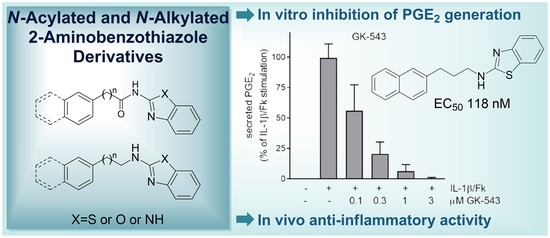
N-Acylated and N-Alkylated 2-Aminobenzothiazoles Are Novel Agents That Suppress the Generation of Prostaglandin E2
Abstract
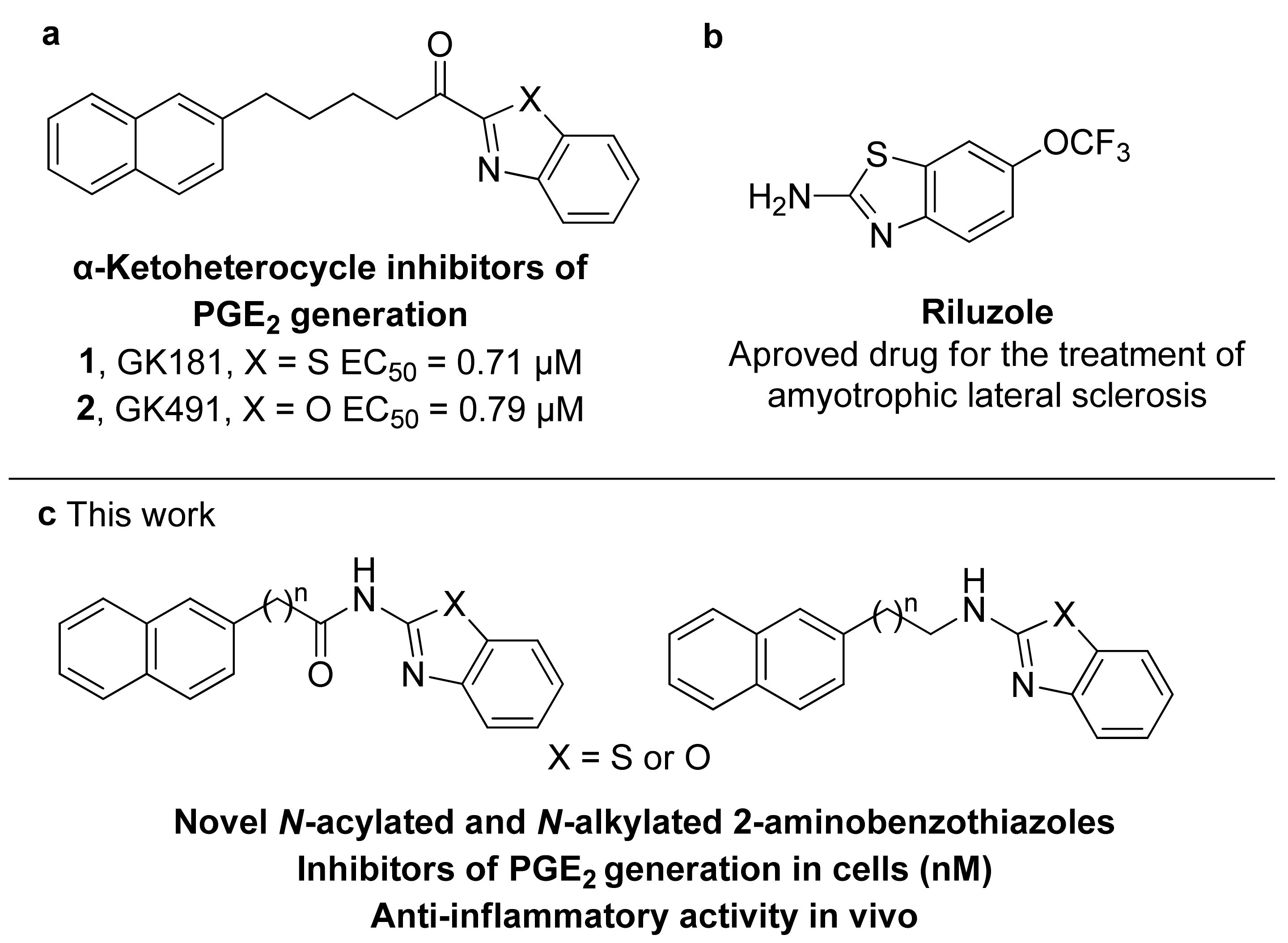
:1. Introduction
2. Materials and Methods
2.1. General Chemistry Methods
2.2. General Procedure for the Synthesis of Amides 5a–f, 7, 9a,b, 14
2.2.1. N-(Benzo[d]Thiazol-2-yl)-4-(Naphthalen-2-yl)Butanamide (5a)
2.2.2. N-(Benzo[d]Thiazol-2-yl)-3-(Naphthalen-2-yl)Propanamide (5b, GK510)
2.2.3. N-(Benzo[d]Oxazol-2-yl)-3-(Naphthalen-2-yl)Propanamide (5c)
2.2.4. N-(Benzo[d]Oxazol-2-yl)-4-(Naphthalen-2-yl)Butanamide (5d)
2.2.5. N-(1H-Benzo[d]Imidazol-2-yl)-3-(Naphthalen-2-yl)Propanamide (5e)
2.2.6. N-(1H-Benzo[d]Imidazol-2-yl)-4-(Naphthalen-2-yl)Butanamide (5f)
2.2.7. N-(Benzo[d]Thiazol-2-yl)-2-(Naphthalen-2-yloxy)Acetamide (7)
2.2.8. (E)-N-(Benzo[d]Thiazol-2-yl)-3-(Naphthalen-2-yl)Acrylamide (9a)
2.2.9. (E)-N-(Benzo[d]Thiazol-2-yl)-3-(4-Methoxyphenyl)Acrylamide (9b)
2.2.10. N-(3-(Naphthalen-2-yl)Propyl)Benzo[d]Thiazole-2-Carboxamide (14)
2.3. N-(Benzo[d]Thiazol-2-yl)-3-(4-Methoxyphenyl)Propanamide (11)
2.4. General Procedure for the Synthesis of Carbamates 16a,b
2.4.1. 4-Methoxybenzyl Benzo[d]Thiazol-2-ylcarbamate (16a)
2.4.2. Naphthalen-2-ylmethyl Benzo[d]Thiazol-2-ylcarbamate (16b)
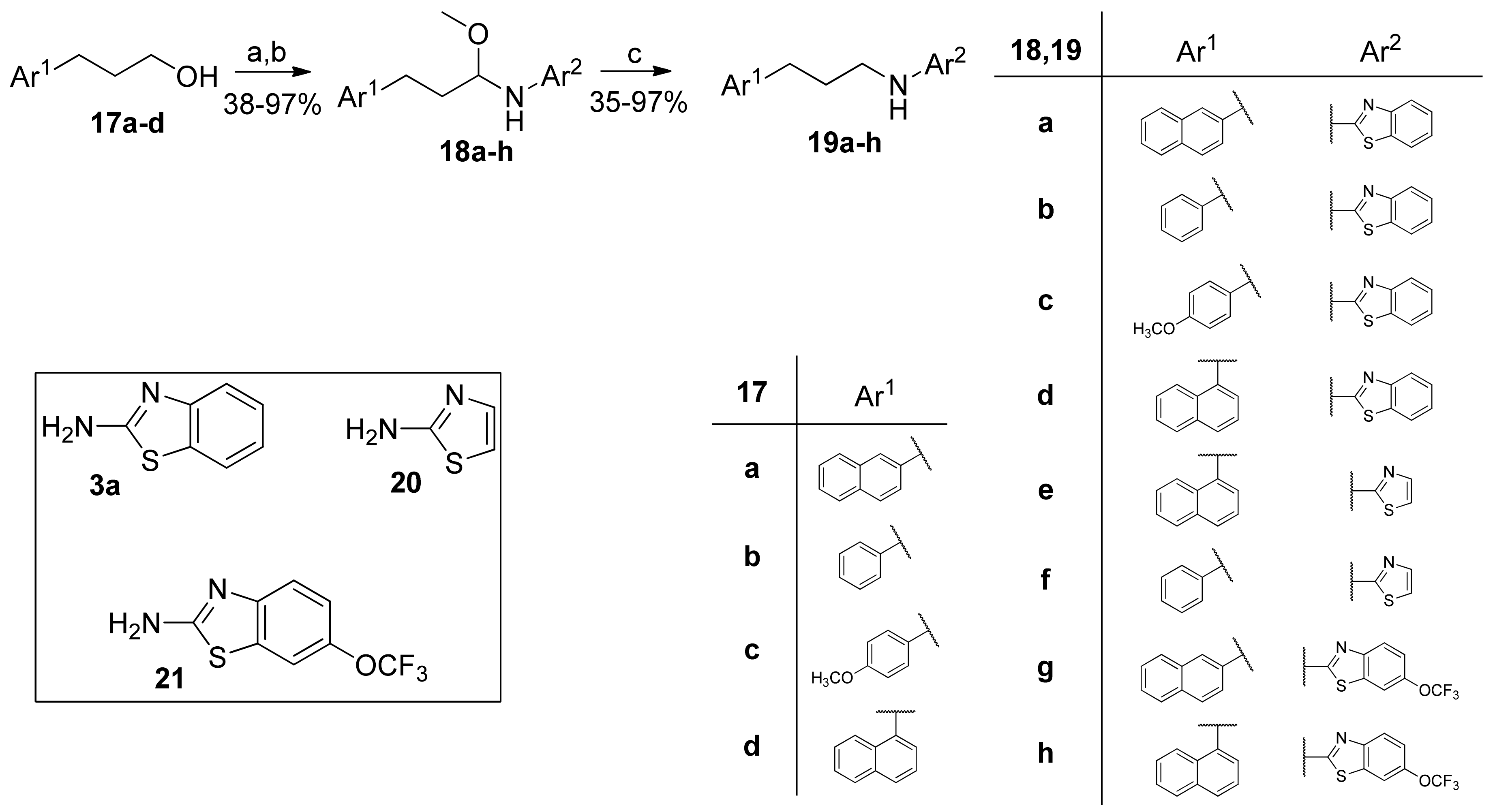
2.5. General Procedure for the Synthesis of Hemiaminal Ethers 18a–h
2.5.1. N-(1-Methoxy-3-(Naphthalen-2-yl)Propyl)Benzo[d]Thiazol-2-Amine (18a)
2.5.2. N-(1-Methoxy-3-Phenylpropyl)Benzo[d]Thiazol-2-Amine (18b)
2.5.3. N-(1-Methoxy-3-(4-Methoxyphenyl)Propyl)Benzo[d]Thiazol-2-Amine (18c)
2.5.4. N-(1-Methoxy-3-(Naphthalen-1-yl)Propyl)Benzo[d]Thiazol-2-Amine (18d)
2.5.5. N-(1-Methoxy-3-(Naphthalen-2-yl)Propyl)-6-(Trifluoromethoxy)Benzo[d]Thiazol-2-Amine (18g)
2.5.6. N-(1-Methoxy-3-(Naphthalen-1-yl)Propyl)-6-(Trifluoromethoxy)Benzo[d]Thiazol-2-Amine (18h)
