Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Wheat Straw Cellulose Production
2.2.2. Cellulose Nanofiber Isolation
2.2.3. Cellulose Nanofiber Characterization
2.2.4. Cellulose Nanofiber-Based Aerogel Production and Characterization
2.2.5. Adsorption Study of Methylene Blue with Cellulose Nanofiber-Based Aerogel
3. Results
3.1. Cellulosic Fiber Chemical Characterization
3.2. Cellulose Nanofiber Characterization
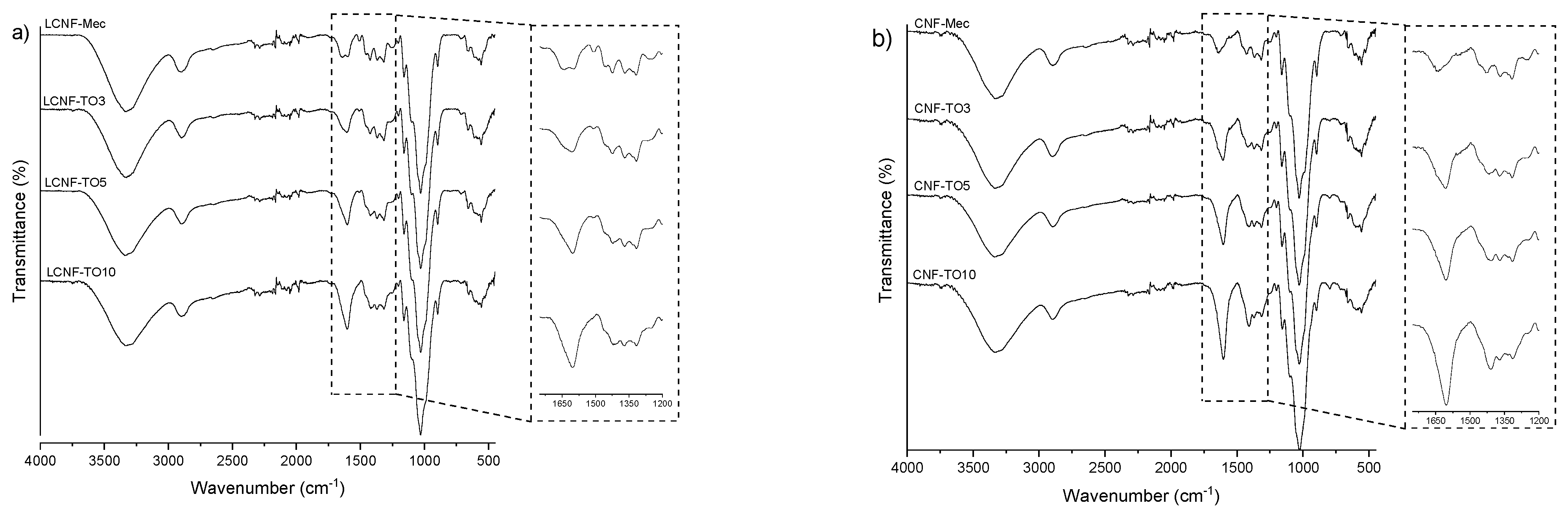
3.2.1. FTIR Analysis
3.2.2. XRD Analysis
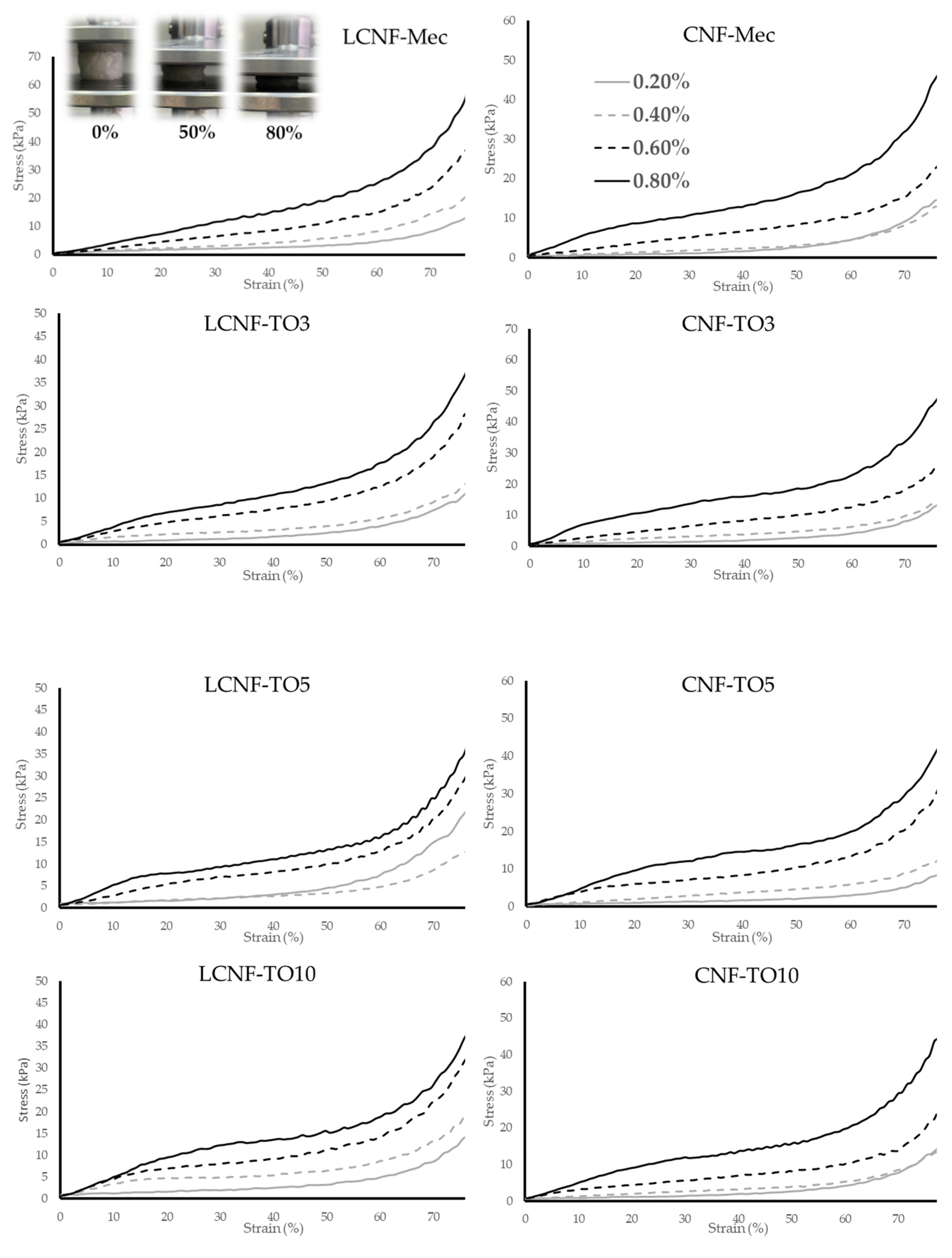
3.3. Cellulose-Based Aerogel Characterization
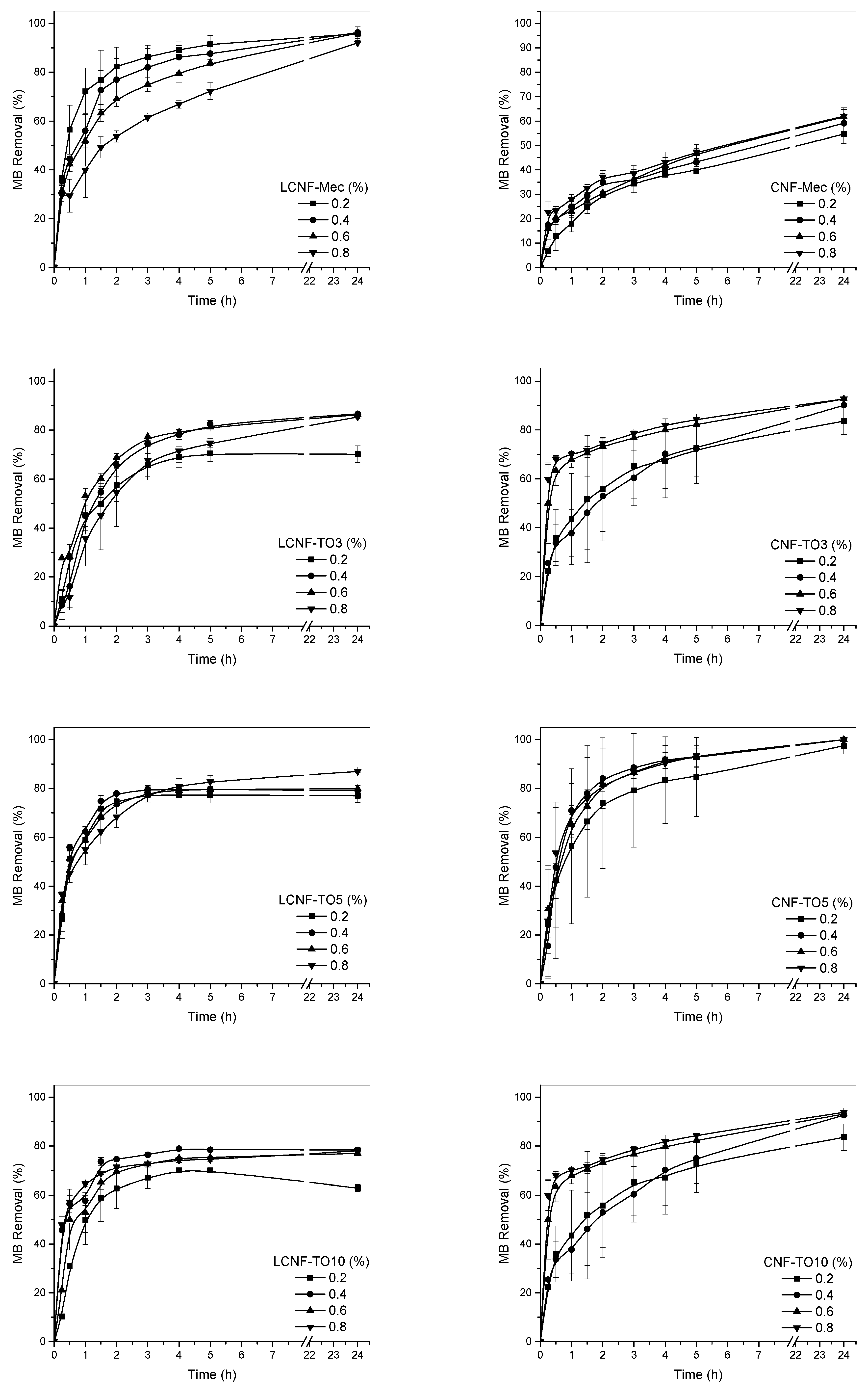
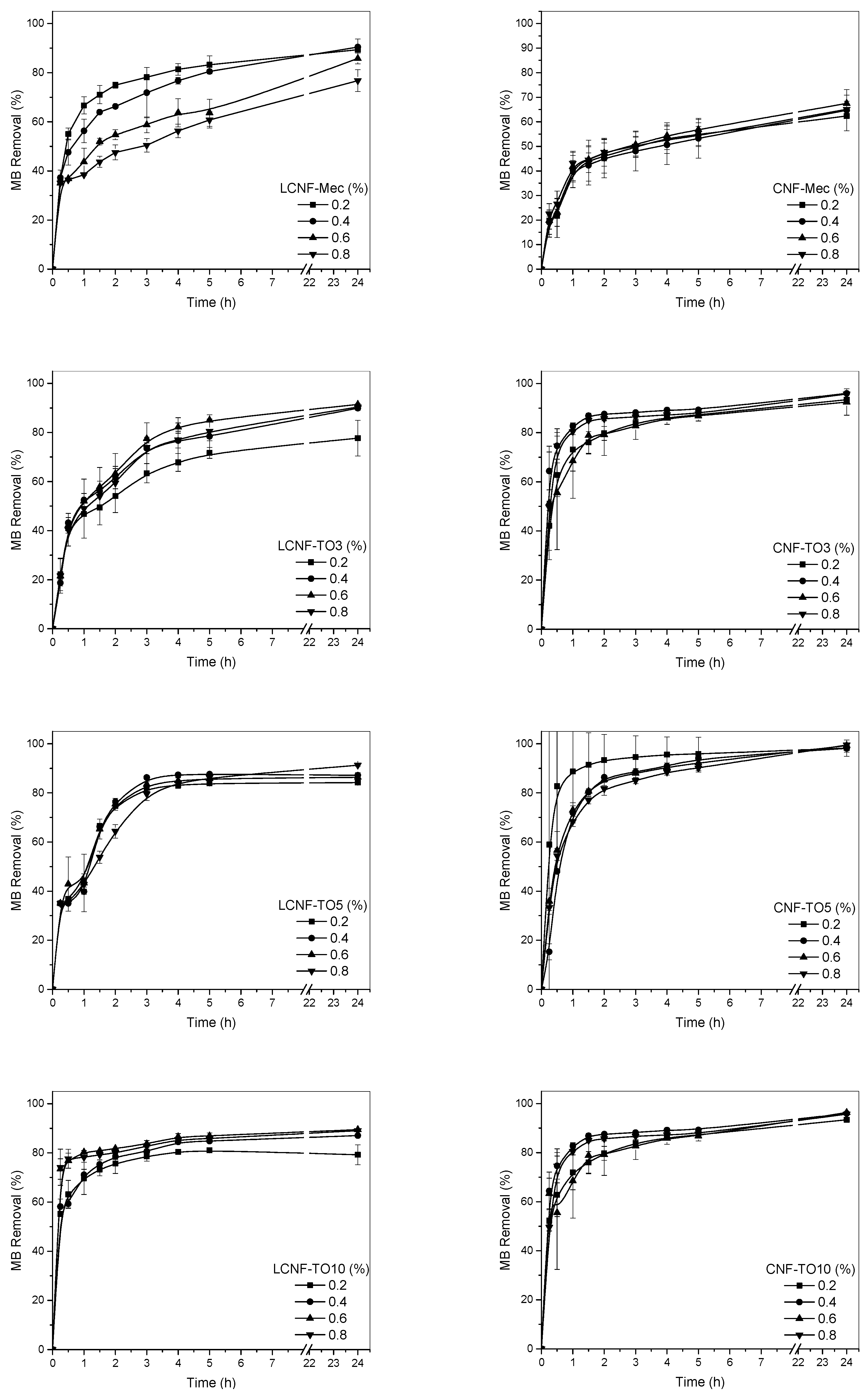
3.4. Cellulose-Based Aerogel Adsorption Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, A.W.; De Lannoy, C.F.; Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, A.; Ghaedi, M.; Agarwal, S.; Tyagi, I.; Gupta, V.K. Removal of basic dye Auramine-O by ZnS:Cu nanoparticles loaded on activated carbon: Optimization of parameters using response surface methodology with central composite design. RSC Adv. 2015, 5, 18438–18450. [Google Scholar] [CrossRef]
