | [57] | DMR6-2 |
| VIT_11s0016g04720 | P12 (Blight Associated) | DEF | [58] | DMR6-1 |
| VIT_06s0004g05800 | PAP2 | DEF | [59] | DMR6-1 |
| VIT_01s0146g00250 | PBS1 (avrPphB Susceptible 1) | DEF | DLO3 | |
| VIT_19s0014g01180 | PR (Pathogenesis Related) | DEF | DMR6-1 | |
| VIT_03s0088g00780 | PR1 | DEF | [60] | DMR6-1 |
| VIT_03s0088g00810 | PR1 | DEF | [60] | DMR6-1, DLO1 |
| VIT_08s0007g06060 | PR2 (Beta-1;3-Glucanase) | DEF | [61,62] | DMR6-1, DLO1 |
| VIT_04s0008g00120 | PR3 (Chitinase, CHI27) | DEF | [61,62] | DMR6-1 |
| VIT_14s0081g00030 | PR4 | DEF | [61] | DMR6-1 |
| VIT_02s0025g04320 | PR5 (Thaumatin-Like) | DEF | [63,64] | DMR6-1 |
| VIT_01s0011g03900 | PR5K | DEF | [65] | DMR6-2 |
| VIT_17s0000g03320 | PR5K-1 | DEF | [65] | DMR6-2 |
| VIT_03s0038g01520 | PRF-Pto | DEF | [66] | DLO2, DLO3 |
| VIT_15s0046g02810 | PRF-Pto | DEF | [66] | DLO3 |
| VIT_00s0231g00040 | RPM1-Interacting Protein 4 | DEF | [67] | DMR6-2 |
| VIT_09s0002g05070 | RPS5 | DEF | DLO3 | |
| VIT_03s0038g02160 | Thaumatin | DEF | DMR6-2 | |
| VIT_18s0117g00400 | TIR-NBS | DEF | DLO2 | |
| VIT_18s0001g06160 | TIR-NBS | DEF | DLO2, DLO3 | |
| VIT_04s0008g06390 | TPR1 (Topless-Related 1) | DEF | [68] | DMR6-2 |
| VIT_04s0008g06400 | TPR1 (Topless-Related 1) | DEF | [68] | DMR6-2 |
| VIT_16s0098g00850 | VviCOMT3 (Caffeic Acid O-Methyltransferase) | DEF | [69] | DMR6-1 |
| VIT_14s0128g00690 | VviGL3 (Germin-Like) | DEF | [52,70] | DMR6-1 |
| VIT_11s0103g00650 | VviHB41 | DEF | DLO2 | |
| VIT_06s0004g03100 | VviMLO13 | DEF | DLO3 | |
| VIT_02s0025g04280 | VviOSM (Osmotin-Like) | DEF | [61,64] | DMR6-1 |
| VIT_01s0244g00090 | FERONIA RLK (FERONIA Receptor-Like Kinase) | DEF, JA | [71] | DLO2 |
| VIT_04s0008g00310 | CLV1 (CLAVATA) | DEV | [72] | DMR6-1 |
| VIT_04s0008g00370 | CLV1 (CLAVATA) | DEV | [72] | DMR6-1 |
| VIT_04s0008g00410 | CLV1 (CLAVATA) | DEV | [72] | DMR6-1 |
| VIT_04s0008g00390 | CLV1 (CLAVATA) | DEV | [72] | DMR6-2 |
| VIT_04s0008g00440 | CLV1 (CLAVATA) | DEV | [72] | DMR6-2 |
| VIT_01s0011g03820 | EMB2454 (Embryo Defective) | DEV | DMR6-2 | |
| VIT_17s0000g01640 | EMB2746 (Embryo Defective) | DEV | DLO2 | |
| VIT_19s0090g01550 | Mo25 | DEV | DMR6-2 | |
| VIT_12s0059g02500 | CONSTANS | f-DEV | DLO1 | |
| VIT_07s0104g01360 | CONSTANS | f-DEV | DMR6-2 | |
| VIT_19s0015g01170 | DIVARICATA | f-DEV | DLO2 | |
| VIT_10s0003g05070 | HEN1 (HUA Enhancer 1) | f-DEV | DMR6-2 | |
| VIT_07s0005g06380 | LEUNIG | f-DEV | DLO2 | |
| VIT_14s0068g01800 | VviFLC2 (SEPALLATA 3) | f-DEV | DLO3 | |
| VIT_17s0000g04990 | VviFUL1 (APETALA 1) | f-DEV | DMR6-2 | |
| VIT_17s0000g05000 | VviSEP2 (SEPALLATA 1) | f-DEV | DMR6-2 | |
| VIT_00s0313g00070 | VviSVP1 (Short Vegetative Phase 1) | f-DEV | DMR6-2 | |
| VIT_03s0167g00190 | CYP714A1 (GA Carboxylase) | GA | [73] | DLO1 |
| VIT_18s0089g00700 | CYP714A1 (GA Carboxylase) | GA | [73] | DMR6-1, DMR6-2, DLO1 |