2.6. General Procedure for the Reduction of Hemiaminal Ethers to 2-Aminobenzothiazoles 19a–h
2.6.1. N-(3-(Naphthalen-2-yl)Propyl)Benzo[d]Thiazol-2-Amine (19a, GK543)
2.6.2. N-(3-Phenylpropyl)Benzo[d]Thiazol-2-Amine (19b, GK562)
2.6.3. N-(3-(4-Methoxyphenyl)Propyl)Benzo[d]Thiazol-2-Amine (19c)
2.6.4. N-(3-(Naphthalen-1-yl)Propyl)Benzo[d]Thiazol-2-Amine (19d)
2.6.5. N-(3-(Naphthalen-1-yl)Propyl)Thiazol-2-Amine (19e)
2.6.6. N-(3-Phenylpropyl)Thiazol-2-Amine (19f)
2.6.7. N-(3-(Naphthalen-2-yl)Propyl)-6-(Trifluoromethoxy)Benzo[d]Thiazol-2-Amine (19g)
2.6.8. N-(3-(Naphthalen-1-yl)Propyl)-6-(Trifluoromethoxy)Benzo[d]Thiazol-2-Amine (19h)
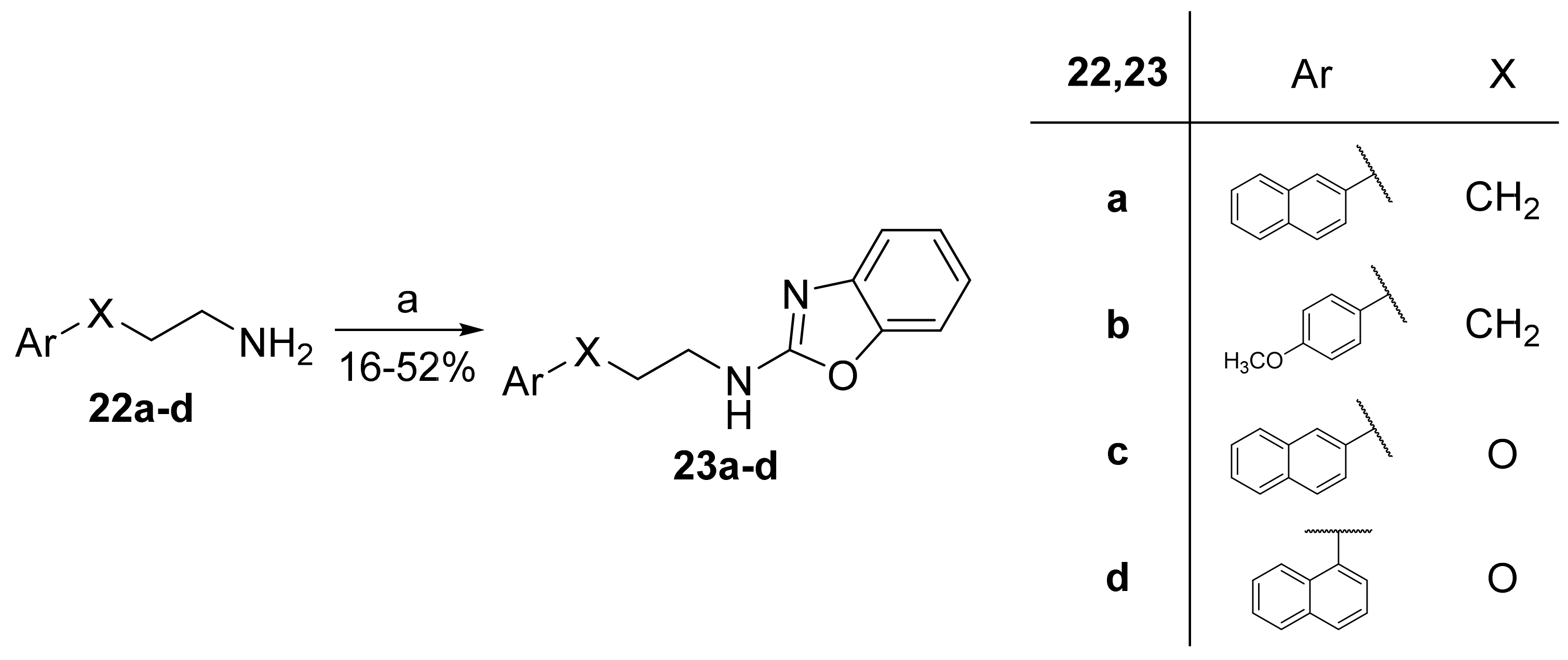
2.7. General Procedure for the Synthesis of 2-Aminobenzoxazoles 23a–d
2.7.1. N-(3-(Naphthalen-2-yl)Propyl)Benzo[d]Oxazol-2-Amine (23a)
2.7.2. N-(3-(4-Methoxyphenyl)Propyl)Benzo[d]Oxazol-2-Amine (23b)
2.7.3. N-(2-(Naphthalen-2-yloxy)Ethyl)Benzo[d]Oxazol-2-Amine (23c)
2.7.4. N-(2-(Naphthalen-1-yloxy)Ethyl)Benzo[d]Oxazol-2-Amine (23d)
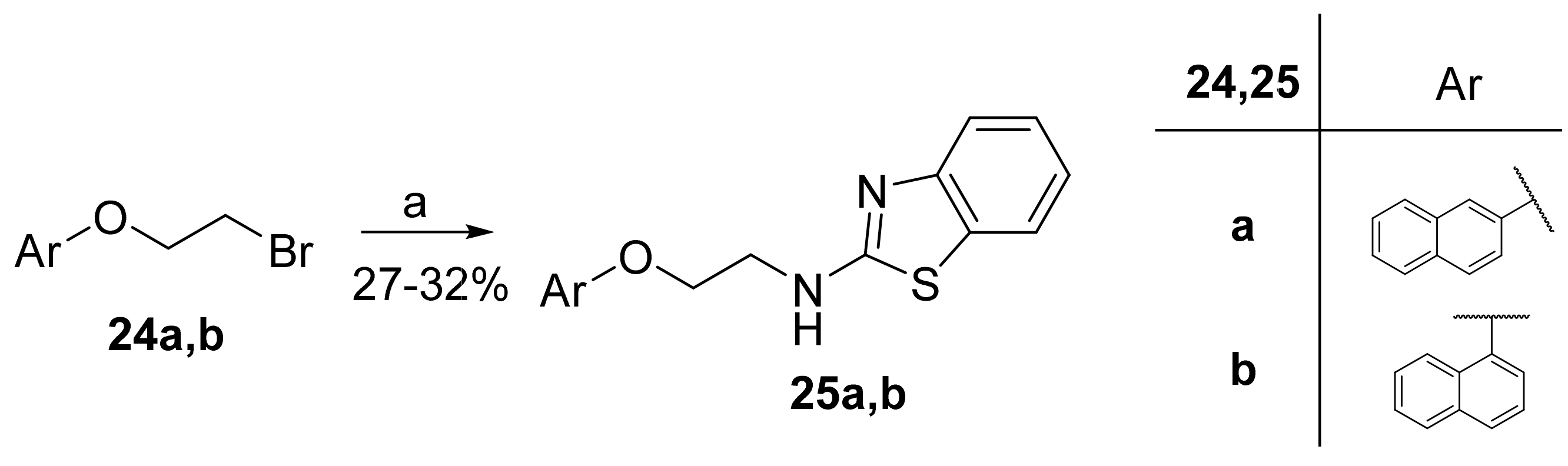
2.8. General Procedure for the Synthesis of 2-Aminobenzothiazoles 25a,b
2.8.1. N-(2-(Naphthalen-2-yloxy)Ethyl)Benzo[d]Thiazol-2-Amine (25a)
2.8.2. N-(2-(Naphthalen-1-yloxy)Ethyl)Benzo[d]Thiazol-2-Amine (25b)
2.9. Biological Assays
2.9.1. Cell Culture
2.9.2. Quantification of PGE2
2.9.3. Statistical Analysis
2.9.4. In Vitro PLA2 Activity Assay
2.9.5. Rat-Paw Carrageenan-Induced-Edema Assay
3. Results and Discussion
3.1. Synthesis of Inhibitors
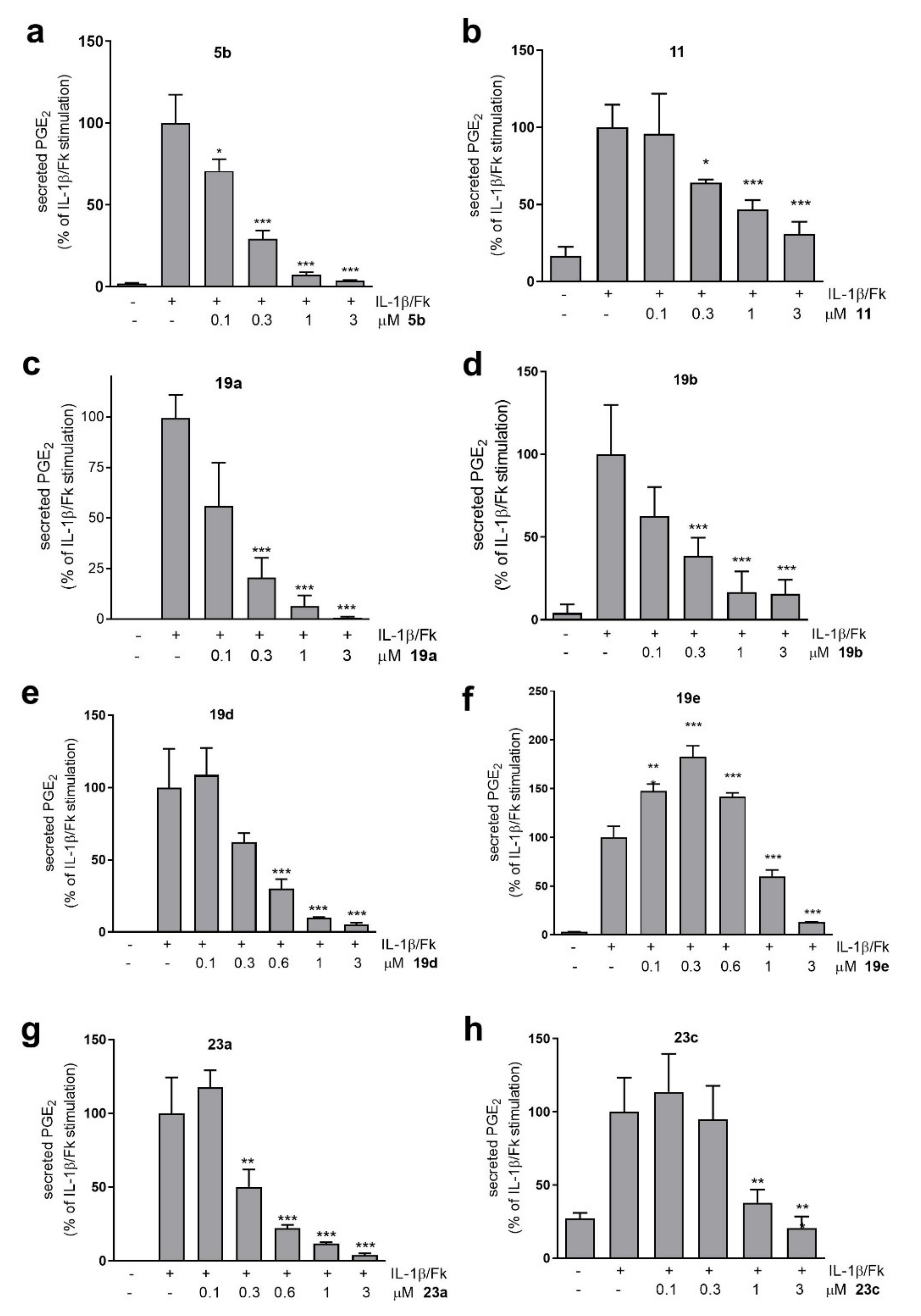
3.2. Study of the Suppression of PGE2 Generation in Mesangial Cells
3.3. Study of the In Vivo Anti-Inflammatory Activity
3.4. Inhibition of Phospholipases A2 by 19a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, S.B. Prostaglandins in health and disease: An overview. Semin. Arthritis Rheum. 2006, 36, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, R.; Kawada, K.; Sakai, Y. Prostaglandin E2/EP signaling in the tumor microenvironment of colorectal cancer. Int. J. Mol. Sci. 2019, 20, 6254. [Google Scholar] [CrossRef] [Green Version]
