Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance
Abstract
:1. Overview of Inflammation
- An increased blood supply to the site of inflammation.
- Elevated capillary permeability due to retraction of endothelial cells.
- Leukocyte migration from the capillaries into the surrounding tissue. This process is facilitated by the release of chemotactic factors from the site of inflammation and from the upregulation of adhesion molecules on the endothelium.
- Release of mediators from leukocytes at the site of inflammation.
Lipid Mediators
2. Inflammation in Cardiovascular Diseases: Focus on Atherosclerosis
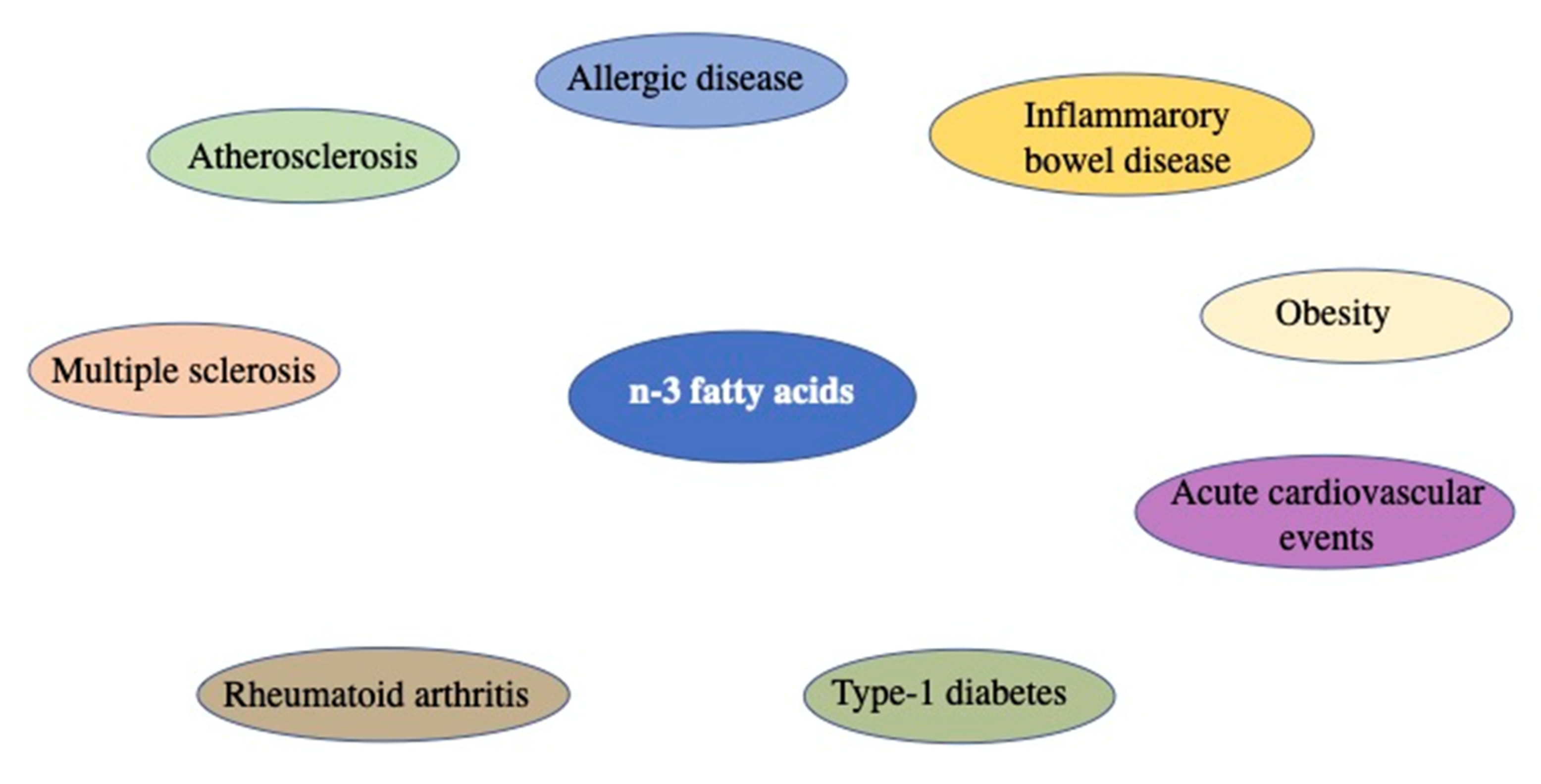
3. Fatty Acids: Composition and Role in Inflammatory Processes
Fatty Acid Sources
- By taking fatty acids we can alter the intracellular concentrations of lipoproteins, metabolites, complex lipids, and hormones, which in turn are modulators of inflammation;
- Fatty acids can undergo oxidation processes and the compounds obtained can act on inflammatory cells by binding to specific receptors;
- Fatty acids can be incorporated into cell membranes where they keep the fluidity of the membranes intact; membrane phospholipids are also substrates for diacylglycerol, and fatty acids can act as transcription factors or precursors for the biosynthesis of lipid mediators.
