Differences According to Age in the Diagnostic Performance of Cardiac Biomarkers to Predict Frailty in Patients with Acute Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Data Collection and Geriatric Assessment
2.3. Statistical Analysis
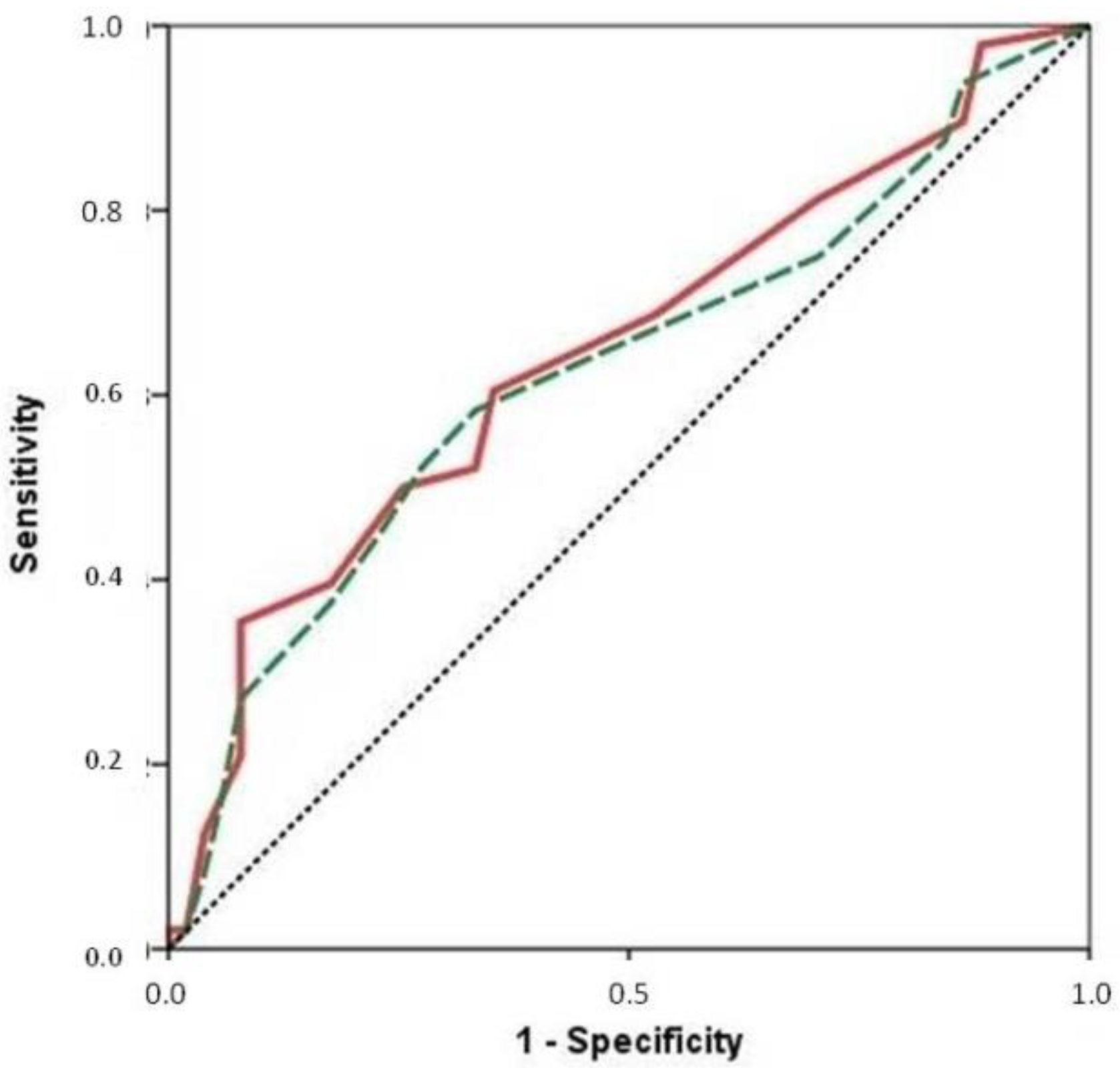
3. Results
3.1. Sample Description
3.2. Analysis of Frailty Determinants in Each Group
3.3. Inclusion of Biomarkers in SPPB Scale
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Roger, V.L. Epidemiology of Heart Failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Jankowska, E.; Hill, L.; Piepoli, M.; Doehner, W.; Anker, S.D.; Lainscak, M.; Jaarsma, T.; Ponikowski, P.; Rosano, G.M.C.; et al. Heart Failure Association of the European Society of Cardiology Position Paper on Frailty in Patients with Heart Failure. Eur. J. Heart Fail. 2019, 21, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Vidán, M.T.; Blaya-Novakova, V.; Sánchez, E.; Ortiz, J.; Serra-Rexach, J.A.; Bueno, H. Prevalence and Prognostic Impact of Frailty and Its Components in Non-Dependent Elderly Patients with Heart Failure. Eur. J. Heart Fail. 2016, 18, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Andrew, M.; Mitnitski, A. A Comparison of Two Approaches to Measuring Frailty in Elderly People. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 738–743. [Google Scholar] [CrossRef]
- Van Kan, G.A.; Rolland, Y.; Bergman, H.; Morley, J.E.; Kritchevsky, S.B.; Vellas, B.; On Behalf of the Geriatric Advisory Panel. The I.A.N.A. Task Force on Frailty Assessment of Older People in Clinical Practice. J. Nutr. Health Aging 2008, 12, 29–37. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Pacho, C.; Domingo, M.; Núñez, R.; Lupón, J.; Núñez, J.; Barallat, J.; Moliner, P.; de Antonio, M.; Santesmases, J.; Cediel, G.; et al. Predictive Biomarkers for Death and Rehospitalization in Comorbid Frail Elderly Heart Failure Patients. BMC Geriatr. 2018, 18, 109. [Google Scholar] [CrossRef] [Green Version]
- Komici, K.; Gnemmi, I.; Bencivenga, L.; Vitale, D.F.; Rengo, G.; Di Stefano, A.; Eleuteri, E. Impact of Galectin-3 Circulating Levels on Frailty in Elderly Patients with Systolic Heart Failure. J. Clin. Med. 2020, 9, 2229. [Google Scholar] [CrossRef]