- Woolbright, B.L.; Pilbeam, C.C.; Taylor, J.A. Prostaglandin E2 as a therapeutic target in bladder cancer: From basic science to clinical trials. Prostaglandins Other Lipid Mediat. 2020, 148, 106409. [Google Scholar] [CrossRef]
- Johansson, J.U.; Woodling, N.S.; Shi, J.; Andreasson, K.I. Inflammatory cyclooxygenase activity and PGE2 signaling in models of Alzheimer’s disease. Curr. Immunol. Rev. 2015, 11, 125–131. [Google Scholar] [CrossRef]
- Grösch, S.; Niederberger, E.; Geisslinger, G. Investigational drugs targeting the prostaglandin E2 signaling pathway for the treatment of inflammatory pain. Expert Opin. Inv. Drugs 2017, 26, 51–61. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [Green Version]
- Koeberle, A.; Laufer, S.A.; Werz, O. Design and development of microsomal prostaglandin E2 synthase-1 inhibitors: Challenges and future directions. J. Med. Chem. 2016, 59, 5970–5986. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.I.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Busquets-Cortés, C.; Capó, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem. 2019, 26, 3225–3241. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kokotou, M.G.; Vasilakaki, S.; Kokotos, G. Small-molecule inhibitors as potential therapeutics and as tools to understand the role of phospholipases A2. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2019, 1864, 941–956. [Google Scholar] [CrossRef]
