6-Hydroxydopamine Induces Abnormal Iron Sequestration in BV2 Microglia by Activating Iron Regulatory Protein 1 and Inhibiting Hepcidin Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. BV2 Microglial Cell Culture
2.3. Drug Treatment
2.4. ELISA of Hepcidin
2.5. Western Blotting
2.6. Total RNA Extraction and Real-Time PCR Analysis
- IL-1β forward: 5′-TCCAGGATGAGGACATGAGCAC-3′,
- reverse: 5′-GAACGTCACACACCAGCAGGTTA-3′.
- TNF-α forward: 5′-TATGGCCCAGACCCTCACA-3′,
- reverse: 5′-GGAGTAGACAAGGTACAACCCATC-3′.
- GAPDH gene was used as the reference gene:
- forward: 5′-AAATGGTGAAGGTCGGTGTGAAC-3′,
- reverse: 5′-CAACAATCTCCACTTTGCCACTG-3′.
2.7. Calcein Loading of Cells and Ferrous Iron Influx and Efflux Assay
2.8. Statistical Analysis
3. Results
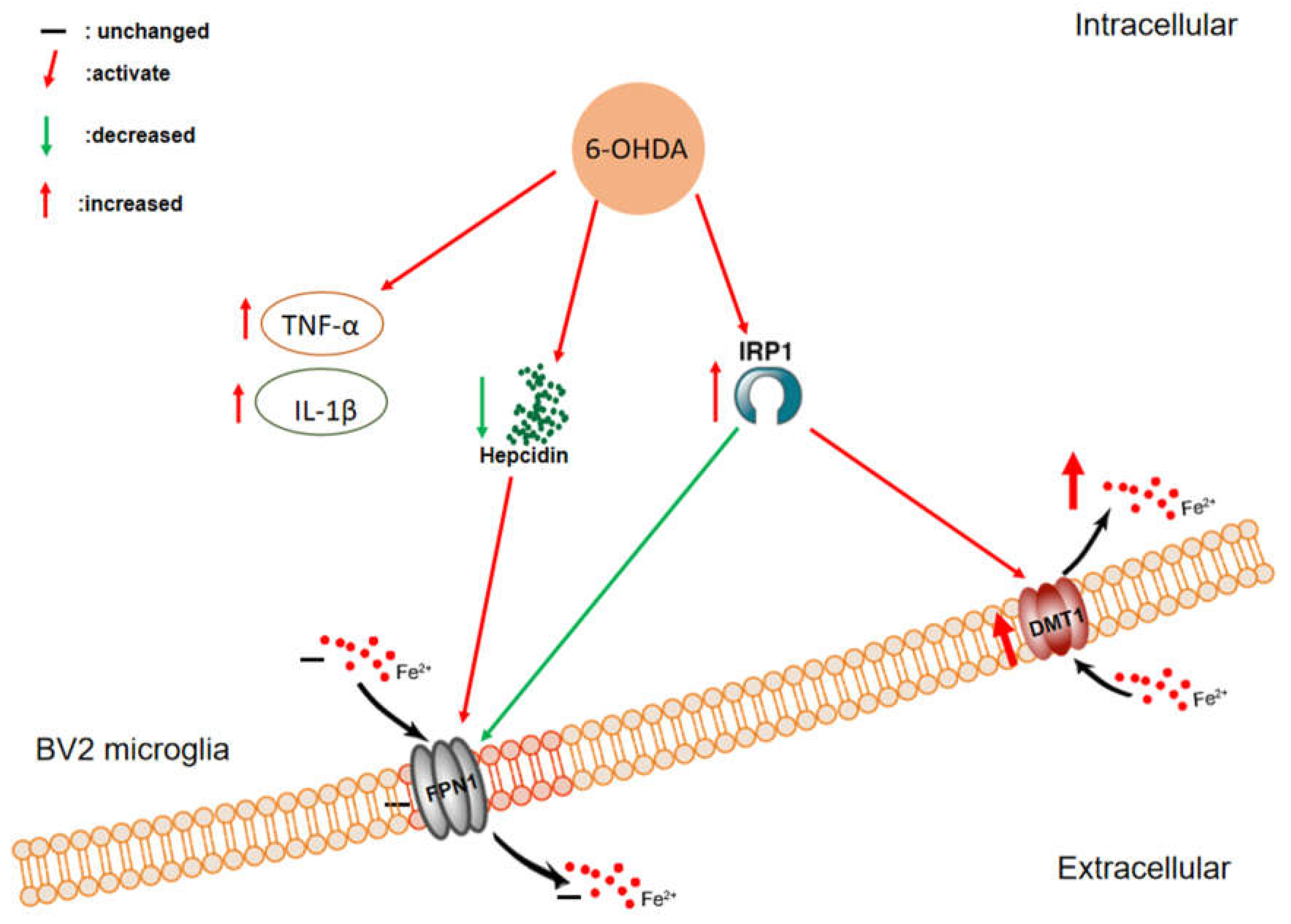
3.1. 6-OHDA Specifically Upregulates the Expression of DMT1 but Not FPN1 in BV2 Microglial Cells
3.2. 6-OHDA Activates the Expression of IRP1 in BV2 Microglial Cells
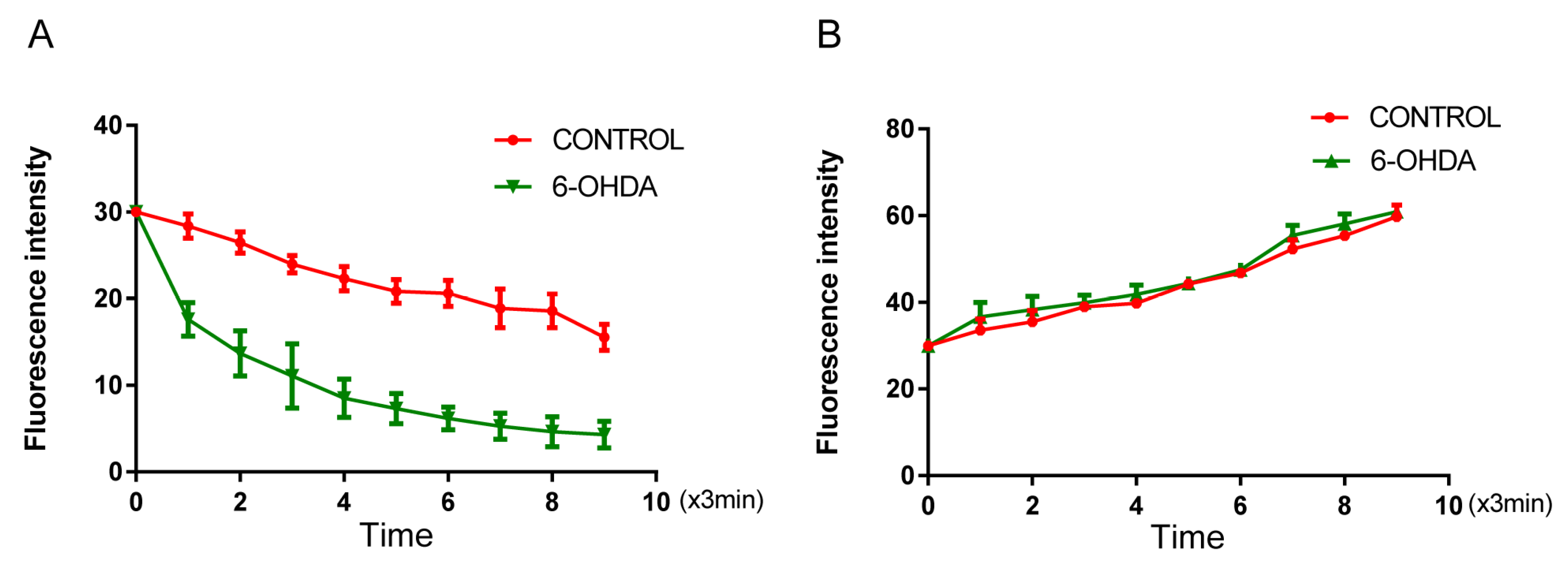
3.3. 6-OHDA Enhances Iron Influx in BV2 Microglial Cells without Affecting Iron Efflux