4. Fatty Acid: Preclinical Studies
5. Fatty Acids: Clinical Studies
EPA: Outcomes Studies
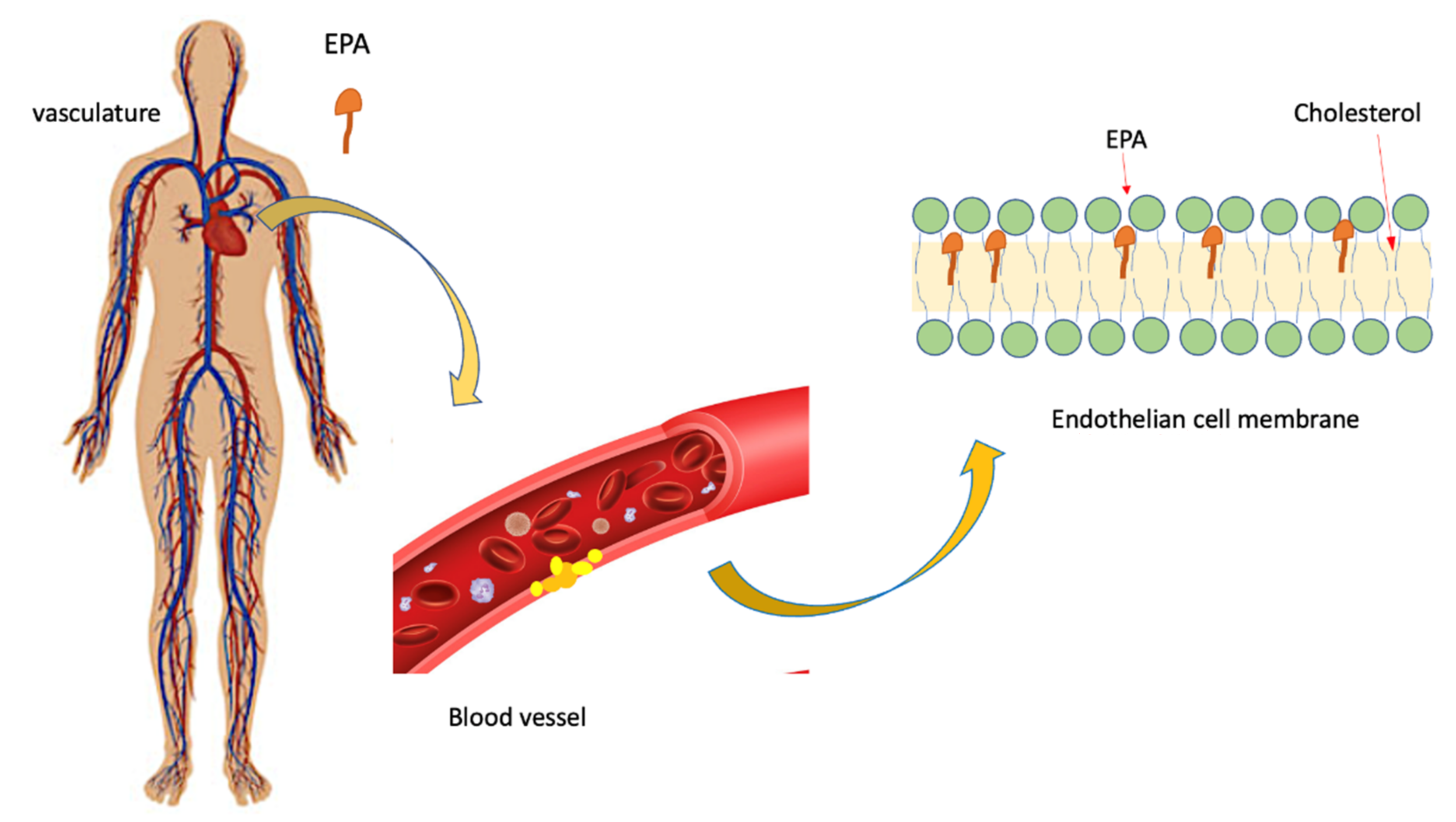
6. Role of EPA on Athero-Inflammatory-Thrombotic Processes
EPA and Atherosclerotic Plaque
7. Conclusions
- EPA protects against oxidative damage and improves endothelium formation [77];
- EPA inhibits monocyte movement into early lesions and subsequent conversion to macrophages and foam cells [78];
- EPA supports anti-oxidant and anti-inflammatory functions of high- density lipoprotein (HDL) [79];
- EPA promotes HDL-mediated cholesterol efflux from macrophages [79];
- EPA reduces atherosclerotic plaque formation, progression, and vulnerability to rupture [80];
- EPA decreases platelet-mediated thrombus formation [81];
- EPA reduces blood pressure, likely attributable to improvement of endothelial function [82]; importantly, many of these effects have been observed with EPA alone or are additive to those of statins.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.H.; Abrams, N.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; Prabhudas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of chronic inflammation in disease development and prevention: Challenges and opportunities. Nat. Immunol. 2017, 18, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Fatty acids and immune function: Relevance to inflammatory bowel diseases. Int. Rev. Immunol. 2009, 28, 506–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.K. Triglyceride-lowering and anti-inflammatory mechanisms of omega-3 polyunsaturated fatty acids for atherosclerotic cardiovascular risk reduction. J. Clin. Lipidol. 2021, 15, 556–568. [Google Scholar] [CrossRef] [PubMed]
- de Winther, M.P.J.; Lutgens, E. The link between hematopoiesis and atherosclerosis. N. Engl. J. Med. 2019, 380, 1869–1871. [Google Scholar] [CrossRef]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef]
- Mehta, N.K.; Abrams, L.R.; Myrskyla, M. US life expectancy stalls due to cardiovascular disease, not drug deaths. Proc. Natl. Acad. Sci. USA 2020, 117, 6998–7000. [Google Scholar] [CrossRef] [Green Version]
- Sergienko, I.V.; Ansheles, A.A.; Drapkina, O.M.; Gornyakova, N.B.; Zubareva, M.Y.; Shepel, R.N.; Kuharchuk, V.V.; Boytsov, S.A. ANICHKOV study: The effect of combined hypotensive and lipid-lowering therapy on cardiovascular complications in patients of high and very high risk. Ter. Arkh. 2019, 91, 90–98. [Google Scholar] [CrossRef]
- Siasos, G.; Skotsimara, G.; Oikonomou, E.; Sagris, M.; Vasiliki-Chara, M.; Bletsa, E.; Stampouloglou, P.; Theofilis, P.; Charalampous, G.; Tousoulis, D. Antithrombotic treatment in diabetes mellitus: A review of the literature about antiplatelet and anticoagulation strategies used for diabetic patients in primary and secondary prevention. Curr. Pharm. Des. 2020, 26, 2780–2788. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef] [Green Version]
- Weintraub, H.S. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad. Med. 2014, 126, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Influence of cell culture conditions on diet-induced changes in lymphocyte fatty acid composition. Biochim. Biophys. Acta 1995, 1255, 333–340. [Google Scholar] [CrossRef]
- Surette, M.E.; Whelan, J.; Lu, G.; Hardard’Ottir, I.; Kinsella, J.E. Dietary n-3 polyunsaturated fatty acids modify Syrian hamster platelet and macrophage phospholipid fatty acyl composition and eicosanoid synthesis: A controlled study. Biochim. Biophys. Acta. 1995, 1255, 185–191. [Google Scholar] [CrossRef]