- Psarra, A.; Nikolaou, A.; Kokotou, M.G.; Limnios, D.; Kokotos, G. Microsomal prostaglandin E2 synthase-1 inhibitors: A patent review. Expert Opin. Ther. Pat. 2017, 27, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, F.; Morgenstern, R.; Jakobsson, P.-J. A review on mPGES-1 inhibitors: From preclinical studies to clinical applications. Prostaglandins Other Lipid Mediat. 2020, 147, 106383. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L.; Kokotos, G.; Svensson, C.I.; Stephens, D.; Kokotos, C.G.; Fitzsimmons, B.; Hadjipavlou-Litina, D.; Hua, X.Y.; Dennis, E.A. Systemic and intrathecal effects of a novel series of phospholipase A2 inhibitors on hyperalgesia and spinal prostaglandin E2 release. J. Pharmacol. Exp. Ther. 2006, 316, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Six, D.A.; Barbayianni, E.; Loukas, V.; Constantinou-Kokotou, V.; Hadjipavlou-Litina, D.; Stephens, D.; Wong, A.C.; Magrioti, V.; Moutevelis-Minakakis, P.; Baker, S.F.; et al. Structure-activity relationship of 2-oxoamide inhibition of group IVA cytosolic phospholipase A2 and group V secreted phospholipase A2. J. Med. Chem. 2007, 50, 4222–4235. [Google Scholar] [CrossRef] [PubMed]
- Kokotos, G.; Feuerherm, A.J.; Barbayianni, E.; Shah, I.; Sæther, M.; Magrioti, V.; Nguyen, T.; Constantinou-Kokotou, V.; Dennis, E.A.; Johansen, B. Inhibition of group IVA cytosolic phospholipase A2 by thiazolyl ketones in vitro, ex vivo, and in vivo. J. Med. Chem. 2014, 57, 7523–7535. [Google Scholar] [CrossRef]
- Vasilakaki, S.; Barbayianni, E.; Magrioti, V.; Pastukhov, O.; Constantinou-Kokotou, V.; Huwiler, A.; Kokotos, G. Inhibitors of secreted phospholipase A2 suppress the release of PGE2 in renal mesangial cells. Bioorg. Med. Chem. 2016, 24, 3029–3034. [Google Scholar] [CrossRef]
- Psarra, A.; Theodoropoulou, M.A.; Erhardt, M.; Mertiri, M.; Mantzourani, C.; Vasilakaki, S.; Magrioti, V.; Huwiler, A.; Kokotos, G. α-Ketoheterocycles able to inhibit the generation of prostaglandin E2 (PGE2) in rat mesangial cells. Biomolecules 2021, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Syed, M.A.; Mohammed, S.M. Therapeutic potential of benzothiazoles: A patent review (2010–2014). Expert Opin. Ther. Pat. 2015, 25, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Rawal, R.K.; Bariwal, J. Recent advances in the chemistry and biology of benzothiazoles. Arch. Pharm. Chem. Life Sci. 2015, 348, 155–178. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207−251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dietrich, J. Privileged scaffolds in lead generation. Expert Opin. Drug Discov. 2015, 10, 781−790. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, M.C. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Chang, H.H.; Medda, F.; Gokhale, V.; Dietrich, J.; Davis, A.; Meuillet, E.J.; Hulme, C. Synthesis and biological activity of 2-aminothiazoles as novel inhibitors of PGE2 production in cells. Bioorg. Med. Chem. Lett. 2012, 22, 3567–3570. [Google Scholar] [CrossRef] [Green Version]
- Chini, M.G.; Giordano, A.; Potenza, M.; Terracciano, S.; Fischer, K.; Vaccaro, M.C.; Colarusso, E.; Bruno, I.; Riccio, R.; Koeberle, A.; et al. Targeting mPGES-1 by a combinatorial approach: Identification of the aminobenzothiazole scaffold to suppress PGE2 levels. ACS Med. Chem. Lett. 2020, 11, 783−789. [Google Scholar] [CrossRef]
- Amnerkar, N.K.; Bhusari, K.P. Synthesis, anticonvulsant activity and 3D-QSAR study of some prop-2-eneamido and 1-acetyl-pyrazolin derivatives of aminobenzothiazole. Eur. J. Med. Chem. 2010, 45, 149–159. [Google Scholar] [CrossRef]
- Li, F.; Shan, H.; Chen, L.; Kang, Q.; Zou, P. Direct N-alkylation of amino-azoles with alcohols catalyzed by an iridium complex/base system. Chem. Commun. 2012, 48, 603–605. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Kurtz, A.; Bauer, C. Role of phospholipase C and protein kinase C in vasoconstrictor-induced prostaglandin synthesis in cultured rat renal mesangial cells. Biochem. J. 1986, 234, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Mouchlis, V.D.; Armando, A.; Dennis, E.A. Substrate-specific inhibition constants for phospholipase A2 acting on unique phospholipid substrates in mixed micelles and membranes using lipidomics. J. Med. Chem. 2019, 62, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Mouchlis, V.D.; Chen, Y.; McCammon, J.A.; Dennis, E.A. Membrane allostery and unique hydrophobic sites promote enzyme substrate specificity. J. Am. Chem. Soc. 2018, 140, 3285–3291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froehr, T.; Sindlinger, C.P.; Kloeckner, U.; Finkbeiner, P.; Nachtsheim, B.J. A metal-free amination of benzoxazoles—The first example of an iodide-catalyzed oxidative amination of heteroarenes. Org. Lett. 2011, 13, 3754−3757. [Google Scholar] [CrossRef]
- Huwiler, A.; Staudt, G.; Kramer, R.M.; Pfeilschifter, J. Cross-talk between secretory phospholipase A2 and cytosolic phospholipase A2 in rat renal mesangial cells. Biochim. Biophys. Acta Lipids Lipid Metab. 1997, 1348, 257–272. [Google Scholar] [CrossRef]
- Huwiler, A.; Feuerherm, A.J.; Sakem, B.; Pastukhov, O.; Filipenko, I.; Nguyen, T.; Johansen, B. The ω3-polyunsaturated fatty acid derivatives AVX001 and AVX002 directly inhibit cytosolic phospholipase A2 and suppress PGE2 formation in mesangial cells: AVX compounds as novel cPLA2 inhibitors. Br. J. Pharmacol. 2012, 167, 1691–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, E.J. Prostagladins: Biphasic dose responses. Crit. Rev. Toxicol. 2001, 31, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, W.; Plüss, C.; Hummel, R.; Pfeilschifter, J. Molecular mechanisms of inducible nitric oxide synthase gene expression by IL-1b and cAMP in rat mesangial cells. J. Immunol. 1998, 160, 4961–4969. [Google Scholar] [PubMed]
- Yeo, J.-F.; Ong, W.-Y.; Ling, S.-F.; Farooqui, A.A. Intracerebroventricular injection of phospholipases A2 inhibitors modulates allodynia after facial carrageenan injection in mice. Pain 2004, 112, 148–155. [Google Scholar] [CrossRef]