- Subbaiah, M.V.; Kim, D.S. Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: Kinetics, isotherms, and thermodynamic studies. Ecotoxicol. Environ. Saf. 2016, 128, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Mansoob Khan, M.; Gupta, V.K.; Mosquera, E.; Gracia, F.; Narayanan, V.; Stephen, A. ZnO/Ag/CdO nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluents. J. Colloid Interface Sci. 2015, 452, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, A.P.; Militky, J. Sustainability in Textile Dyeing: Recent Developments. In Sustainability in the Textile and Apparel Industries; Senthilkannan Muthu, S., Gardetti, M.A., Eds.; Springer: Cham, Switzerland, 2016; ISBN 9783030385446. [Google Scholar]
- Parlayici, Ş. Alginate-coated perlite beads for the efficient removal of methylene blue, malachite green, and methyl violet from aqueous solutions: Kinetic, thermodynamic, and equilibrium studies. J. Anal. Sci. Technol. 2019, 10, 4. [Google Scholar] [CrossRef]
- Ayranci, E.; Duman, O. In-Situ UV-visible spectroscopic study on the adsorption of some dyes onto activated carbon cloth. Sep. Sci. Technol. 2009, 44, 3735–3752. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoǧlan, B.K.I. Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef]
- Suzuki, N.; Okazaki, A.; Takagi, K.; Serizawa, I.; Hirami, Y.; Noguchi, H.; Pitchaimuthu, S.; Terashima, C.; Suzuki, T.; Ishida, N.; et al. Complete decomposition of sulfamethoxazole during an advanced oxidation process in a simple water treatment system. Chemosphere 2022, 287, 132029. [Google Scholar] [CrossRef]
