Effects of Phytohormone-Producing Rhizobacteria on Casparian Band Formation, Ion Homeostasis and Salt Tolerance of Durum Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Bacterial Strains and Culture Media
2.2. Experimental Design
2.3. Elemental Analysis
2.4. Determination of Hormones
2.5. Measurement of Photosynthetic Activity
2.6. Visualization of Lignin and Suberin
2.7. Statistics
3. Results
3.1. Lignifications of Xylem Cell Walls and Formation of Casparian Bands
3.2. Effects of Salinity and Bacteria on the Mass of Plants
3.3. Effects of Bacteria on the Hormone Concentration in the Plants
3.4. Elemental Analysis
4. Discussion
4.1. Effect of Salt Stress and Bacterial Inoculation on Sodium Concentration in Wheat Plants and Its Relation to Formation of Apoplast Barriers
4.2. Relative Importance of Bacterial Control of Na, K and P Levels for Promotion of Salt-Stressed Plant Growth and Its Possible Dependence on Bacterial Effects on Plant Hormones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lambers, H. Introduction, dryland salinity: A key environmental issue in southern Australia. Plant Soil 2003, 257, 5–7. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, A.K.; Basak, N.; Sundha, P. Saline and Sodic Ecosystems in the Changing World. In Soil Science: Fundamentals to Recent Advances; Rakshit, A., Singh, S., Abhilash, P., Biswas, A., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Ruzzia, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Jabborova, D.; Räsänen, L.A.; Liao, H. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J. Plant Interact. 2017, 12, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant. Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Chandran, H.; Meena, M.; Swapnil, P. Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture. Sustainability 2021, 13, 10986. [Google Scholar] [CrossRef]
- Kumar, M.; Giri, V.P.; Pandey, S.; Gupta, A.; Patel, M.K.; Bajpai, A.B.; Jenkins, S.; Siddique, K.H.M. Plant-Growth-Promoting Rhizobacteria Emerging as an Effective Bioinoculant to Improve the Growth, Production, and Stress Tolerance of Vegetable Crops. Int. J. Mol. Sci. 2021, 22, 12245. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40(1), 326–345. [Google Scholar] [CrossRef] [Green Version]