- Singh, M.; Alexander, K.; Roger, V.L.; Rihal, C.S.; Whitson, H.E.; Lerman, A.; Jahangir, A.; Nair, K.S. Frailty and Its Potential Relevance to Cardiovascular Care. Mayo Clin. Proc. 2008, 83, 1146–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, E.; Vidán, M.T.; Serra, J.A.; Fernández-Avilés, F.; Bueno, H. Prevalence of Geriatric Syndromes and Impact on Clinical and Functional Outcomes in Older Patients with Acute Cardiac Diseases. Heart Br. Card. Soc. 2011, 97, 1602–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.U.; Tsai, V.; Klein, L.; Heidenreich, P.A. Characteristics and Outcomes of Very Elderly Patients after First Hospitalization for Heart Failure. Circ. Heart Fail. 2011, 4, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A Simple Frailty Questionnaire (FRAIL) Predicts Outcomes in Middle Aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower Extremity Function and Subsequent Disability: Consistency across Studies, Predictive Models, and Value of Gait Speed Alone Compared with the Short Physical Performance Battery. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef] [Green Version]
- Cacciatore, F.; Amarelli, C.; Maiello, C.; Mattucci, I.; Salerno, G.; Di Maio, M.; Palmieri, V.; Curcio, F.; Pirozzi, F.; Mercurio, V.; et al. Sacubitril/Valsartan in Patients Listed for Heart Transplantation: Effect on Physical Frailty. ESC Heart Fail. 2020, 7, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Bertschi, D.; Moser, A.; Stortecky, S.; Zwahlen, M.; Windecker, S.; Carrel, T.; Stuck, A.E.; Schoenenberger, A.W. Evolution of Basic Activities of Daily Living Function in Older Patients One Year After Transcatheter Aortic Valve Implantation. J. Am. Geriatr. Soc. 2021, 69, 500–505. [Google Scholar] [CrossRef]
- Denfeld, Q.E.; Winters-Stone, K.; Mudd, J.O.; Gelow, J.M.; Kurdi, S.; Lee, C.S. The Prevalence of Frailty in Heart Failure: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 236, 283–289. [Google Scholar] [CrossRef]
- Zheng, P.-P.; Yao, S.-M.; Shi, J.; Wan, Y.-H.; Guo, D.; Cui, L.-L.; Sun, N.; Wang, H.; Yang, J.-F. Prevalence and Prognostic Significance of Frailty in Gerontal Inpatients with Pre-Clinical Heart Failure: A Subgroup Analysis of a Prospective Observational Cohort Study in China. Front. Cardiovasc. Med. 2020, 7, 607439. [Google Scholar] [CrossRef]
- Murad, K.; Kitzman, D.W. Frailty and Multiple Comorbidities in the Elderly Patient with Heart Failure: Implications for Management. Heart Fail. Rev. 2012, 17, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Aprahamian, I.; Sassaki, E.; Dos Santos, M.F.; Izbicki, R.; Pulgrossi, R.C.; Biella, M.M.; Borges, A.C.N.; Sassaki, M.M.; Torres, L.M.; Fernandez, Í.S.; et al. Hypertension and Frailty in Older Adults. J. Clin. Hypertens. 2018, 20, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assar, M.E.; Laosa, O.; Rodríguez Mañas, L. Diabetes and Frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 52–57. [Google Scholar] [CrossRef]