- Gorito, A.M.; Pesqueira, J.F.J.R.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Nunes, O.C.; Almeida, C.M.R.; Silva, A.M.T. Ozone-based water treatment (O3, O3/UV, O3/H2O2) for removal of organic micropollutants, bacteria inactivation and regrowth prevention. J. Environ. Chem. Eng. 2021, 9, 10–14. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Kumar, J.S.; Kamyab, H.; Sujana, J.A.J.; Al-Khashman, O.A.; Kuslu, Y.; Ene, A.; Suresh Kumar, B. Modern enabling techniques and adsorbents based dye removal with sustainability concerns in textile industrial sector-A comprehensive review. J. Clean. Prod. 2020, 272, 122636. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Ebenezer Selvakumari, I.A.; Iyyappan, J.; Varjani, S. Itaconic acid: An effective sorbent for removal of pollutants from dye industry effluents. Curr. Opin. Environ. Sci. Health 2019, 12, 6–17. [Google Scholar] [CrossRef]
- Roa, K.; Oyarce, E.; Boulett, A.; ALSamman, M.; Oyarzún, D.; Pizarro, G.D.C.; Sánchez, J. Lignocellulose-based materials and their application in the removal of dyes from water: A review. Sustain. Mater. Technol. 2021, 29, e00320. [Google Scholar] [CrossRef]