- Bomle, D.V.; Kiran, A.; Kumar, J.K.; Nagaraj, L.S.; Pradeep, C.K.; Ansari, M.A.; Alghamdi, S.; Kabrah, A.; Assaggaf, H.; Dablool, A.S.; et al. Plants Saline Environment in Perception with Rhizosphere Bacteria Containing 1-Aminocyclopropane1-Carboxylate Deaminase. Int. J. Mol. Sci. 2021, 22, 11461. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, A.; Rubio, F. High-affinity potassium and sodium transport systems in plants. J. Exp. Bot. 2006, 57, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Yousefirad, S.; Soltanloo, H.; Ramezanpour, S.S.; Nezhad, K.Z.; Shariati, V. The RNA-seq transcriptomic analysis reveals genes mediating salt tolerance through rapid triggering of ion transporters in a mutant barley. PLoS ONE 2020, 15, e0229513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilev, S. Plant-growth-promoting bacteria mitigating soil salinity stress in plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Mellidou, I.; Ainalidou, A.; Papadopoulou, A.; Leontidou, K.; Genitsaris, S.; Karagiannis, E.; Van de Poel, B.; Karamanoli, K. Comparative Transcriptomics and Metabolomics Reveal an Intricate Priming Mechanism Involved in PGPR-Mediated Salt Tolerance in Tomato. Front. Plant Sci. 2021, 12, 713984. [Google Scholar] [CrossRef]
- Chen, T.; Cai, X.; Wu, X.; Karahara, I.; Schreiber, L.; Lin, J. Casparian strip development and its potential function in salt tolerance. Plant Signal. Behav. 2011, 6, 1499–1502. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Cui, B.; Liu, R.; Flowers, T.J.; Song, J. Casparian bands and suberin lamellae: Key targets for breeding salt tolerant crops? Environ. Exp. Bot. 2021, 191, 104600. [Google Scholar] [CrossRef]
- Verbon, E.H.; Liberman, L.M. Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Qin, X.; Qi, J.; Dou, W.; Dunand, C.; Chen, S.; He, Y. CsPrx25, a class III peroxidase in Citrus sinensis, confers resistance to citrus bacterial canker through the maintenance of ROS homeostasis and cell wall lignification. Hort. Res. 2020, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.E.; Albacete, A.; Smigocki, A.C.; Frebort, I.; Pospısilova, H.; Martınez-Andujar, C.; Acosta, M.; Sanchez-Bravo, J.; Lutts, S.; Dodd, I.C.; et al. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2011, 62, 125–140. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Melentiev, A.I.; Martynenko, E.V.; Arkhipova, T.N.; Shendel, G.V.; Kuz’mina, L.Y.; Dodd, I.C.; Veselov, S.Y. Cytokinin producing bacteria stimulates amino acid deposition by wheat roots. Plant Physiol. Biochem. 2014, 83, 285–291. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Arkhipova, T.N.; Kuzmina, L.Y.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants. Acta Physiol. Plant. 2017, 39, 253. [Google Scholar] [CrossRef]
- Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of plant growth promoting rhizobacteria on the content of abscisic acid and salt resistance of wheat plants. Plants 2020, 9, 1429. [Google Scholar] [CrossRef]
- Belimov, A.A.; Shaposhnikov, A.I.; Syrova, D.S.; Kichko, A.A.; Guro, P.V.; Yuzikhin, O.S.; Azarova, T.S.; Sazanova, A.L.; Sekste, E.A.; Litvinskiy, V.A.; et al. The Role of Symbiotic Microorganisms, Nutrient Uptake and Rhizosphere Bacterial Community in Response of Pea (Pisum sativum L.). Genotypes Elev. Conc. Soil Plants 2020, 9, 1801. [Google Scholar] [CrossRef]
- Korobova, A.; Kuluev, B.; Möhlmann, T.; Veselov, D.; Kudoyarova, G. Limitation of Cytokinin Export to the Shoots by Nucleoside Transporter ENT3 and Its Linkage with Root Elongation in Arabidopsis. Cells 2021, 10, 350. [Google Scholar] [CrossRef]
- Vysotskaya, L.; Akhiyarova, G.; Feoktistova, A.; Akhtyamova, Z.; Korobova, A.; Ivanov, I.; Dodd, I.; Kuluev, B.; Kudoyarova, G. Effects of Phosphate Shortage on Root Growth and Hormone Content of Barley Depend on Capacity of the Roots to Accumulate ABA. Plants 2020, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, T.N.; Evseeva, N.V.; Tkachenko, O.V.; Burygin, G.L.; Vysotskaya, L.B.; Akhtyamova, Z.A.; Kudoyarova, G.R. Rhizobacteria Inoculation Effects on Phytohormone Status of Potato Microclones Cultivated In Vitro under Osmotic Stress. Biomolecules 2020, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Musielak, T.J.; Schenkel, L.; Kolb, M.; Henschen, A.; Bayer, M. A simple and versatile cell wall staining protocol to study plant reproduction. Plant Reprod. 2015, 28, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamaya, N.J.; Shavrukov, Y.; Langridge Roy, P.S.J.; Tester, M. Genetics of Na+ exclusion and salinity tolerance in Afghani durum wheat landraces. BMC Plant Biol. 2017, 17, 209. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.L.; Aziz, M.; Qiao, Y.; Han, Q.Q.; Li, J.; Wang, Y.Q.; Shen, X.; Wang, S.M.; Pare, P.W. Soil microbe Bacillus subtilis (GB03) induces biomass accumulation and salt tolerance with lower sodium accumulation in wheat. Crop Pasture Sci. 2014, 65, 423–427. [Google Scholar] [CrossRef]