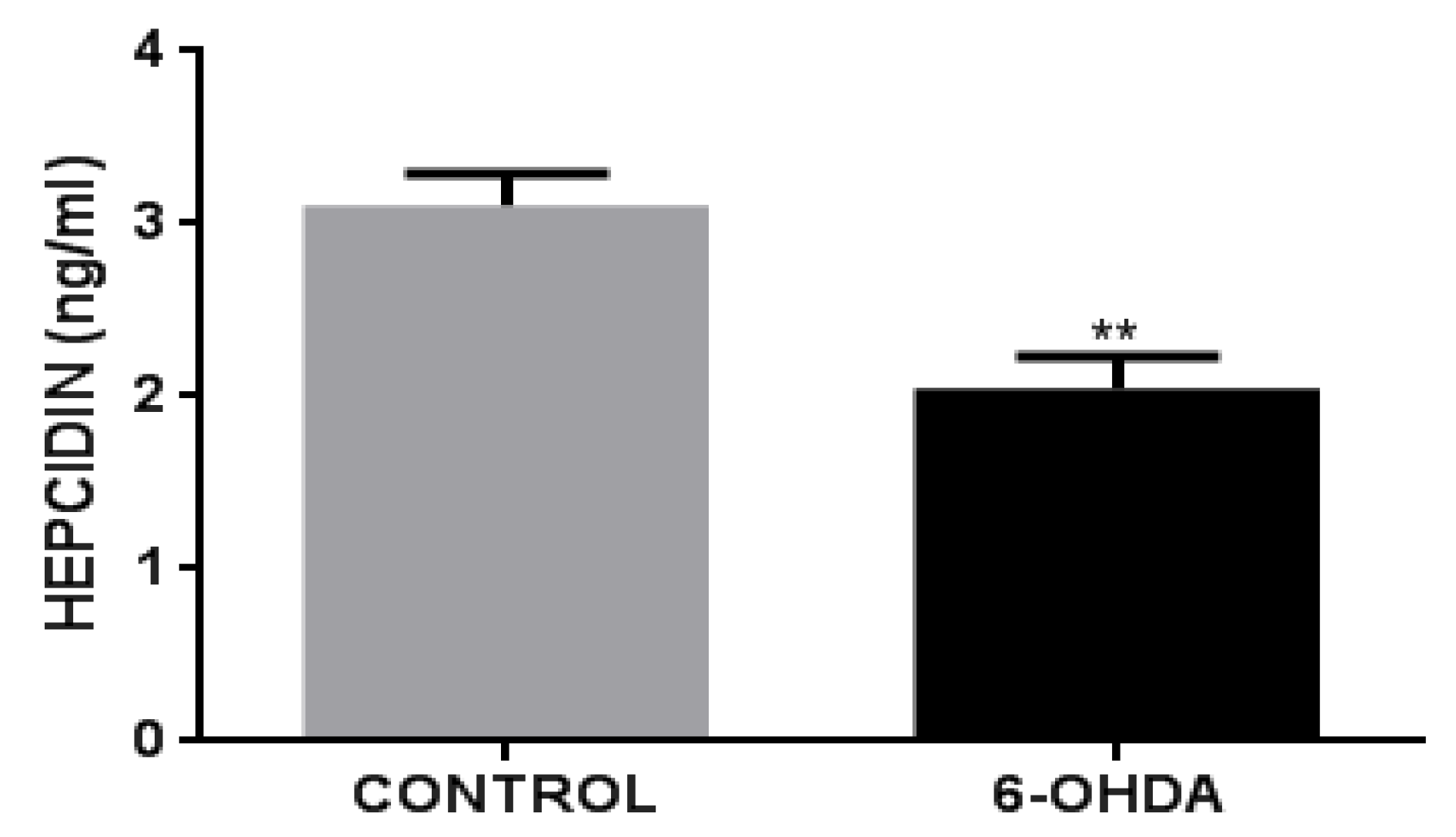
3.4. 6-OHDA Decreases Hepcidin Release in BV2 Cells
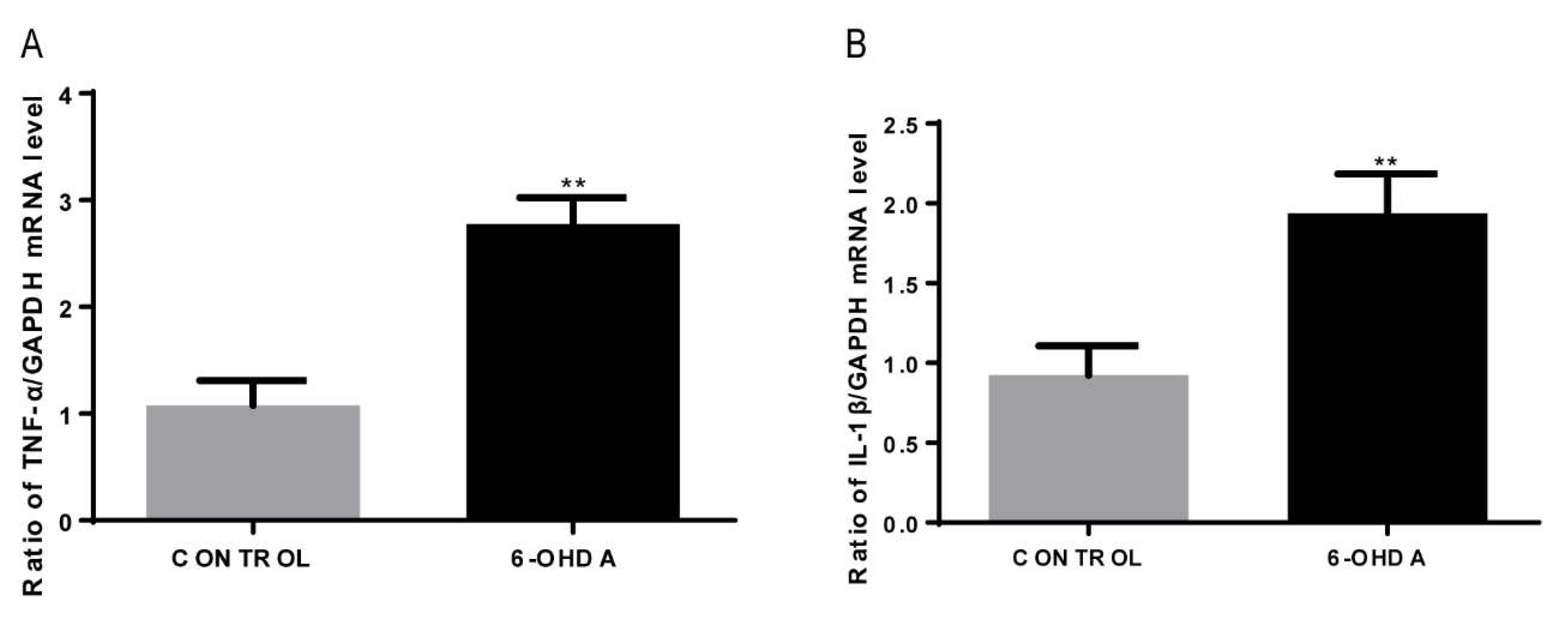
3.5. 6-OHDA Increases the mRNA Expression of IL-1β and TNF-α in BV2 Cells
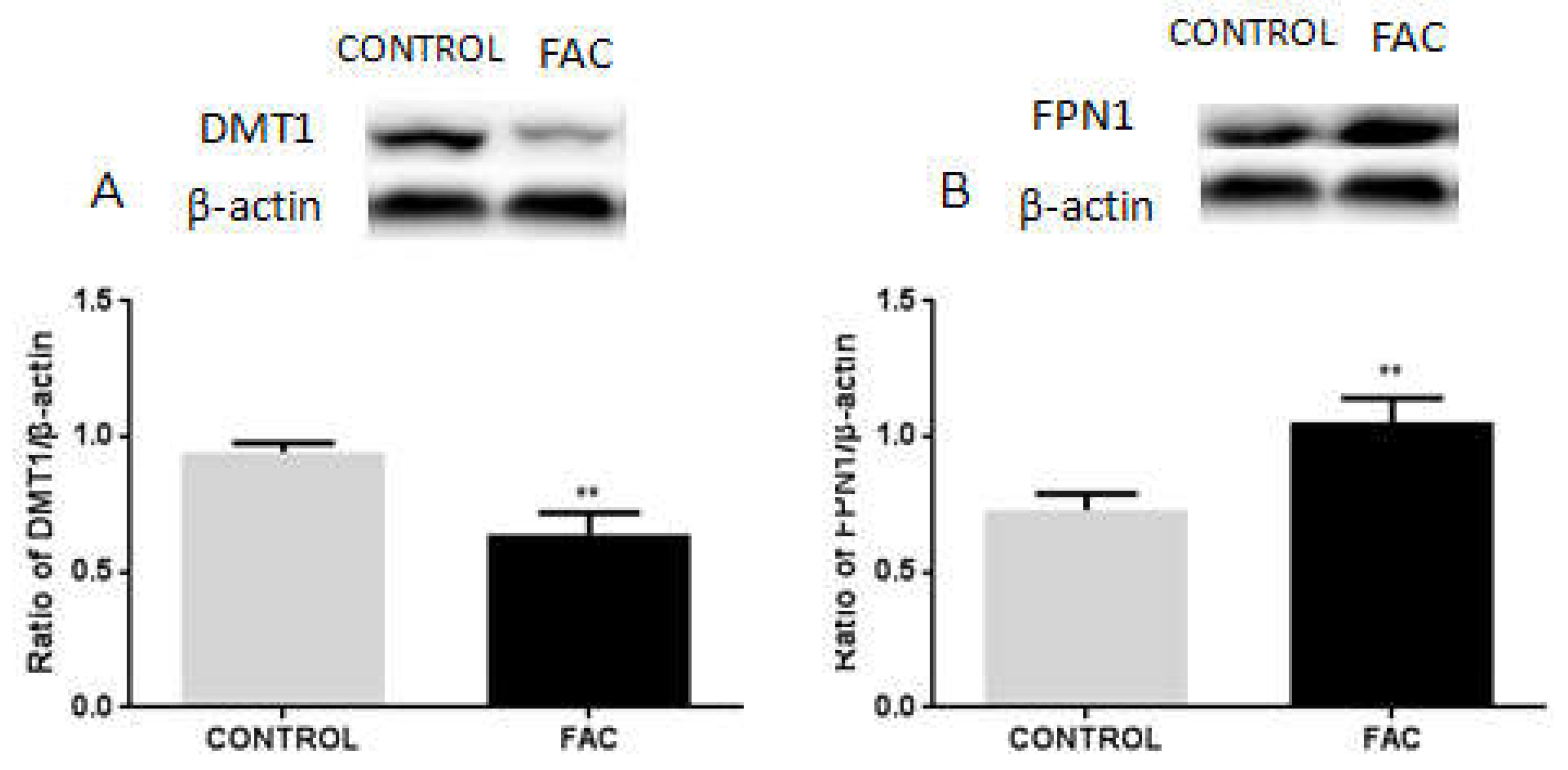
3.6. FAC Decreases the Expression of DMT1 and Increases FPN1 Expression in BV2 Cells
3.7. FAC Decreases the Expression of IRP1 in BV2 Cells
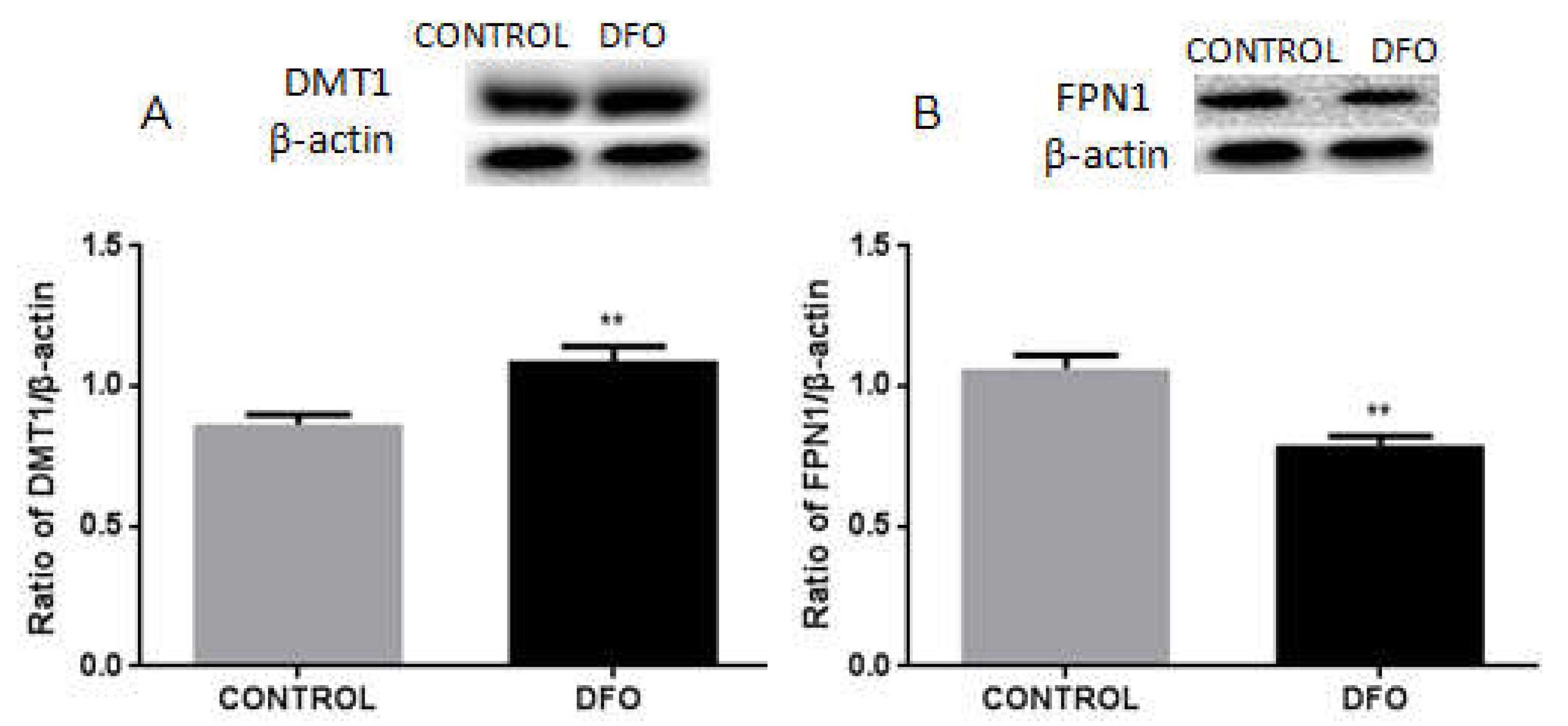
3.8. DFO Increases the Expression of DMT1 but Decreases FPN1 Expression in BV2 Cells
3.9. DFO Increases the Expression of IRP1 in BV2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toulouse, A.; Sullivan, A.M. Progress in Parkinson’s disease-where do we stand? Prog. Neurobiol. 2008, 85, 376–392. [Google Scholar] [CrossRef]
- Lawton, M.; Baig, F.; Toulson, G.; Morovat, A.; Evetts, S.G.; Ben-Shlomo, Y.; Hu, M.T. Blood biomarkers with Parkinson’s disease clusters and prognosis: The oxford discovery cohort. Mov. Disord. 2020, 35, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef]