- Liu, X.; Pang, H.; Liu, X.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.; Hu, B.; Yang, H.; Wang, X. Orderly Porous Covalent Organic Frameworks-based Materials: Superior Adsorbents for Pollutants Removal from Aqueous Solutions. Innovation 2021, 2, 100076. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Zeolite nanoparticle as a superior adsorbent with high capacity: Synthesis, surface modification and pollutant adsorption ability from wastewater. Microchem. J. 2019, 145, 74–83. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Simchi, A.; Shahriyari Far, H. Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: Synthesis, adsorption mechanism and kinetics studies. J. Ind. Eng. Chem. 2020, 81, 405–414. [Google Scholar] [CrossRef]
- De Lima Barizão, A.C.; Silva, M.F.; Andrade, M.; Brito, F.C.; Gomes, R.G.; Bergamasco, R. Green synthesis of iron oxide nanoparticles for tartrazine and bordeaux red dye removal. J. Environ. Chem. Eng. 2020, 8, 103618. [Google Scholar] [CrossRef]
- Boorboor Azimi, E.; Badiei, A.; Ghasemi, J.B. Efficient removal of malachite green from wastewater by using boron-doped mesoporous carbon nitride. Appl. Surf. Sci. 2019, 469, 236–245. [Google Scholar] [CrossRef]