- Calder, P.C.; Bond, J.A.; Harvey, D.J.; Gordon, S.; Newsholme, E.A. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem. J. 1990, 269, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Palombo, J.D.; Demichele, S.J.; Lydon, E.; Bistrian, B.R. Cyclic vs continuous enteral feeding with omega-3 and gamma-linolenic fatty acids: Effects on modulation of phospholipid fatty acids in rat lung and liver immune cells. JPEN J. Parenter. Enter. Nutr. 1997, 21, 123–132. [Google Scholar] [CrossRef]
- Careaga-Houck, M.; Sprecher, H. Effect of a fish oil diet on the composition of rat neutrophil lipids and the molecular species of choline and ethanolamine glycerophospholipids. J. Lipid Res. 1989, 30, 77–87. [Google Scholar] [CrossRef]
- Lokesh, B.R.; Hsieh, H.L.; Kinsella, J.E. Peritoneal macrophages from mice fed dietary (n-3) polyunsaturated fatty acids secrete low levels of prostaglandins. J. Nutr. 1986, 116, 2547–2552. [Google Scholar] [CrossRef] [Green Version]
- Chapkin, R.; Hubbard, N.E.; Erickson, K.L. 5-series peptido-leukotriene synthesis in mouse peritoneal macrophages: Modulation by dietary n-3 fatty acids. Biochem. Biophys. Res. Commun. 1990, 171, 764–769. [Google Scholar] [CrossRef]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; Van Der Meer, J.W.M.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C.; et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Thies, F.; Nebe-von-Caron, G.; Powell, J.R.; Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J. Nutr. 2001, 131, 1918–1927. [Google Scholar] [CrossRef] [Green Version]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, E.A.; Banerjee, T.; Calder, P.C. The influence of different combinations of gamma-linolenic, stearidonic and eicosapentaenoic acids on the fatty acid composition of blood lipids and mononuclear cells in human volunteers. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 529–538. [Google Scholar] [CrossRef]
- Matsumoto, M.; Sata, M.; Fukuda, D.; Tanaka, K.; Soma, M.; Hirata, Y.; Nagai, R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis 2008, 197, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Salic, K.; Morrison, M.C.; Verschuren, L.; Wielinga, P.Y.; Wu, L.; Kleemann, R.; Gjorstrup, P.; Kooistra, T. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 2016, 250, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, K.; Yamashita, T.; Kita, T.; Takeda, M.; Sasaki, N.; Kasahara, K.; Shinohara, M.; Rikitake, Y.; Ishida, T.; Yokoyama, M.; et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arter. Thromb. Vasc. Biol. 2011, 31, 1963–1972. [Google Scholar] [CrossRef] [Green Version]
- Lau, A.C.; Jongstra-Bilen, J.; Cybulsky, M.I. Eicosapentaenoic acid and regression of atherosclerotic lesions: A role for dendritic cells. Arter. Thromb. Vasc. Biol. 2011, 31, 1943–1945. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—Executive summary. J. Clin. Lipidol. 2014, 8, 473–488. [Google Scholar] [CrossRef] [Green Version]