- Giasson, B.I.; Ischiropoulos, H.; Lee, V.M.; Trojanowski, J.Q. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer’s and Parkinson’s diseases. Free Radic. Biol. Med. 2002, 32, 1264–1275. [Google Scholar] [CrossRef]
- Veldman, B.A.; Wijn, A.M.; Knoers, N.; Praamstra, P.; Horstink, M.W. Genetic and environmental risk factors in Parkinson’s disease. Clin. Neurol. Neurosurg. 1998, 100, 15–26. [Google Scholar] [CrossRef]
- De la Fuente-Fernández, R.; Calne, D.B. Evidence for environmental causation of Parkinson’s disease. Parkinsonism Relat. Disord. 2002, 8, 235–241. [Google Scholar] [CrossRef]
- Chen, L.L.; Huang, Y.J.; Cui, J.T.; Song, N.; Xie, J. Iron Dysregulation in Parkinson’s Disease: Focused on the Autophagy-Lysosome Pathway. ACS Chem. Neurosci. 2019, 10, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, C.; Xie, J. Axonal Iron Transport might Contribute to Iron Deposition in Parkinson’s Disease. Neurosci. Bull. 2021, 37, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, H.; Xie, J.X. Ferroportin1 and hephaestin are involved in the nigral iron accumulation of 6-OHDA-lesioned rats. Eur. J. Neurosci. 2007, 25, 2766–2772. [Google Scholar] [CrossRef]