- Chaari, I.; Fakhfakh, E.; Medhioub, M.; Jamoussi, F. Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. J. Mol. Struct. 2019, 1179, 672–677. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Campbell, P.G.; Baumann, T.F.; Worsley, M.A. Carbon aerogel evolution: Allotrope, graphene-inspired, and 3D-printed aerogels. J. Mater. Res. 2017, 32, 4166–4185. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Xu, Z.; Gao, C. Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.A.B.; Alemán, C.R.; Hanson, K.; Ramirez, N.; Vivod, S.L.; Wilmoth, N.; McCorkle, L. Polyimide aerogels with amide cross-links: A low cost alternative for mechanically strong polymer aerogels. ACS Appl. Mater. Interfaces 2015, 7, 1240–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigacci, A.; Marechal, J.C.; Repoux, M.; Moreno, M.; Achard, P. Preparation of polyurethane-based aerogels and xerogels for thermal superinsulation. J. Non. Cryst. Solids 2004, 350, 372–378. [Google Scholar] [CrossRef]
- Williams, J.C.; Meador, M.A.B.; McCorkle, L.; Mueller, C.; Wilmoth, N. Synthesis and properties of step-growth polyamide aerogels cross-linked with triacid chlorides. Chem. Mater. 2014, 26, 4163–4171. [Google Scholar] [CrossRef]
- Food Agriculture Organization Cereal Supply and Demand Briefing Note. Available online: http://www.fao.org/worldfoodsituation/csdb/es/ (accessed on 8 October 2021).
