Neural Differentiation of Human Dental Mesenchymal Stem Cells Induced by ATRA and UDP-4: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Culture Conditions
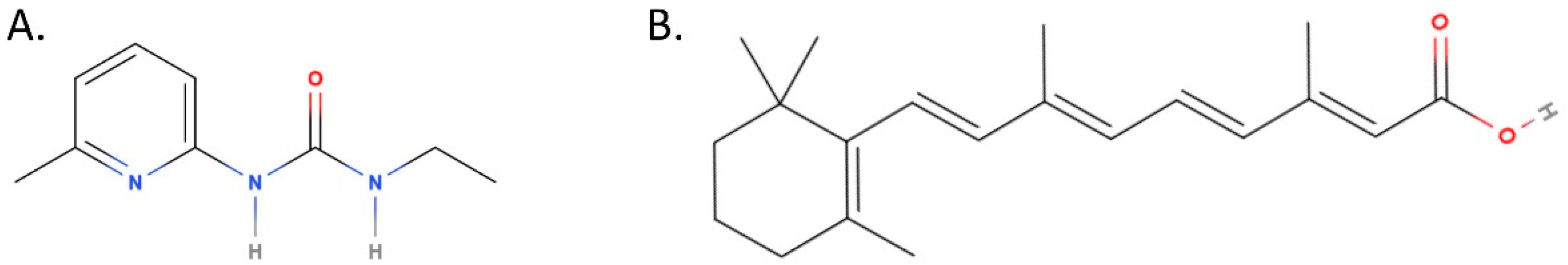
2.2. Compounds
2.3. Evaluation of Gene Expression with RT–PCR
- ENO2 (NM_001975.2)–Sense: TGTGCACAGGCCAGATCAAG
- // Antisense: GTGCGGAACCCCAATGAGTA and Ta = 60 °C
- NF1 (NM_001042492.2)–Sense: AACGAGTGTCTCATGGGCAG
- // Antisense: GTGCAGAGGCGAGTCCTTAT and Ta = 60 °C
- GAPDH (NM_002046.5)–Sense: GAAGGTGAAGGTCGGAGT
- // Antisense: GAAGATGGTGATGGGATTTC and Ta = 56 °C
- TH (NM_000360.3)–Sense: CCAAGTTCGACCCTGACCTG
- // Antisense: CAATGTCCTGCGAGAACTGC and Ta = 58 °C
- CHAT (NM_020549.4)–Sense: TGGGCTCTTCTCCTCCTACC
- // Antisense: CCGGTTGGTGGAGTCTTTCA and Ta = 58 °C
- SLC32A1 (NM_080552.2)–Sense: GCCATCCAGGGCATGTTCG
- // Antisense: AGCTCGATGATCTGCGCTAC and Ta = 58 °C
2.4. Immunocytochemistry
2.5. Statistical Analysis
3. Results
3.1. Treatment of SCAP with ATRA or UDP-4 Affects Cell Growth and Viability
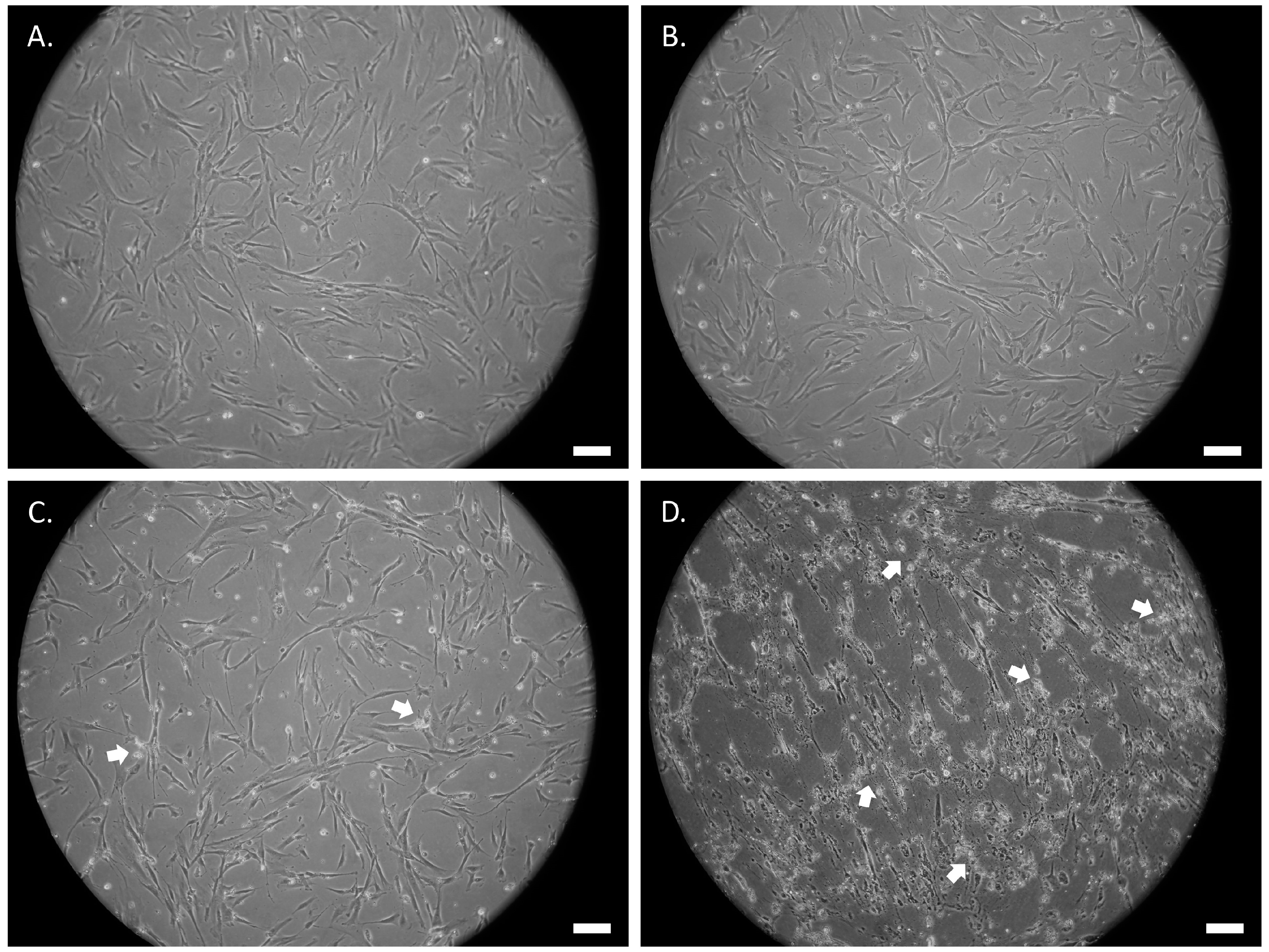
3.2. ATRA or UDP-4 Treatment Changes Cellular Morphology into Neural Phenotypes
3.3. UDP-4 Treatment Increases Neuronal-Specific Gene Expression of ENO2 and NF1
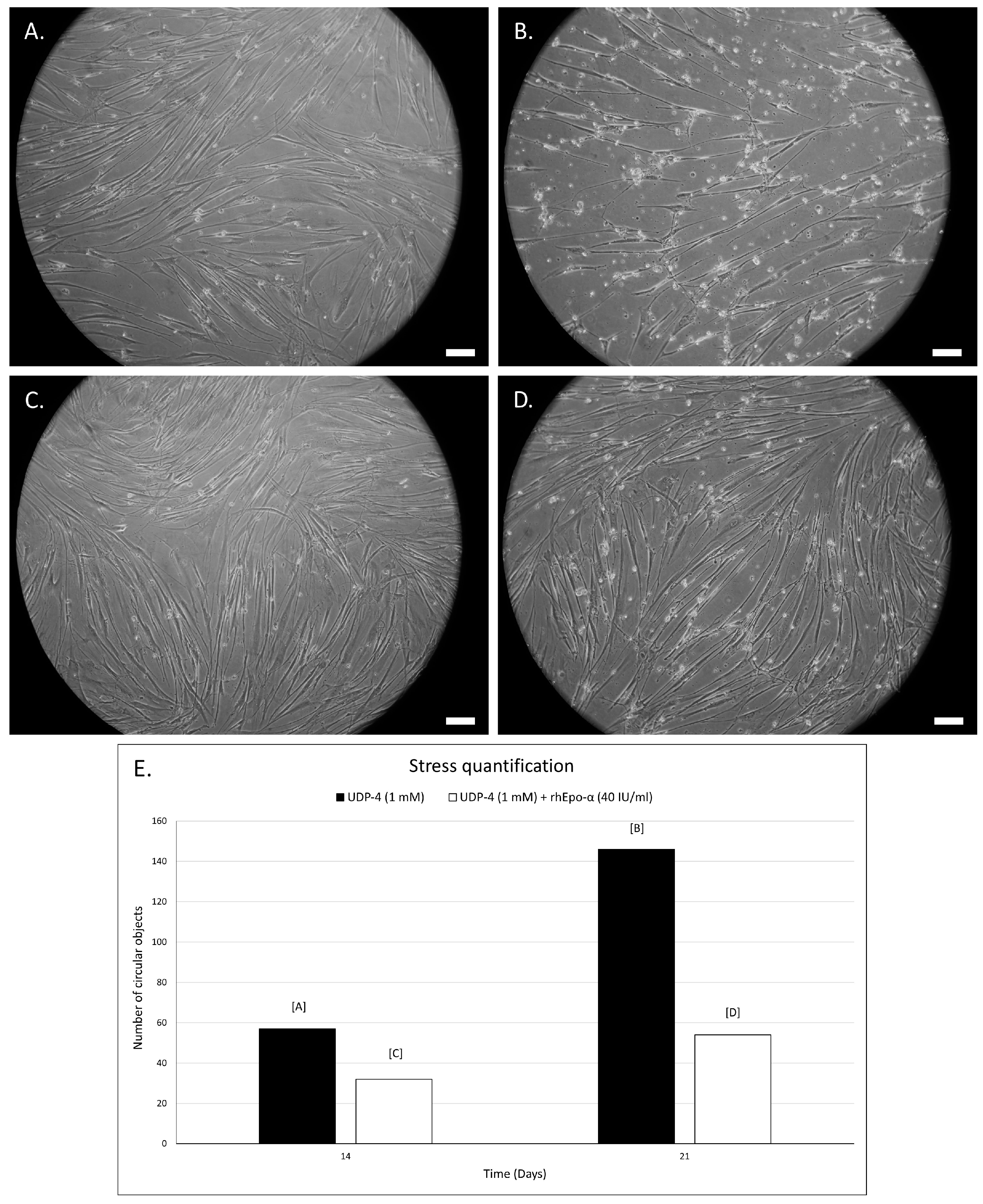
3.4. Erythropoietin Increases Survival of UDP-4 Treated Cells
3.5. UDP-4 Treatment Increases the Neuronal-Specific Expression of GFAP and NeuN
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mason, C.; Dunhill, A. Brief Definition of Regenerative Medicine: Editorial. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Committee for Advanced Therapies (CAT); Schneider, C.K.; Salmikangas, P.; Jilma, B.; Flamion, B.; Todorova, L.R.; Paphitou, A.; Haunerova, I.; Maimets, T.; Trouvin, J.-H.; et al. Challenges with advanced therapy medicinal products and how to meet them. Nat. Rev. Drug Discov. 