- Li, P.-S.; Kong, W.-L.; Wu, X.-Q.; Zhang, Y. Volatile Organic Compounds of the Plant Growth-Promoting Rhizobacteria JZ-GX1 Enhanced the Tolerance of Robinia pseudoacacia to Salt Stress. Front. Plant Sci. 2021, 12, 753332. [Google Scholar] [CrossRef]
- Lee, M.-H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.-J.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef]
- Gamez, R.M.; Rodríguez, F.; Vidal, N.M.; Ramirez, S.; Alvarez, R.V.; Landsman, D.; Mariño-Ramírez, L. Banana (Musa acuminata) transcriptome profiling in response to rhizobacteria: Bacillus amyloliquefaciens Bs006 and Pseudomonas fluorescens Ps006. BMC Genom. 2019, 20, 378. [Google Scholar] [CrossRef] [Green Version]
- Ursache, R.; De Jesus Vieira Teixeira, C.; Dénervaud Tendon, V.; Gully, K.; De Bellis, D.; Schmid-Siegert, E.; Andersen, T.G.; Shekhar, V.; Calderon, S.; Pradervand, S.; et al. GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants 2021, 7, 353–364. [Google Scholar] [CrossRef]
- Wu, H.; Shabala, L.; Azzarello, E.; Huang, Y.; Pandolfi, C.; Su, N.; Wu, Q.; Cai, S.; Bazihizina, N.; Wang, L.; et al. Na+ extrusion from the cytosol and tissue-specific Na+ sequestration in roots confer differential salt stress tolerance between durum and bread wheat. J. Exp. Bot. 2018, 69, 3987–4001. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; James, R.A.; Gilliham, M.; Flowers, T.J.; Colmer, T.D. Tissue tolerance: An essetial but elusive trait for salt-tolerant crops. Funct. Plant Biol. 2016, 43, 1103–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, M.; Tang, R.-J.; Tang, Y.; Tian, W.; Hou, C.; Zhao, F.; Lan, W.; Luan, S. Transport and homeostasis of potassium and phosphate: Limiting factors for sustainable crop production. J. Exp. Bot. 2017, 68, 3091–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtyamova, Z.; Arkhipova, T.; Martynenko, E.; Nuzhnaya, T.; Kuzmina, L.; Kudoyarova, G.; Veselov, D. Growth-Promoting Effect of Rhizobacterium (Bacillus subtilis IB22) in Salt-Stressed Barley Depends on Abscisic Acid Accumulation in the Roots. Int. J. Mol. Sci. 2021, 22, 10680. [Google Scholar] [CrossRef] [PubMed]
- Pons, R.; Cornejo, M.J.; Sanz, A. Is ABA involved in tolerance responses to salinity by affecting cytoplasm ion homeostasis in rice cell lines? Plant Physiol. Biochem. 2013, 62, 88–94. [Google Scholar] [CrossRef] [PubMed]
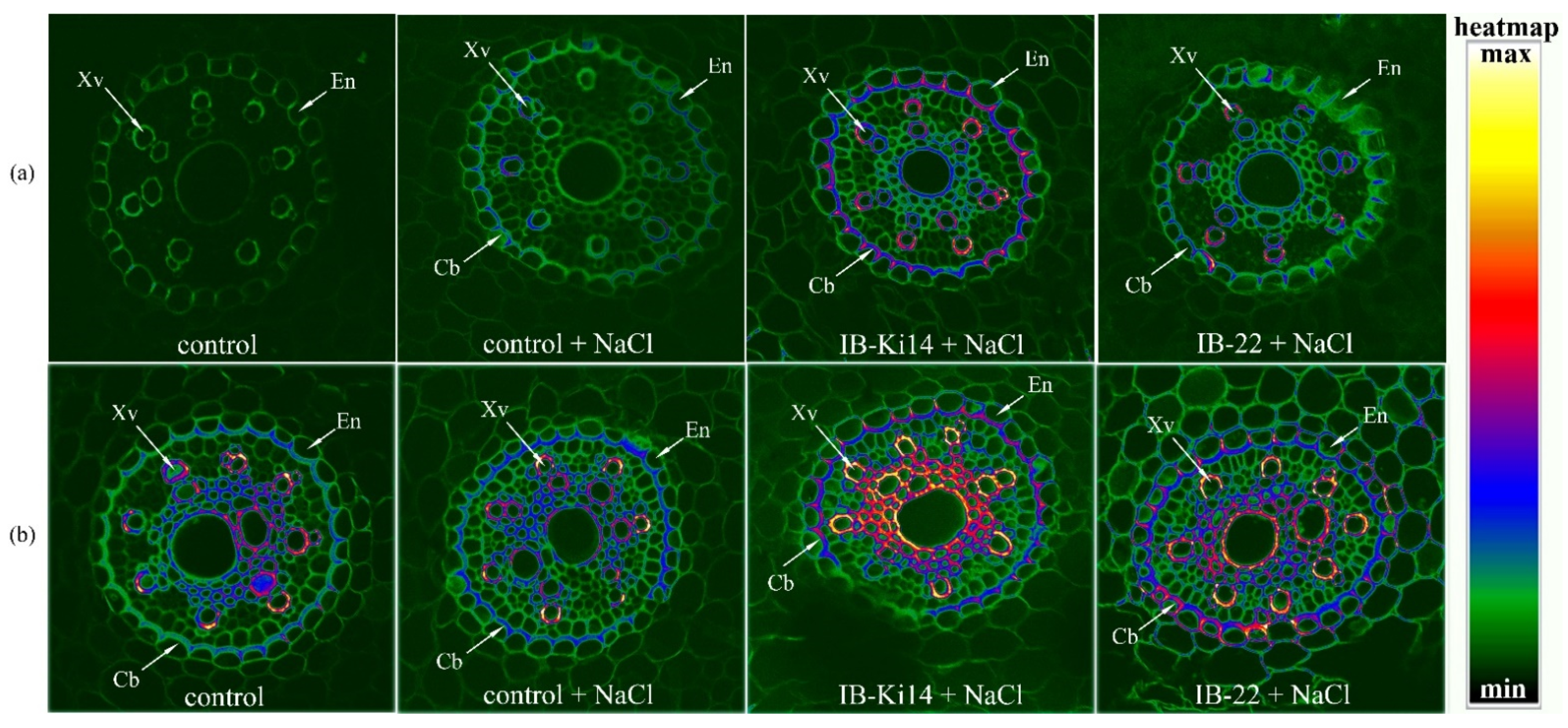

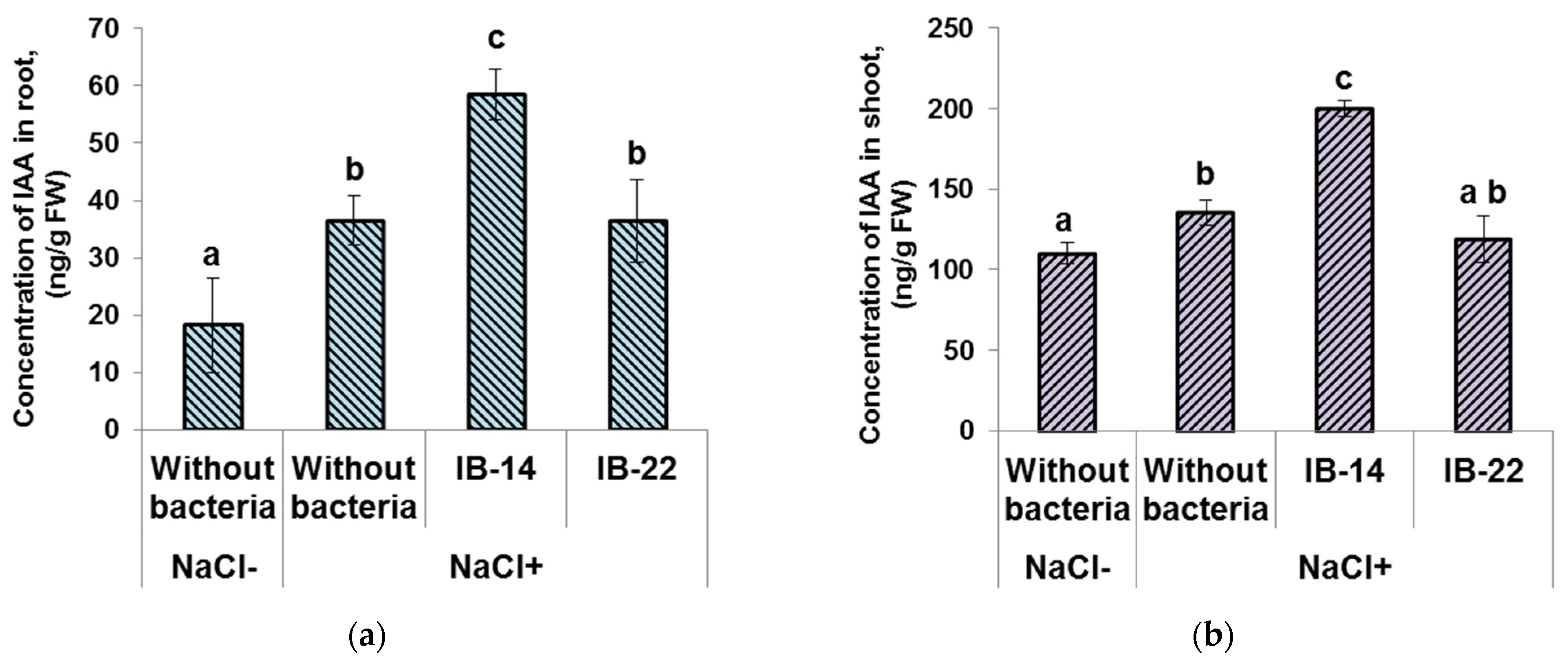
| NaCl Concentration, mM | Inoculation | Fresh Mass, mg | |