- Espinosa, E.; Tarrés, Q.; Delgado-Aguilar, M.; González, I.; Mutjé, P.; Rodríguez, A. Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 2016, 23, 837–852. [Google Scholar] [CrossRef]
- Isci, A.; Thieme, N.; Lamp, A.; Zverlov, V.; Kaltschmitt, M. Production of xylo-oligosaccharides from wheat straw using microwave assisted deep eutectic solvent pretreatment. Ind. Crops Prod. 2021, 164, 113393. [Google Scholar] [CrossRef]
- Liu, L.; Zou, S.; Li, H.; Deng, L.; Bai, C.; Zhang, X.; Wang, S.; Li, N. Experimental physical properties of an eco-friendly bio-insulation material based on wheat straw for buildings. Energy Build. 2019, 201, 19–36. [Google Scholar] [CrossRef]
- Serna-Loaiza, S.; Zikeli, F.; Adamcyk, J.; Friedl, A. Towards a wheat straw biorefinery: Combination of Organosolv and Liquid Hot Water for the improved production of sugars from hemicellulose and lignin hydrolysis. Bioresour. Technol. Rep. 2021, 14, 100667. [Google Scholar] [CrossRef]
- Ziaei-Rad, Z.; Fooladi, J.; Pazouki, M.; Gummadi, S.N. Lignocellulosic biomass pre-treatment using low-cost ionic liquid for bioethanol production: An economically viable method for wheat straw fractionation. Biomass Bioenergy 2021, 151, 106140. [Google Scholar] [CrossRef]
- Yang, S.; Bai, S.; Wang, Q. Sustainable packaging biocomposites from polylactic acid and wheat straw: Enhanced physical performance by solid state shear milling process. Compos. Sci. Technol. 2018, 158, 34–42. [Google Scholar] [CrossRef]
- Dixit, S.; Yadav, V.L. Optimization of polyethylene/polypropylene/alkali modified wheat straw composites for packaging application using RSM. J. Clean. Prod. 2019, 240, 118228. [Google Scholar] [CrossRef]
- Berthet, M.A.; Angellier-Coussy, H.; Chea, V.; Guillard, V.; Gastaldi, E.; Gontard, N. Sustainable food packaging: Valorising wheat straw fibres for tuning PHBV-based composites properties. Compos. Part A Appl. Sci. Manuf. 2015, 72, 139–147. [Google Scholar] [CrossRef]
- Espinosa, E.; Rol, F.; Bras, J.; Rodríguez, A. Production of lignocellulose nanofibers from wheat straw by different fibrillation methods. Comparison of its viability in cardboard recycling process. J. Clean. Prod. 2019, 239, 118083. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, B.K.; Rubiyah, H.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.L.; Shi, C.; Yu, H.Q.; Sheng, G.P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, P.; Min, L.; Tang, J.; Sun, H. Synthesis of cellulose carbon aerogel via combined technology of wet ball-milling and TEMPO-mediated oxidation and its supersorption performance to ionic dyes. Bioresour. Technol. 2020, 315, 123815. [Google Scholar] [CrossRef]
- Espinosa, E.; Domínguez-Robles, J.; Sánchez, R.; Tarrés, Q.; Rodríguez, A. The effect of pre-treatment on the production of lignocellulosic nanofibers and their application as a reinforcing agent in paper. Cellulose 2017, 24, 2605–2618. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Carrasco, F.; Mutjé, P.; Pelach, M.A. Control of retention in paper-making by colloid titration and zeta potential techniques. Wood Sci. Technol. 1998, 32, 145–155. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Marx-Figini, M. The acid-catalyzed degradation of cellulose linters in distinct ranges of degree of polymerization. J. Appl. Polym. Sci. 1987, 33, 2097–2105. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Vargas, F.; González, Z.; Sánchez, R.; Jiménez, L.; Rodríguez, A. Cellulosic pulps of cereal straws as raw material for the manufacture of ecological packaging. BioResources 2012, 7, 4161–4170. [Google Scholar] [CrossRef]