- Teramoto, T.; Sasaki, J.; Ishibashi, S.; Birou, S.; Daida, H.; Dohi, S.; Egusa, G.; Hiro, T.; Hirobe, K.; Iida, M.; et al. Executive summary of the Japan atherosclerosis society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan 2012 version. J. Atheroscler. Thromb. 2013, 20, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- ORIGIN Trial Investigators. n-3 Fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Risk and Prevention Study Collaborative Group. n-3 Fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013, 368, 1800–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M. n-3 Fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef]
- ASCEND Study Collaborative Group. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.; Koenig, W.; McGuire, D.K.; et al. Effect of high-dose omega-3 fatty acids vs. corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: The STRENGTH randomized clinical trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef]
- Elbadawi, A.; Olorunfemi, O.; Ogunbayo, G.O.; Saad, M.; Elgendy, I.Y.; Arif, Z.; Badran, H.; Saheed, D.; Ahmed, H.M.A.; Rao, M. Cardiovascular outcomes with surgical left atrial appendage exclusion in patients with atrial fibrillation who underwent valvular heart surgery (from the national inpatient sample database). Am. J. Cardiol. 2017, 119, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine omega-3 supplementation and cardiovascular disease: An updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J. Am. Heart Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef] [PubMed]
- Orringer, C.E.; Jacobson, T.A.; Maki, K.C. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J. Clin. Lipidol. 2019, 13, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Aim-High Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keech, A.C.; Simes, R.J.; Barter, P.J.; Best, J.; Scott, R.A.P.; Taskinen, M.-R.; Forder, P.M.; Pillai, A.; Davis, T.M.; Glasziou, P.; et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Paynter, N.P.; Everett, B.M.; Glynn, R.J.; Amarenco, P.; Elam, M.; Ginsberg, H.; Hiatt, W.R.; Ishibashi, S.; Koenig, W.; et al. Rationale and design of the pemafibrate to reduce cardiovascular outcomes by reducing triglycerides in patients with diabetes (PROMINENT) study. Am. Hear. J. 2018, 206, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Montezano, A.; Touyz, R.M. Reactive oxygen species and endothelial function—Role of nitric oxide synthase uncoupling and nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin. Pharmacol. Toxicol. 2012, 110, 87–94. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, S.-D.; Ou, H.-C.; Lai, S.-C.; Cheng, Y.-J. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway. Int. J. Mol. Sci. 2014, 15, 10334–10349. [Google Scholar] [CrossRef] [Green Version]
- Mason, R.P.; Jacob, R.F. Eicosapentaenoic acid inhibits glucose-induced membrane cholesterol crystalline domain formation through a potent antioxidant mechanism. Biochim. Biophys. Acta 2015, 1848, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.-C.; Chiang, E.-P.I.; Tsai, S.-Y.; Wang, F.-Y.; Pai, M.-H.; Syu, J.-N.; Cheng, C.-C.; Rodriguez, R.L.; Tang, F.-Y. Eicosapentaenoic acid induces neovasculogenesis in human endothelial progenitor cells by modulating c-kit protein and PI3-K/Akt/eNOS signaling pathways. J. Nutr. Biochem. 2014, 25, 934–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bays, H.E.; Ballantyne, C.M.; Braeckman, R.A.; Stirtan, W.G.; Soni, P.N. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: Effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am. J. Cardiovasc. Drugs 2013, 13, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Toyama, K.; Nishioka, T.; Isshiki, A.; Ando, T.; Inoue, Y.; Kirimura, M.; Kamiyama, T.; Sasaki, O.; Ito, H.; Maruyama, Y.; et al. Eicosapentaenoic acid combined with optimal statin therapy improves endothelial dysfunction in patients with coronary artery disease. Cardiovasc. Drugs Ther. 2014, 28, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, K.; Shimabukuro, M.; Higa, N.; Asahi, T.; Ohba, K.; Arasaki, O.; Higa, M.; Oshiro, Y.; Yoshida, H.; Higa, T.; et al. Eicosapentaenoic acid supplementation changes fatty acid composition and corrects endothelial dysfunction in hyperlipidemic patients. Cardiol. Res. Pract. 2012, 2012, 754181. [Google Scholar] [CrossRef]
- Takaki, A.; Umemoto, S.; Ono, K.; Seki, K.; Ryoke, T.; Fujii, A.; Itagaki, T.; Harada, M.; Tanaka, M.; Yonezawa, T.; et al. Add-on therapy of EPA reduces oxidative stress and inhibits the progression of aortic stiffness in patients with coronary artery disease and statin therapy: A randomized controlled study. J. Atheroscler. Thromb. 2011, 18, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Ballantyne, C.M.; Bays, H.E.; Philip, S.; Doyle, R.T.; Braeckman, R.A.; Stirtan, W.G.; Soni, P.N.; Juliano, R.A. Icosapent ethyl (eicosapentaenoic acid ethyl ester): Effects on remnant-like particle cholesterol from the MARINE and ANCHOR studies. Atherosclerosis 2016, 253, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Brinton, E.A.; Ballantyne, C.M.; Bays, H.E.; Kastelein, J.J.; Braeckman, R.A.; Soni, P.N. Effects of icosapent ethyl on lipid and inflammatory parameters in patients with diabetes mellitus-2, residual elevated triglycerides (200–500 mg/dL), and on statin therapy at LDL-C goal: The ANCHOR study. Cardiovasc. Diabetol. 2013, 12, 100. [Google Scholar] [CrossRef] [Green Version]
- Satoh, N.; Shimatsu, A.; Kotani, K.; Sakane, N.; Yamada, K.; Suganami, T.; Kuzuya, H.; Ogawa, Y. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care 2007, 30, 144–146. [Google Scholar] [CrossRef] [Green Version]
- Karyagina, A.S.; Grunina, T.; Lyaschuk, A.M.; Voronina, E.V.; Marigin, R.A.; Cherepushkin, S.; Trusova, I.N.; Grishin, A.V.; Poponova, M.S.; Orlova, P.A.; et al. Recombinant human erythropoietin proteins synthesized in escherichia coli cells: Effects of additional domains on the in vitro and in vivo activities. Biochemistry 2019, 84, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Takashima, H.; Ozaki, Y.; Yasukawa, T.; Waseda, K.; Asai, K.; Wakita, Y.; Kuroda, Y.; Kosaka, T.; Kuhara, Y.; Ito, T. Impact of lipid-lowering therapy with pitavastatin, a new HMG-CoA reductase inhibitor, on regression of coronary atherosclerotic plaque. Circ. J. 2007, 71, 1678–1684. [Google Scholar] [CrossRef] [Green Version]
- Goto, Y.Y.; Kawasaki, T.; Koga, N.; Tanaka, H.; Koga, H.; Orita, Y.; Ikeda, S.; Shintani, Y.; Kajiwara, M.; Fukuyama, T. Plaque distribution patterns in left main trunk bifurcations: Prediction of branch vessel compromise by multidetector row computed topography after percutaneous coronary intervention. EuroIntervention 2012, 8, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Philip, S.; Reynolds, K.; Granowitz, C.B.; Fazio, S. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin-controlled LDL cholesterol. Diabetes Obes. Metab. 2019, 21, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Ballantyne, C.M.; Bays, H.E.; Kastelein, J.J.; Stein, E.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am. J. Cardiol. 2012, 110, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Chowdhury, S.; Nelson, J.R.; Everett, P.B.; Hulme-Lowe, C.K.; Schmier, J.K. A novel cost-effectiveness model of prescription eicosapentaenoic acid extrapolated to secondary prevention of cardiovascular diseases in the United States. J. Med. Econ. 2016, 19, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res. 2008, 52, 885–897. [Google Scholar] [CrossRef]