- Martínez-Sellés, M.; Teresa Vidán, M.; López-Palop, R.; Rexach, L.; Sánchez, E.; Datino, T.; Cornide, M.; Carrillo, P.; Ribera, J.M.; Díaz-Castro, Ó.; et al. End-Stage Heart Disease in the Elderly. Rev. Esp. Cardiol. Engl. Ed. 2009, 62, 409–421. [Google Scholar] [CrossRef]
- Molvin, J.; Jujic, A.; Bachus, E.; Gallo, W.; Tasevska-Dinevska, G.; Holm, H.; Melander, O.; Fedorowski, A.; Magnusson, M. Cardiovascular Biomarkers Predict Post-Discharge Re-Hospitalization Risk and Mortality among Swedish Heart Failure Patients. ESC Heart Fail. 2019, 6, 992–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, M.; Honda, H.; Takahashi, K.; Shishido, K.; Shibata, T. N-Terminal Pro-B-Type Natriuretic Peptide as a Biomarker for Loss of Muscle Mass in Prevalent Hemodialysis Patients. PLoS ONE 2016, 11, e0166804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Peet, P.G.; de Craen, A.J.M.; Gussekloo, J.; de Ruijter, W. Plasma NT-ProBNP as Predictor of Change in Functional Status, Cardiovascular Morbidity and Mortality in the Oldest Old: The Leiden 85-plus Study. AGE 2014, 36, 9660. [Google Scholar] [CrossRef] [Green Version]
- Graf, C.E.; Herrmann, F.R.; Genton, L. Relation of Disease with Standardized Phase Angle among Older Patients. J. Nutr. Health Aging 2018, 22, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Kohara, K.; Tabara, Y.; Ochi, M.; Nagai, T.; Igase, M.; Miki, T. Sarcopenic Obesity and Arterial Stiffness, Pressure Wave Reflection and Central Pulse Pressure: The J-SHIPP Study. Int. J. Cardiol. 2014, 174, 214–217. [Google Scholar] [CrossRef]
- Moro, C.; Lafontan, M. Natriuretic Peptides and CGMP Signaling Control of Energy Homeostasis. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H358–H368. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.; Yang, X.; Tin Lui, L.; Li, Q.; Fai Cheng, K.; Fan, Y.; Yau, F.; Lee, A.P.W.; Lee, J.S.W.; Fung, E. Utility of the FRAIL Questionnaire in Detecting Heart Failure with Preserved Ejection Fraction. J. Nutr. Health Aging 2019, 23, 373–377. [Google Scholar] [CrossRef]
- Cannizzo, E.S.; Clement, C.C.; Sahu, R.; Follo, C.; Santambrogio, L. Oxidative Stress, Inflamm-Aging and Immunosenescence. J. Proteom. 2011, 74, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.; Collerton, J.; Martin-Ruiz, C.; Jagger, C.; von Zglinicki, T.; Rockwood, K.; Kirkwood, T.B.L. Age-Related Frailty and Its Association with Biological Markers of Ageing. BMC Med. 2015, 13, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Frail Patients (n: 17; 16.67%) | Non-Frail Patients (n: 85; 83.33%) | p |