- Chaker, A.; Alila, S.; Mutjé, P.; Vilar, M.R.; Boufi, S. Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose 2013, 20, 2863–2875. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between length and degree of polymerization of TEMPO-oxidized cellulose nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of cellulose nanofibers from olive tree harvest—A residue with wide applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-Robles, J.; Rodríguez, A. A comparative study of the suitability of different cereal straws for lignocellulose nanofibers isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef]
- Isogai, A.; Bergström, L. Preparation of cellulose nanofibers using green and sustainable chemistry. Curr. Opin. Green Sustain. Chem. 2018, 12, 15–21. [Google Scholar] [CrossRef]
- Ferrer, A.; Quintana, E.; Filpponen, I.; Solala, I.; Vidal, T.; Rodríguez, A.; Laine, J.; Rojas, O.J. Effect of residual lignin and heteropolysaccharides in nanofibrillar cellulose and nanopaper from wood fibers. Cellulose 2012, 19, 2179–2193. [Google Scholar] [CrossRef]
- Solala, I.; Iglesias, M.C.; Peresin, M.S. On the potential of lignin-containing cellulose nanofibrils (LCNFs): A review on properties and applications. Cellulose 2020, 27, 1853–1877. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Cui, F.; Li, H.; Chen, C.; Wang, Z.; Liu, X.; Jiang, G.; Cheng, T.; Bai, R.; Song, L. Cattail fibers as source of cellulose to prepare a novel type of composite aerogel adsorbent for the removal of enrofloxacin in wastewater. Int. J. Biol. Macromol. 2021, 191, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Saito, T.; Isogai, A. Cellulose nanofibrils prepared from softwood cellulose by TEMPO/NaClO/NaClO2 systems in water at pH 4.8 or 6.8. Int. J. Biol. Macromol. 2012, 51, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Puangsin, B.; Yang, Q.; Saito, T.; Isogai, A. Comparative characterization of TEMPO-oxidized cellulose nanofibril films prepared from non-wood resources. Int. J. Biol. Macromol. 2013, 59, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Mäkilä, E.; Willför, S.; Xu, C. Ultralight and porous cellulose nanofibers / polyethyleneimine composite aerogels with exceptional performance for selective anionic dye adsorption. Ind. Crops Prod. 2022, 177, 114513. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Q.; Zhu, J.; Li, J.; Cai, Z.; Chen, L.; Gong, S. Mechanically strong fully biobased anisotropic cellulose aerogels. RSC Adv. 2016, 6, 96518–96526. [Google Scholar] [CrossRef]
- Lázár, I.; Kalmár, J.; Peter, A.; Szilágyi, A.; Gyori, E.; Ditrói, T.; Fábián, I. Photocatalytic performance of highly amorphous titania-silica aerogels with mesopores: The adverse effect of the in situ adsorption of some organic substrates during photodegradation. Appl. Surf. Sci. 2015, 356, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Sehaqui, H.; Salajková, M.; Zhou, Q.; Berglund, L.A. Mechanical performance tailoring of tough ultra-high porosity foams prepared from cellulose i nanofiber suspensions. Soft Matter 2010, 6, 1824–1832. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically cross-linked cellulose nanocrystal aerogels with shape recovery and superabsorbent properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef]
- Do, N.H.N.; Truong, B.Y.; Nguyen, P.T.X.; Le, K.A.; Duong, H.M.; Le, P.K. Composite aerogels of TEMPO-oxidized pineapple leaf pulp and chitosan for dyes removal. Sep. Purif. Technol. 2022, 283, 120200. [Google Scholar] [CrossRef]