2010, 9, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Kritis, A.; Andreadis, D.; Papachristou, E.; Leyhausen, G.; Koidis, P.; Geurtsen, W.; Tsiftsoglou, A. Angiogenic Potential and Secretome of Human Apical Papilla Mesenchymal Stem Cells in Various Stress Microenvironments. Stem Cells Dev. 2015, 24, 2496–2512. [Google Scholar] [CrossRef] [Green Version]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult Human Dental Pulp Stem Cells Differentiate Toward Functionally Active Neurons Under Appropriate Environmental Cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; He, Y.; Wang, X.; Key, B.; Lee, B.H.; Li, H.; Ye, Q. Potential Roles of Dental Pulp Stem Cells in Neural Regeneration and Repair. Stem Cells Int. 2018, 2018, 1731289. [Google Scholar] [CrossRef] [Green Version]
- Solis-Castro, O.O.; Boissonade, F.M.; Rivolta, M.N. Establishment and neural differentiation of neural crest-derived stem cells from human dental pulp in serum-free conditions. Stem Cells Transl. Med. 2020, 9, 1462–1476. [Google Scholar] [CrossRef]
- Varga, G.; Gerber, G. Mesenchymal stem cells of dental origin as promising tools for neuroregeneration. Stem Cell Res. Ther. 2014, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Kakkar, A.; Sharma, R.; Kharbanda, O.P.; Monga, N.; Kumar, M.; Chowdhary, S.; Airan, B.; Mohanty, S. Synergistic Effect of BDNF and FGF2 in Efficient Generation of Functional Dopaminergic Neurons from human Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 10378. [Google Scholar] [CrossRef] [Green Version]
- Urrutia, D.N.; Caviedes, P.; Mardones, R.; Minguell, J.J.; Vega-Letter, A.M.; Jofre, C.M. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS ONE 2019, 14, e0213032. [Google Scholar] [CrossRef] [PubMed]