- Bone, R.N.; Gai, Y.; Magrioti, V.; Kokotou, M.G.; Ali, T.; Lei, X.; Tse, H.M.; Kokotos, G.; Ramanadham, S. Inhibition of Ca2+-independent phospholipase A2β (iPLA2β) ameliorates islet infiltration and incidence of diabetes in NOD mice. Diabetes 2015, 64, 541–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]

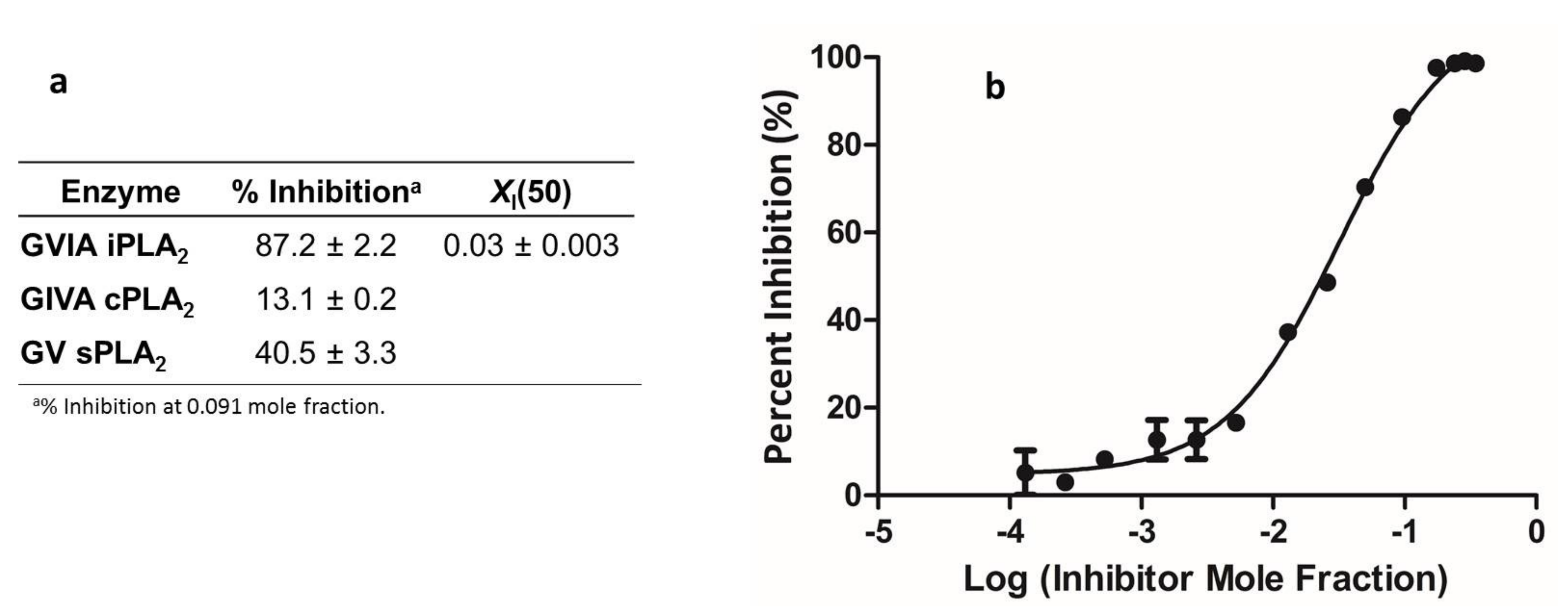
| Entry | Compound | Structure | % Inhibition (at 3 μM) | EC50 (μM) |
|---|---|---|---|---|
| 1 | 14 | 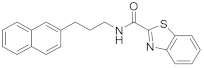 | 64 | |
| 2 | 5a |  | 50 | |
| 3 | 5b |  | 96 | 0.173 |
| 4 | 9a |  | 28 | |
| 5 | 16b |  | 39 | |
| 6 | 5d |  | 73 | |
| 7 | 5c | 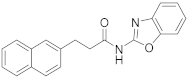 | 49 | |
| 8 | 5f |  | 66 | |
| 9 | 5e | 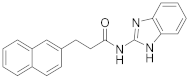 | 29 | |
| 10 | 11 | 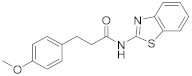 | 80 | 0.916 |
| 11 | 9b |  | 25 | |
| 12 | 16a | 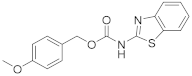 | 15 | |
| 13 | 7 | 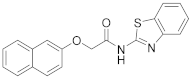 | 41 |
| Entry | Compound | Structure | % Inhibition (at 3 μM) | EC50 (μM) |
|---|---|---|---|---|
| 1 | 18a |  | 46 | |
| 2 | 19a | 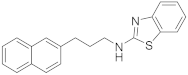 | 98 | 0.118 |
| 3 | 23a | 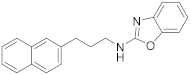 | 95 | 0.336 |
| 4 | 25a |  | 63 | |
| 5 | 23c | 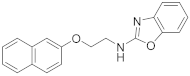 | 87 | 0.894 |
| 6 | 19d | 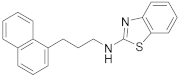 | 82 | 0.399 |
| 7 | 25b |  | 57 | |
| 8 | 23d | 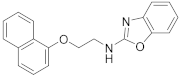 | 45 | |
| 9 | 18c | 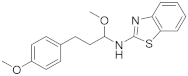 | 0 | |
| 10 | 18b | 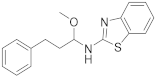 | 0 | |
| 11 | 19c | 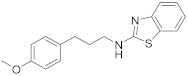 | 62 | |
| 12 | 19b |  | 96 | 0.177 |
| 13 | 23b | 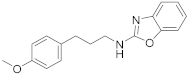 | 79 | |
| 14 | 19e | 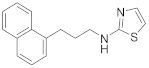 | 88 | |