- Kremmyda, L.-S.; Vlachava, M.; Noakes, P.S.; Diaper, N.D.; Miles, E.A.; Calder, P.C. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: A systematic review. Clin. Rev. Allergy Immunol. 2011, 41, 36–66. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef]
- Krauss-Etschmann, S.; Hartl, D.; Rzehak, P.; Heinrich, J.; Shadid, R.; del Carmen Ramírez-Tortosa, M.; Campoy, C.; Pardillo, S.; Schendel, D.J.; Decsi, T.; et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J. Allergy Clin. Immunol. 2008, 121, 464–470 e6. [Google Scholar] [CrossRef]
- Olsen, S.F.; Østerdal, M.L.; Salvig, J.D.; Mortensen, L.M.; Rytter, D.; Secher, N.J.; Henriksen, T.B. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Sala-Vila, A.; Miles, E.A.; Calder, P. Fatty acid composition abnormalities in atopic disease: Evidence explored and role in the disease process examined. Clin. Exp. Allergy 2008, 38, 1432–1450. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Omega-3 (n-3) fatty acids, cardiovascular disease and stability of atherosclerotic plaques. Cell. Mol. Biol. 2010, 56, 28–37. [Google Scholar]
- Calder, P.C. n-3 Fatty acids and cardiovascular disease: Evidence explained and mechanisms explored. Clin. Sci. 2004, 107, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thies, F.; Garry, J.M.C.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef]
- Calder, P.C.; Campoy, C.; Eilander, A.; Fleith, M.; Forsyth, S.; Larsson, P.-O.; Schelkle, B.; Lohner, S.; Szommer, A.; Van De Heijning, B.J.M.; et al. A systematic review of the effects of increasing arachidonic acid intake on PUFA status, metabolism and health-related outcomes in humans. Br. J. Nutr. 2019, 121, 1201–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, J.M.; Lawrence, D.A.; Jubiz, W.; Digiacomo, R.; Rynes, R.; Bartholomew, L.E.; Sherman, M. Dietary fish oil and olive oil supplementation in patients with Rheumatoid Arthritis clinical and immunologic effects. Arthritis Care Res. 1990, 33, 810–820. [Google Scholar] [CrossRef]
- Bays, H.E.; Ballantyne, C.M.; Kastelein, J.J.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the multi-center, plAcebo-controlled, randomized, double-blINd, 12-week study with an open-label extension [MARINE] trial). Am. J. Cardiol. 2011, 108, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Walter, M.F.; Jacob, R.F. Effects of HMG-CoA reductase inhibitors on endothelial function: Role of microdomains and oxidative stress. Circulation 2004, 109, II34–II41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawood, A.L.; Ding, R.; Napper, F.L.; Young, R.H.; Williams, J.A.; Ward, M.J.; Gudmundsen, O.; Vige, R.; Payne, S.P.; Ye, S.; et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis 2010, 212, 252–259. [Google Scholar] [CrossRef]
- Tanaka, N.; Ishida, T.; Nagao, M.; Mori, T.; Monguchi, T.; Sasaki, M.; Mori, K.; Kondo, K.; Nakajima, H.; Honjo, T.; et al. Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis 2014, 237, 577–583. [Google Scholar] [CrossRef]