|---|---|---|---|
| Root | Shoot | ||
| 0 | Without bacteria | 94 ± 6.4 b | 305 ± 8.3 f |
| 100 | Without bacteria | 70 ± 3.3 a | 198 ± 7.7 c |
| 100 | P. mandelii IB-Ki14 | 77 ± 3.8 ab | 243 ± 6.9 d |
| 100 | B. subtilis IB-22 | 81 ± 4.5 b | 266 ± 6.6 e |
| Treatment | Sodium Concentration, mg/g DW | Potassium Concentration, mg/g DW | Phosphorus Concentration, mg/g DW | |
|---|---|---|---|---|
| Roots | Without bacteria, NaCl− | 1.24 ± 0.21 a | 10.6 ± 0.8 b | 33.5 ± 1.1 a |
| Without bacteria, NaCl+ | 4.48 ± 0.42 b | 7.8 ± 0.8 a | 33.2 ± 2.7 a | |
| P. mandelii IB-Ki14, NaCl+ | 4.29 ± 0.27 b | 7.8 ± 0.5 a | 40.3 ± 1.2 b | |
| B. subtilis IB-22, NaCl+ | 3.99 ± 0.47 b | 10.2 ± 0.1 b | 36.1 ± 0.6 a | |
| Shoots | Without bacteria, NaCl− | 2.81 ± 0.07 b | 30.9 ± 0.6 cd | 86.3 ± 1.7 d |
| Without bacteria, NaCl+ | 10.13 ± 0.08 d | 30.3 ± 0.8 cd | 63.0 ± 2.1 c | |
| P. mandelii IB-Ki14, NaCl+ | 8.11 ± 0.39 c | 29.1 ± 0.6 c | 60.0 ± 0.4 c | |
| B. subtilis IB-22, NaCl+ | 9.85 ± 0.57 d | 31.7 ± 0.5 d | 85.7 ± 1.6 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martynenko, E.; Arkhipova, T.; Safronova, V.; Seldimirova, O.; Galin, I.; Akhtyamova, Z.; Veselov, D.; Ivanov, R.; Kudoyarova, G. Effects of Phytohormone-Producing Rhizobacteria on Casparian Band Formation, Ion Homeostasis and Salt Tolerance of Durum Wheat. Biomolecules 2022, 12, 230. https://doi.org/10.3390/biom12020230
Martynenko E, Arkhipova T, Safronova V, Seldimirova O, Galin I, Akhtyamova Z, Veselov D, Ivanov R, Kudoyarova G. Effects of Phytohormone-Producing Rhizobacteria on Casparian Band Formation, Ion Homeostasis and Salt Tolerance of Durum Wheat. Biomolecules. 2022; 12(2):230. https://doi.org/10.3390/biom12020230
Chicago/Turabian StyleMartynenko, Elena, Tatiana Arkhipova, Vera Safronova, Oksana Seldimirova, Ilshat Galin, Zarina Akhtyamova, Dmitry Veselov, Ruslan Ivanov, and Guzel Kudoyarova. 2022. "Effects of Phytohormone-Producing Rhizobacteria on Casparian Band Formation, Ion Homeostasis and Salt Tolerance of Durum Wheat" Biomolecules 12, no. 2: 230. https://doi.org/10.3390/biom12020230
APA StyleMartynenko, E., Arkhipova, T., Safronova, V., Seldimirova, O., Galin, I., Akhtyamova, Z., Veselov, D., Ivanov, R., & Kudoyarova, G. (2022). Effects of Phytohormone-Producing Rhizobacteria on Casparian Band Formation, Ion Homeostasis and Salt Tolerance of Durum Wheat. Biomolecules, 12(2), 230. https://doi.org/10.3390/biom12020230