| 15 | 19f |  | 20 | |
| 16 | 19g | 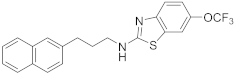 | 0 | |
| 17 | 19h |  | 0 |
| Compound | Structure | % Reduction of Rat-Paw Edema a |
|---|---|---|
| 5b |  | 48.4 ** ± 2.16 |
| 19a | 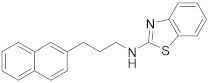 | 47.3 * ± 3.06 |
| 19c |  | 41.4 * ± 2.16 |
| Indomethacin | 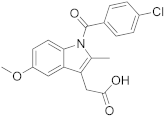 | 37.3 * ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodoropoulou, M.A.; Psarra, A.; Erhardt, M.; Nikolaou, A.; Gerogiannopoulou, A.-D.D.; Hadjipavlou-Litina, D.; Hayashi, D.; Dennis, E.A.; Huwiler, A.; Kokotos, G. N-Acylated and N-Alkylated 2-Aminobenzothiazoles Are Novel Agents That Suppress the Generation of Prostaglandin E2. Biomolecules 2022, 12, 267. https://doi.org/10.3390/biom12020267
Theodoropoulou MA, Psarra A, Erhardt M, Nikolaou A, Gerogiannopoulou A-DD, Hadjipavlou-Litina D, Hayashi D, Dennis EA, Huwiler A, Kokotos G. N-Acylated and N-Alkylated 2-Aminobenzothiazoles Are Novel Agents That Suppress the Generation of Prostaglandin E2. Biomolecules. 2022; 12(2):267. https://doi.org/10.3390/biom12020267
Chicago/Turabian StyleTheodoropoulou, Maria A., Anastasia Psarra, Martin Erhardt, Aikaterini Nikolaou, Anna-Dimitra D. Gerogiannopoulou, Dimitra Hadjipavlou-Litina, Daiki Hayashi, Edward A. Dennis, Andrea Huwiler, and George Kokotos. 2022. "N-Acylated and N-Alkylated 2-Aminobenzothiazoles Are Novel Agents That Suppress the Generation of Prostaglandin E2" Biomolecules 12, no. 2: 267. https://doi.org/10.3390/biom12020267
APA StyleTheodoropoulou, M. A., Psarra, A., Erhardt, M., Nikolaou, A., Gerogiannopoulou, A.-D. D., Hadjipavlou-Litina, D., Hayashi, D., Dennis, E. A., Huwiler, A., & Kokotos, G. (2022). N-Acylated and N-Alkylated 2-Aminobenzothiazoles Are Novel Agents That Suppress the Generation of Prostaglandin E2. Biomolecules, 12(2), 267. https://doi.org/10.3390/biom12020267