- Subirós, N.; del Barco, D.G.; Coro-Antich, R.M. Erythropoietin: Still on the neuroprotection road. Ther. Adv. Neurol. Disord. 2012, 5, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. [Google Scholar] [CrossRef]
- Koutsoumparis, A.; Vassili, A.; Bakopoulou, A.; Ziouta, A.; Tsiftsoglou, A.S. Erythropoietin (rhEPOa) promotes endothelial transdifferentiation of stem cells of the apical papilla (SCAP). Arch. Oral Biol. 2018, 96, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Pappas, I.S.; Ioannis, N.; Tsiftsoglou, A.S. Ureido Derivatives of Pyridine: A New Class of Inducers of Murine Erythroleu-kemia Cell Differentiation. Anti-Cancer Drug Des. 1992, 7, 153–161. [Google Scholar]
- Pappas, I.S.; Lambris, J.D.; Vizirianakis, I.; Winters, M.S.; Tsiftsoglou, A.S. Mechanisms of Action of Differentiation Inducers: Detection of Inducer Binding Protein(s) in Murine Erythroleukemia Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2005, 15, 21–37. [Google Scholar] [CrossRef]
- Allenby, G.; Janocha, R.; Kazmer, S.; Speck, J.; Grippo, J.; Levin, A. Binding of 9-cis-retinoic acid and all-trans-retinoic acid to retinoic acid receptors alpha, beta, and gamma. Retinoic acid receptor gamma binds all-trans-retinoic acid preferentially over 9-cis-retinoic acid. J. Biol. Chem. 1994, 269, 16689–16695. [Google Scholar] [CrossRef]
- Guan, K.; Chang, H.; Rolletschek, A.; Wobus, A.M. Embryonic stem cell-derived neurogenesis. Cell Tissue Res. 2001, 305, 171–176. [Google Scholar] [CrossRef]
- Lenz, M.; Kruse, P.; Eichler, A.; Straehle, J.; Beck, J.; Deller, T.; Vlachos, A. All-trans retinoic acid induces synaptic plasticity in human cortical neurons. eLife 2021, 10, e63026. [Google Scholar] [CrossRef]
- Dräger, U.C. Retinoic Acid Signaling in the Functioning Brain. Sci. STKE 2006, 2006, pe10. [Google Scholar] [CrossRef] [PubMed]