- Lim, Z.E.; Thai, Q.B.; Le, D.K.; Luu, T.P.; Nguyen, P.T.T.; Do, N.H.N.; Le, P.K.; Phan-Thien, N.; Goh, X.Y.; Duong, H.M. Functionalized pineapple aerogels for ethylene gas adsorption and nickel (II) ion removal applications. J. Environ. Chem. Eng. 2020, 8, 104524. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Guo, W.; Niu, H.; Song, L.; Hu, Y. Eco-friendly thermally insulating cellulose aerogels with exceptional flame retardancy, mechanical property and thermal stability. J. Taiwan Inst. Chem. Eng. 2021, 131, 104159. [Google Scholar] [CrossRef]
- Luo, M.; Wang, M.; Pang, H.; Zhang, R.; Huang, J.; Liang, K.; Chen, P.; Sun, P.; Kong, B. Super-assembled highly compressible and flexible cellulose aerogels for methylene blue removal from water. Chin. Chem. Lett. 2021, 32, 2091–2096. [Google Scholar] [CrossRef]
- Saha, T.K. Adsorption of Methyl Orange onto Chitosan from Aqueous Solution. J. Water Resour. Prot. 2010, 2, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Robles, J.; Peresin, M.S.; Tamminen, T.; Rodríguez, A.; Larrañeta, E.; Jääskeläinen, A.S. Lignin-based hydrogels with “super-swelling” capacities for dye removal. Int. J. Biol. Macromol. 2018, 115, 1249–1259. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, H.; Zirakjou, A.; McClements, D.J.; Goodarzi, V.; Chen, W.H. Removal of methylene blue from wastewater using ternary nanocomposite aerogel systems: Carboxymethyl cellulose grafted by polyacrylic acid and decorated with graphene oxide. J. Hazard. Mater. 2022, 421, 126752. [Google Scholar] [CrossRef] [PubMed]

| Sample | Extractables (%) | Lignin (%) | Hemicelluloses (%) | α-Cellulose (%) | Ashes (%) |
|---|---|---|---|---|---|
| WS | 19.8 ± 0.6 | 15.7 ± 1.7 | 29.8 ± 0.5 | 33.4 ± 0.4 | 7.2 ± 0.1 |
| WS-UP | 12.0 ± 0.7 | 9.9 ± 0.8 | 24.9 ± 0.2 | 50.9 ± 0.3 | 3.7 ± 0.0 |
| WS-BP | 7.1 ± 0.8 | 2.5 ± 0.2 | 23.5 ± 0.3 | 62.7 ± 3.9 | 2.86 ± 0.0 |
| Pre-Treatment | Sample | ɳ 1 (%) | CD 2 (μeq/g) | CC 3 (μeq/g) | σspec (m2/g) | Length 4 (nm) | Diameter (nm) | Aspect Ratio |
|---|---|---|---|---|---|---|---|---|
| Mec | CNF | 27.1 ± 5.3 | 328.7 ± 37.2 | <74.4 | 124 | 4121 | 20 | 206 |
| LCNF | 55.6 ± 4.1 | 441.1 ± 7.4 | <74.4 | 179 | 4224 | 14 | 301 | |
| TO3 | CNF | 85.6 ± 0.0 | 1160.8 ± 20.0 | 330.9 ± 1.3 | 404 | 1907 | 6 | 318 |
| LCNF | 68.8 ± 0.8 | 728.6 ± 70.9 | 359.3 ± 1.0 | 180 | 1962 | 14 | 140 | |
| TO5 | CNF | 89.1 ± 1.5 | 1210.0 ± 15.5 | 359.9 ± 0.1 | 414 | 1563 | 6 | 260 |
| LCNF | 87.6 ± 0.1 | 925.7 ± 19.8 | 360.4 ± 0.3 | 275 | 1238 | 9 | 138 | |
| TO10 | CNF | 96.4 ± 0.4 | 1440.1 ± 20.1 | 369.5 ± 2.9 | 521 | 1033 | 5 | 207 |
| LCNF | 88.4 ± 1.1 | 1136.3 ± 59.9 | 366.6 ± 0.7 | 375 | 905 | 7 | 129 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcillo-Martín, R.; Espinosa, E.; Rabasco-Vílchez, L.; Sanchez, L.M.; de Haro, J.; Rodríguez, A. Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity. Biomolecules 2022, 12, 232. https://doi.org/10.3390/biom12020232
Morcillo-Martín R, Espinosa E, Rabasco-Vílchez L, Sanchez LM, de Haro J, Rodríguez A. Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity. Biomolecules. 2022; 12(2):232. https://doi.org/10.3390/biom12020232
Chicago/Turabian StyleMorcillo-Martín, Ramón, Eduardo Espinosa, Laura Rabasco-Vílchez, Laura M. Sanchez, Jorge de Haro, and Alejandro Rodríguez. 2022. "Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity" Biomolecules 12, no. 2: 232. https://doi.org/10.3390/biom12020232
APA StyleMorcillo-Martín, R., Espinosa, E., Rabasco-Vílchez, L., Sanchez, L. M., de Haro, J., & Rodríguez, A. (2022). Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity. Biomolecules, 12(2), 232. https://doi.org/10.3390/biom12020232