- Jiang, H.; Song, N.; Xu, H.; Zhang, S.; Wang, J.; Xie, J. Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res. 2010, 20, 345–356. [Google Scholar] [CrossRef]
- Song, N.; Wang, J.; Jiang, H.; Xie, J. Ferroportin 1 but not hephaestin contributes to iron accumulation in a cell model of Parkinson’s disease. Free Radic. Biol. Med. 2010, 48, 332–341. [Google Scholar] [CrossRef]
- Junxia, X.; Hong, J.; Wenfang, C.; Ming, Q. Dopamine release rather than content in the caudate putamen is associated with behavioral changes in the iron rat model of Parkinson’s disease. Exp. Neurol. 2003, 182, 483–489. [Google Scholar] [CrossRef]
- Singh, N.; Haldar, S.; Tripathi, A.K.; McElwee, M.K.; Horback, K.; Beserra, A. Iron in neurodegenerative disorders of protein misfolding: A case of prion disorders and Parkinson’s disease. Antioxid. Redox Signal. 2014, 21, 471–484. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.L.; Wang, Y.B.; Song, J.X.; Deng, W.K.; Lu, J.H.; Ma, L.L.; Yang, C.B.; Li, M.; Xue, Y. Phosphoproteome-based kinase activity profiling reveals the critical role of MAP2K2 and PLK1 in neuronal autophagy. Autophagy 2017, 13, 1969–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanatori, I.; Kishi, F. DMT1 and iron transport. Free Radic. Biol. Med. 2019, 133, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.L.; Gelbart, T.; West, C.; Halloran, C.; Beutler, E. The human Nramp2 gene: Characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol. Dis. 1998, 24, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, R.S. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu. Rev. Nutr. 2000, 20, 627–662. [Google Scholar] [CrossRef]
- Galy, B.; Ferring-Appel, D.; Kaden, S.; Gröne, H.J.; Hentze, M.W. Iron regulatory proteins are essential for intestinal function and control key iron absorption molecules in the duodenum. Cell Metab. 2008, 7, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T. Cellular iron: Ferroportin is the only way out. Cell Metab. 2005, 1, 155–157. [Google Scholar] [CrossRef] [Green Version]