- Pappas, I.S.; Sophianos, D.; Tzartos, S.; Tsiftsoglou, A.S. Expression of Memory, Differentiation, and Repression in c-myc and p53 Genes in Human RD/TE-671 Cells Inuded by a Ureido-Derivative of Pyridine (UDP-4). Cell Growth Differ. 1996, 7, 797–809. [Google Scholar] [PubMed]
- Marangos, P.J.; Schmechel, D.E. Neuron Specific Enolase, A Clinically Useful Marker For Neurons And Neuroendocrine Cells. Annu. Rev. Neurosci. 1987, 10, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Lee, P.S.; Oka, K.; Levin, V.A.; Tanase, S.; Morino, Y.; Saya, H. Differential expression of two types of the neurofibromatosis type 1 (NF1) gene transcripts related to neuronal differentiation. Oncogene 1991, 6, 1555–1559. [Google Scholar] [PubMed]
- Bekiari, C.; Grivas, I.; Tsingotjidou, A.; Papadopoulos, G.C. Adult neurogenesis and gliogenesis in the dorsal and ventral canine hippocampus. J. Comp. Neurol. 2019, 528, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Wang, D.J.; Yang, Y.; He, Y. Neuroimaging of Brain Networks and Function. BioMed Res. Int. 2015, 2015, 509141. [Google Scholar] [CrossRef]
- Brazelton, T.R.; Rossi, F.M.V.; Keshet, G.I.; Blau, H.M. From Marrow to Brain: Expression of Neuronal Phenotypes in Adult Mice. Science 2000, 290, 1775–1779. [Google Scholar] [CrossRef] [Green Version]
- Messam, C.A.; Hou, J.; Major, E.O. Coexpression of Nestin in Neural and Glial Cells in the Developing Human CNS Defined by a Human-Specific Anti-nestin Antibody. Exp. Neurol. 2000, 161, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Um, S.; Song, I.-S.; Kim, H.Y.; Seo, B.M. Neurogenic differentiation of human dental stem cellsin vitro. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Bi, Y.; Jiang, W.; Zhang, Y.; Chen, L.; Hou, N.; Chen, J.; Li, T. Retinoic acid receptor beta mediates all-trans retinoic acid facilitation of mesenchymal stem cells neuronal differentiation. Int. J. Biochem. Cell Biol. 2013, 45, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Wiskott, L.; Gage, F.H. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004, 14, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A.; Alvarez-Buylla, A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, A.; Arranz, L. Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cell. Mol. Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Concise Review: Dental Pulp Stem Cells: A Novel Cell Therapy for Retinal and Central Nervous System Repair. Stem Cells 2016, 35, 61–67. [Google Scholar] [CrossRef] [Green Version]




| GFAP | NeuN | ||
|---|---|---|---|
| Untreated | + | +/− | |
| UDP-4 | 1 week | + | +/− |
| 2 weeks | + | + | |
| 3 weeks | ++ | ++ | |
| 4 weeks | +++ | ++ | |
| ATRA | 1 week | + | +/− |
| 2 weeks | + | +/− | |
| 3 weeks | ++ | +/− | |
| 4 weeks | +/− | +/− | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutsoumparis, A.E.; Patsiarika, A.; Tsingotjidou, A.; Pappas, I.; Tsiftsoglou, A.S. Neural Differentiation of Human Dental Mesenchymal Stem Cells Induced by ATRA and UDP-4: A Comparative Study. Biomolecules 2022, 12, 218. https://doi.org/10.3390/biom12020218
Koutsoumparis AE, Patsiarika A, Tsingotjidou A, Pappas I, Tsiftsoglou AS. Neural Differentiation of Human Dental Mesenchymal Stem Cells Induced by ATRA and UDP-4: A Comparative Study. Biomolecules. 2022; 12(2):218. https://doi.org/10.3390/biom12020218
Chicago/Turabian StyleKoutsoumparis, Anastasios E., Anastasia Patsiarika, Anastasia Tsingotjidou, Ioannis Pappas, and Asterios S. Tsiftsoglou. 2022. "Neural Differentiation of Human Dental Mesenchymal Stem Cells Induced by ATRA and UDP-4: A Comparative Study" Biomolecules 12, no. 2: 218. https://doi.org/10.3390/biom12020218