| VIT_01s0011g05260 | GAI1_3 (DELLA Protein) | GA | DMR6-2 | |
| VIT_06s0004g05050 | KAO1 (Ent-Kaurenoic Acid oxidase) | GA | [74] | DLO2 |
| VIT_00s0203g00080 | MFT (Mother of Flowering Locus T) | GA | DLO2, DLO3 | |
| VIT_07s0005g01500 | RGL2_2 (DELLA RGA-Like 2) | GA | DLO3 | |
| VIT_18s0041g01880 | VviAGL11 (SEEDSTICK) | GA | DMR6-2 | |
| VIT_04s0044g01650 | VviGA20ox2 | GA | [75] | DLO1 |
| VIT_19s0177g00030 | VviGA2ox6 | GA | [75] | DMR6-2 |
| VIT_10s0116g00410 | VviGA2ox7 | GA | [75] | DMR6-2, DLO2 |
| VIT_19s0085g00830 | VviTPS152 (Ent-Kaurene Synthase) | GA | [74] | DMR6-2 |
| VIT_13s0047g00450 | VvibHLH053 | JA | DMR6-1 | |
| VIT_13s0073g00400 | VvibHLH056 | JA | DMR6-1 | |
| VIT_01s0146g00480 | JAZ10 | JA | DLO3 | |
| VIT_17s0000g02230 | VviJAZ11 | JA | DMR6-1 | |
| VIT_09s0002g00890 | VviJAZ4 | JA | DLO3 | |
| VIT_10s0003g03790 | VviJAZ5 | JA | DLO3 | |
| VIT_11s0016g03690 | AIM1 | JA, SA | [76] | DLO2, DLO3 |
| VIT_02s0012g00090 | PIP5K (Phosphatidylinositol 4-Phosphate 5-Kinase) | SA | [77] | DMR6-1 |
| VIT_08s0007g04700 | PIP5K (Phosphatidylinositol 4-Phosphate 5-Kinase) | SA | [77] | DMR6-1 |
| VIT_01s0011g05890 | SAMT | SA | DMR6-1 | |
| VIT_12s0057g01070 | SAMT | SA | DMR6-1 | |
| VIT_17s0000g02870 | SAMT | SA | [78] | DMR6-1 |
| VIT_04s0023g02260 | SAMTBSCMT | SA | DLO2 | |
| VIT_18s0001g12900 | SAMTBSCMT | SA | DLO2 | |
| VIT_04s0008g06570 | VviCM2 (Chorismate Mutase) | SA | DLO3 | |
| VIT_14s0006g01210 | SAG (Senescence-Associated Gene) | SEN | DLO1 | |
| VIT_09s0002g00420 | SAG (Senescence-Associated Gene) | SEN | DLO2, DLO3 | |
| VIT_05s0020g01310 | SRG1 (Senescence Related Gene) | SEN | [79] | DMR6-2 |
| VIT_07s0031g01710 | VviWRKY22 | SEN | [80] | DMR6-1, DLO2 |
| VIT_05s0077g00500 | VviMYB108A | [81,82] | DLO1 | |
| VIT_04s0008g05760 | VviWRKY08 | [80] | DLO1 | |
| VIT_08s0058g01390 | VviWRKY25 | [80] | DLO1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirrello, C.; Malacarne, G.; Moretto, M.; Lenzi, L.; Perazzolli, M.; Zeilmaker, T.; Van den Ackerveken, G.; Pilati, S.; Moser, C.; Giacomelli, L. Grapevine DMR6-1 Is a Candidate Gene for Susceptibility to Downy Mildew. Biomolecules 2022, 12, 182. https://doi.org/10.3390/biom12020182
Pirrello C, Malacarne G, Moretto M, Lenzi L, Perazzolli M, Zeilmaker T, Van den Ackerveken G, Pilati S, Moser C, Giacomelli L. Grapevine DMR6-1 Is a Candidate Gene for Susceptibility to Downy Mildew. Biomolecules. 2022; 12(2):182. https://doi.org/10.3390/biom12020182
Chicago/Turabian StylePirrello, Carlotta, Giulia Malacarne, Marco Moretto, Luisa Lenzi, Michele Perazzolli, Tieme Zeilmaker, Guido Van den Ackerveken, Stefania Pilati, Claudio Moser, and Lisa Giacomelli. 2022. "Grapevine DMR6-1 Is a Candidate Gene for Susceptibility to Downy Mildew" Biomolecules 12, no. 2: 182. https://doi.org/10.3390/biom12020182