|---|---|---|---|
| Age (years old) | 68 ± 5 | 62 ± 9 | <0.001 |
| Women | 6 (35.3%) | 25 (29.4%) | 0.630 |
| Hypertension | 14 (82.4%) | 48 (56.5%) | 0.046 |
| Diabetes | 10 (58.8%) | 21 (24.7%) | 0.005 |
| Atrial fibrillation | 8 (47.1%) | 31 (36.5%) | 0.412 |
| LVEF < 40% | 7 (41.2%) | 41 (48.2%) | 0.595 |
| ST2 (ng/mL) | 128.11 ± 162.54 | 81.92 ± 83.01 | 0.300 |
| Troponin T us (ng/L) | 98.62 ± 246.36 | 73.66 ± 170.82 | 0.620 |
| CRP (mg/L) | 25.03 ± 21.21 | 21.72 ± 34.70 | 0.707 |
| NT-proBNP (pg/mL) | 8704.94 ± 8090.29 | 6028.73 ± 915.02 | 0.161 |
| GFR (mL/min/1.73 m2) | 56.56 ± 23.23 | 63.87 ± 23.74 | 0.142 |
| Hemoglobin (g/dL) | 12.93 ± 2.50 | 13.43 ± 2.32 | 0.417 |
| Variable | Frail Patients (n: 51; 51.51%) | Non-Frail Patients (n: 48; 48.48%) | p |
|---|---|---|---|
| Age (years old) | 84 ± 5 | 82 ± 5 | 0.069 |
| Women | 22 (43.1%) | 25 (52.1%) | 0.373 |
| Hypertension | 44 (83.3%) | 34 (70.8%) | 0.060 |
| Diabetes | 18 (35.3%) | 15 (31.2%) | 0.670 |
| Atrial fibrillation | 31 (60.8%) | 20 (41.7%) | 0.057 |
| LVEF < 40% | 20 (39.2%) | 17 (35.4%) | 0.696 |
| ST2 (ng/mL) | 108.17 ± 84.16 | 85.49 ± 72.69 | 0.180 |
| Troponin T us (ng/L) | 149.55 ± 401.09 | 130.31 ± 245.94 | 0.776 |
| CRP (mg/L) | 26.30 ± 49.44 | 22.15 ± 35.07 | 0.636 |
| NT-proBNP (pg/mL) | 12,297.61 ± 13,710.20 | 7709.40 ± 8374.26 | 0.046 |
| GFR (mL/min/1.73 m2) | 46.24 ± 18.85 | 51.84 ± 17.23 | 0.127 |
| Hemoglobin (g/dL) | 12.74 ± 2.09 | 12.46 ± 1.85 | 0.480 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Iglesias, L.; Merino-Merino, A.; Sanchez-Corral, E.; Garcia-Sanchez, M.-J.; Santos-Sanchez, I.; Saez-Maleta, R.; Perez-Rivera, J.-A. Differences According to Age in the Diagnostic Performance of Cardiac Biomarkers to Predict Frailty in Patients with Acute Heart Failure. Biomolecules 2022, 12, 245. https://doi.org/10.3390/biom12020245
Aguilar-Iglesias L, Merino-Merino A, Sanchez-Corral E, Garcia-Sanchez M-J, Santos-Sanchez I, Saez-Maleta R, Perez-Rivera J-A. Differences According to Age in the Diagnostic Performance of Cardiac Biomarkers to Predict Frailty in Patients with Acute Heart Failure. Biomolecules. 2022; 12(2):245. https://doi.org/10.3390/biom12020245
Chicago/Turabian StyleAguilar-Iglesias, Lara, Ana Merino-Merino, Ester Sanchez-Corral, Maria-Jesus Garcia-Sanchez, Isabel Santos-Sanchez, Ruth Saez-Maleta, and Jose-Angel Perez-Rivera. 2022. "Differences According to Age in the Diagnostic Performance of Cardiac Biomarkers to Predict Frailty in Patients with Acute Heart Failure" Biomolecules 12, no. 2: 245. https://doi.org/10.3390/biom12020245