- Liu, L.; Yang, W.; Nagahara, Y.; Li, Y.; Lamooki, S.R.; Muramatsu, T.; Kitslaar, P.; Sarai, M.; Ozaki, Y.; Barlis, P.; et al. The impact of image resolution on computation of fractional flow reserve: Coronary computed tomography angiography versus 3-dimensional quantitative coronary angiography. Int. J. Cardiovasc. Imaging 2016, 32, 513–523. [Google Scholar] [CrossRef]
- Gajos, G.; Rostoff, P.; Undas, A.; Piwowarska, W. Effects of polyunsaturated omega-3 fatty acids on responsiveness to dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: The OMEGA-PCI (OMEGA-3 fatty acids after pci to modify responsiveness to dual antiplatelet therapy) study. J. Am. Coll. Cardiol. 2010, 55, 1671–1678. [Google Scholar] [PubMed] [Green Version]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sources of Dietary n-3 PUFA | Sources of Dietary n-3 PUFA | ALA (α-Linolenic Acid) | EPA (Eicosapentaenoic Acid) | DHA (Docosahexaenoic Acid) | Ref. |
|---|---|---|---|---|---|
| Fish oil | Menhaden (oil) Salmon (oil) Herring (oil) Sardine (oil) | - - - - | 13.18 13.3 6.28 10.15 | 8.56 18.23 4.21 10.66 | [10,11] |
| Fish raw | Salmon (raw) Sardine (raw) Cod (dried) Trout (raw) Herring (raw) | 0.09 - - 0.1 0.19 | 0.89 0.51 0.02 0.15 1.09 | 1.19 1.16 0.62 0.5 1.01 | [11] |
| Beef | New Zealand, liver (raw) New Zealand, kidney (cooked) | 0.05 0.08 | 0.11 0.15 | 0.04 0.03 | [10] |
| Oils | Soybean (oil) Wheat germ (oil) Sunflower (oil) Flaxseed (oil) Safflower (oil) Corn (oil) Canola (oil) | 7.3 5.3 0.33 53.37 0.1 0.6 9.15 | - - - - - - - | - - - - - - - | [10,11] |
| Seed and nuts | Chia (dried/ground) Hazelnut (dried/ground) Almond (dried/ground) Hemp seed (hulled) Brazil nuts (dried) Walnut (dried/ground) | 17.83 0.11 0.3 8.68 0.02 6.64 | - - - - - - | - - - - - - | [10,11] |
| Clinical Trial | Patient Characteristics | Dose PUFA | Outcomes | Ref |
|---|---|---|---|---|
| (GISSI)-Prevention study | Men and women (15%) after myocardial infarction | 850 mg EPA/DHA | The group treated with omega-3 PUFAs were shown to have a 20% reduction in major CV events, a 30% reduction of CV death, and a 45% reduction in SCD | [3] |
| JELIS trial | Hypercholesterolemic men and women (69%), with and without CHD, already receiving statin therapy | 1800 mg EPA | Treatment was associated with a 19% reduction in major CV events | [29] |
| GISSI-Heart Failure study | Men and women (22%) with congestive heart failure | 850 mg EPA/DHA | Treatment was associated with a 6% reduction in CV death or hospitalization | [30] |
| REDUCE-IT | Middle-aged, history of CVD or DM; TG 135–499 mg/dL; LDL-C 40–100 mg/dL with statin | 4000 mg EPA | Treatment was associated with a reduction risk of ischemic events | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crupi, R.; Cuzzocrea, S. Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules 2022, 12, 242. https://doi.org/10.3390/biom12020242
Crupi R, Cuzzocrea S. Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules. 2022; 12(2):242. https://doi.org/10.3390/biom12020242
Chicago/Turabian StyleCrupi, Rosalia, and Salvatore Cuzzocrea. 2022. "Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance" Biomolecules 12, no. 2: 242. https://doi.org/10.3390/biom12020242
APA StyleCrupi, R., & Cuzzocrea, S. (2022). Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules, 12(2), 242. https://doi.org/10.3390/biom12020242