- Muckenthaler, M.; Gray, N.K.; Hentze, M.W. IRP-1 binding to ferritin mRNA prevents the recruitment of the small ribosomal subunit by the cap-binding complex eIF4F. Mol. Cell 1998, 2, 383–388. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.L.; Senecal, T.; Ghosh, M.C.; Ollivierre-Wilson, H.; Tu, T.; Rouault, T.A. Hepcidin regulates ferroportin expression and intracellular iron homeostasis of erythroblasts. Blood 2011, 118, 2868–2877. [Google Scholar] [CrossRef] [Green Version]
- Berg, D.; Hochstrasser, H. Iron metabolism in Parkinsonian syndromes. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 1299–1310. [Google Scholar] [CrossRef]
- Sui, Y.; Stanić, D.; Tomas, D.; Jarrott, B.; Horne, M.K. Meloxicam reduces lipopolysaccharide-induced degeneration of dopaminergic neurons in the rat substantia nigra pars compacta. Neurosci. Lett. 2009, 460, 121–125. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, N.D.; Song, N.; Xu, H.M.; Shi, L.M.; Jiang, H.; Xie, J.X. 6-Hydroxydopamine promotes iron traffic in primary cultured astrocytes. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2013, 26, 705–714. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Song, N.; Jiang, H.; Bi, M.X.; Xie, J.X. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor inhibit ferrous iron influx via divalent metal transporter 1 and iron regulatory protein 1 regulation in ventral mesencephalic neurons. Biochim. Biophys. Acta 2014, 1843, 2967–2975. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, X.; You, L.H.; Ci, Y.Z.; Chang, S.; Yu, P.; Gao, G.; Chang, Y.Z. Hepcidin and iron regulatory proteins coordinately regulate ferroportin 1 expression in the brain of mice. J. Cell. Physiol. 2019, 234, 7600–7607. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Holscher, C.; Chen, B.B.; Zhang, Z.F.; Liu, Y.Z. Hepcidin treatment modulates the expression of divalent metal transporter-1, ceruloplasmin, and ferroportin-1 in the rat cerebral cortex and hippocampus. Biol. Trace Elem. Res. 2011, 143, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.C.; Sosa, J.C.; Gardeck, A.M.; Baez, A.S.; Lee, C.H.; Wessling-Resnick, M. Inflammation-induced iron transport and metabolism by brain microglia. J. Biol. Chem. 2018, 293, 7853–7863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 2013, 61, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 10, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. Glial reactions in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Li, Y.; Meng, D.; Zhang, D.; Wang, B.; Xie, J.; Wang, J. 6-Hydroxydopamine Induces Abnormal Iron Sequestration in BV2 Microglia by Activating Iron Regulatory Protein 1 and Inhibiting Hepcidin Release. Biomolecules 2022, 12, 266. https://doi.org/10.3390/biom12020266
Xu M, Li Y, Meng D, Zhang D, Wang B, Xie J, Wang J. 6-Hydroxydopamine Induces Abnormal Iron Sequestration in BV2 Microglia by Activating Iron Regulatory Protein 1 and Inhibiting Hepcidin Release. Biomolecules. 2022; 12(2):266. https://doi.org/10.3390/biom12020266
Chicago/Turabian StyleXu, Manman, Yinghui Li, Dapeng Meng, Danyang Zhang, Bingjing Wang, Junxia Xie, and Jun Wang. 2022. "6-Hydroxydopamine Induces Abnormal Iron Sequestration in BV2 Microglia by Activating Iron Regulatory Protein 1 and Inhibiting Hepcidin Release" Biomolecules 12, no. 2: 266. https://doi.org/10.3390/biom12020266





