Vasorelaxant Activity of AP39, a Mitochondria-Targeted H2S Donor, on Mouse Mesenteric Artery Rings In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vitro Vascular Response
2.3. Materials
2.4. H2S Producing Enzyme Expression
2.5. In Vitro H2S Production
2.6. Statistical Analysis
2.7. Data Availability
3. Results
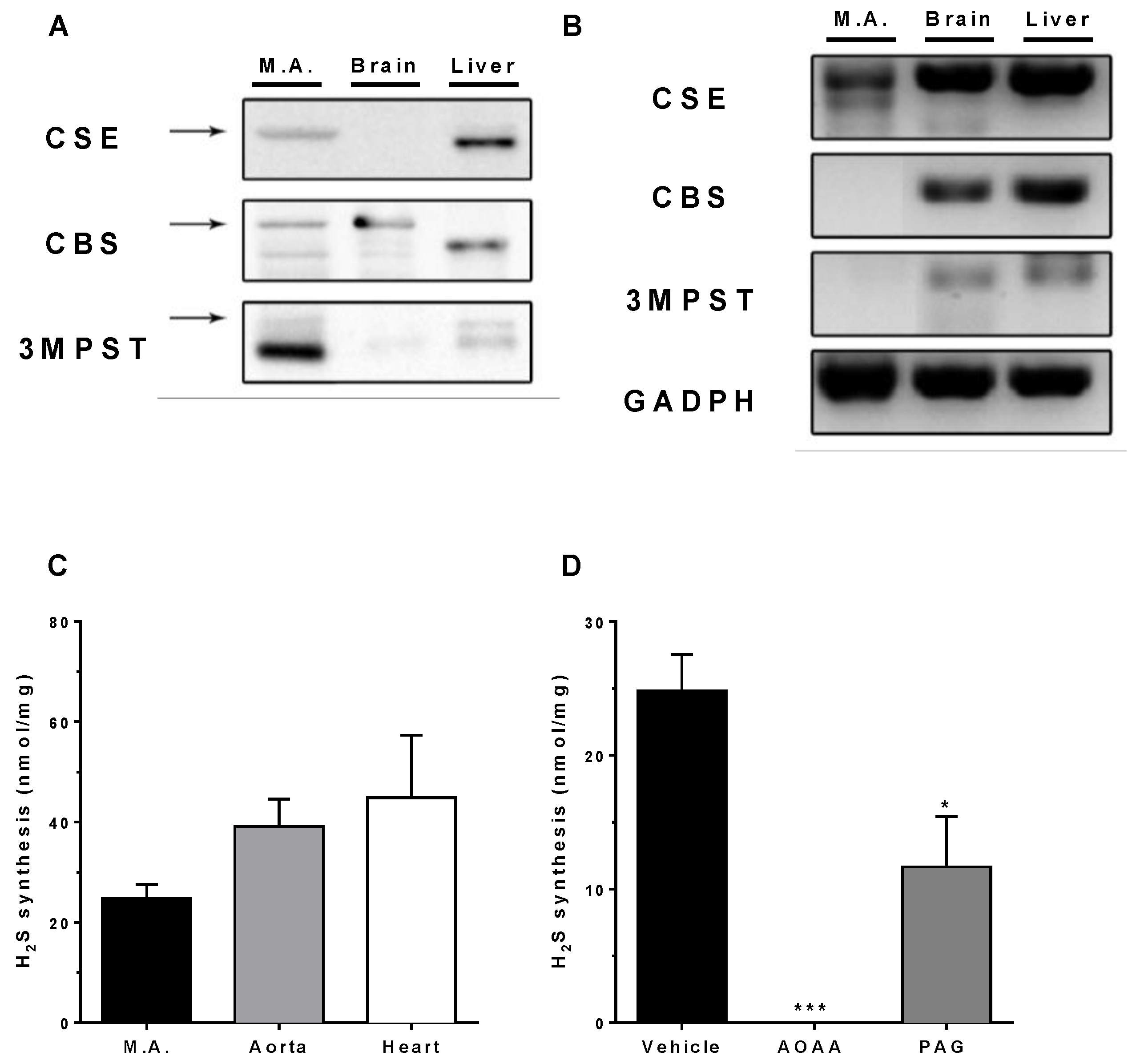
3.1. Expression of H2S-Producing Enzymes
3.2. In Vitro H2S Generation by Mesenteric Artery Homogenates
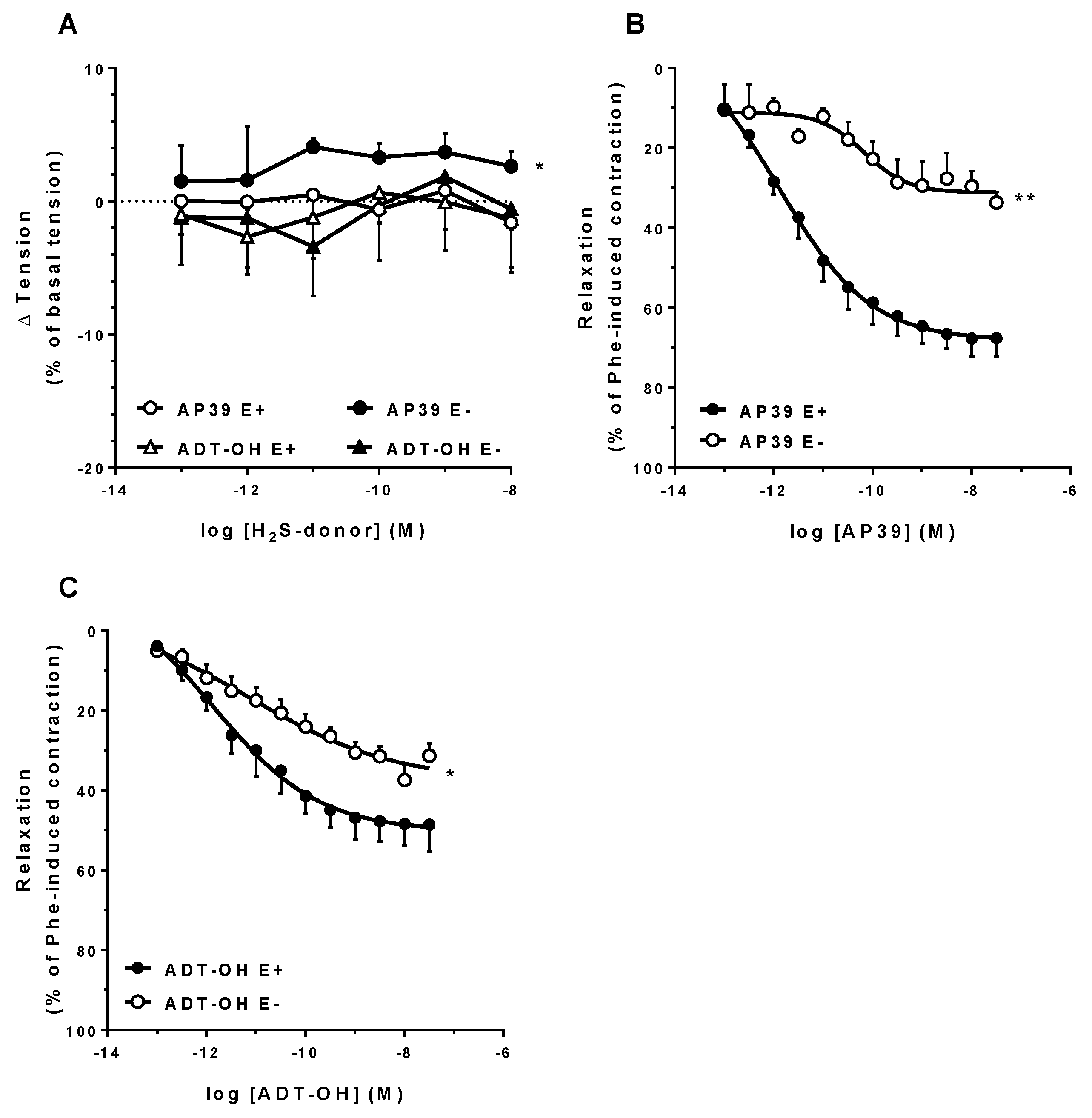
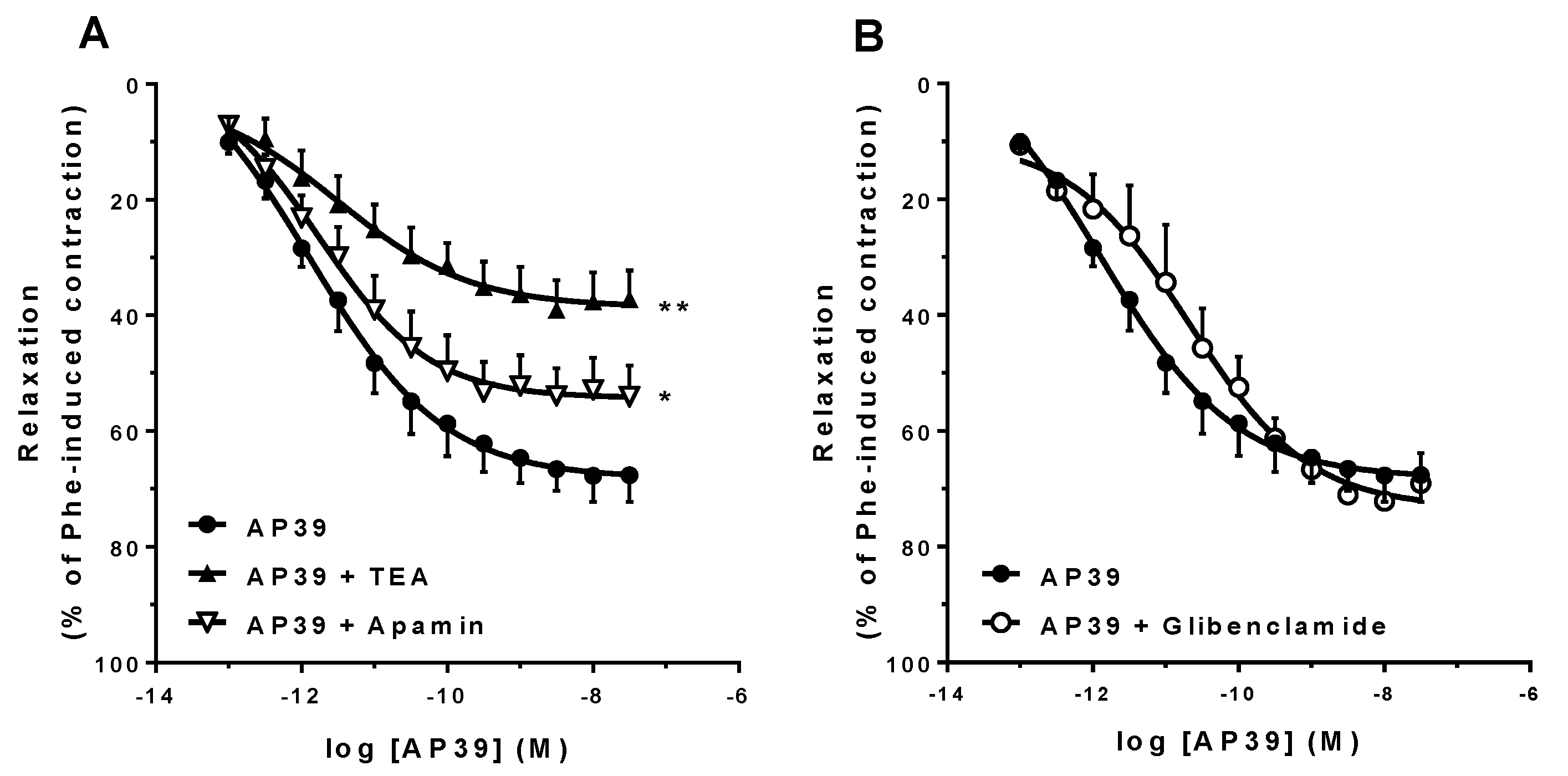
3.3. In Vitro Vascular Effects of AP39 and ADT-OH
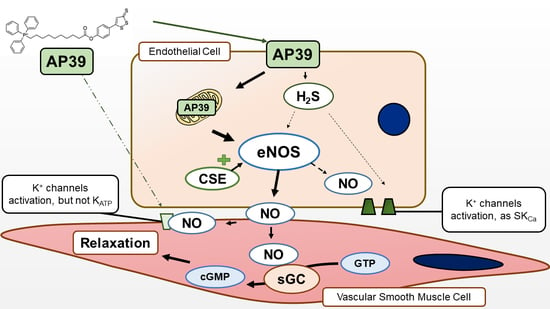
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Circ. Physiol. 2002, 283, H474–H480. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L.; Qin, M.; Liu, X.H.; Zhu, Y.Z. The role of hydrogen sulfide on cardiovascular homeostasis: An overview with update on immunomodulation. Front. Pharmacol. 2017, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine -Lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef]
- Cheng, Y.; Ndisang, J.F.; Tang, G.; Cao, K.; Wang, R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Circ. Physiol. 2004, 287, H2316–H2323. [Google Scholar] [CrossRef]
- Hedegaard, E.R.; Gouliaev, A.; Winther, A.K.; Arcanjo, D.D.R.; Aalling, M.; Renaltan, N.S.; Wood, M.E.; Whiteman, M.; Skovgaard, N.; Simonsen, U. Involvement of Potassium Channels and Calcium-Independent Mechanisms in Hydrogen Sulfide-Induced Relaxation of Rat Mesenteric Small Arteries. J. Pharmacol. Exp. Ther. 2015, 356, 53–63. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Breschi, M.C.; Lawson, K.; McKay, N.G.; Miceli, F.; Taglialatela, M.; Calderone, V.; Testai, L.; Miceli, F.; et al. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol. Res. 2013, 70, 27–34. [Google Scholar] [CrossRef]
- Jackson-Weaver, O.; Osmond, J.M.; Riddle, M.A.; Naik, J.S.; Bosc, L.V.G.; Walker, B.R.; Kanagy, N.L. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. AJP Hear. Circ. Physiol. 2013, 304, H1446–H1454. [Google Scholar] [CrossRef] [PubMed]
- Bibli, S.-I.; Yang, G.; Zhou, Z.; Wang, R.; Topouzis, S.; Papapetropoulos, A. Role of cGMP in hydrogen sulfide signaling. Nitric Oxide 2015, 46, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Modis, K.; Panopoulos, P.; Asimakopoulou, A.; Gero, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Greaney, J.L.; Kutz, J.L.; Shank, S.W.; Jandu, S.; Santhanam, L.; Alexander, L.M. Impaired Hydrogen Sulfide-Mediated Vasodilation Contributes to Microvascular Endothelial Dysfunction in Hypertensive Adults. Hypertension 2017, 69, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R.; et al. Hydrogen Sulfide as Endothelium-Derived Hyperpolarizing Factor Sulfhydrates Potassium Channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef]
- Meng, G.; Zhao, S.; Xie, L.; Han, Y.; Ji, Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br. J. Pharmacol. 2018, 175, 1146–1156. [Google Scholar] [CrossRef]
- Wang, R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr. Opin. Nephrol. Hypertens. 2011, 20, 107–112. [Google Scholar] [CrossRef]
- Bibli, S.I.; Hu, J.; Leisegang, M.S.; Wittig, J.; Zukunft, S.; Kapasakalidi, A.; Fisslthaler, B.; Tsilimigras, D.; Zografos, G.; Filis, K.; et al. Shear stress regulates cystathionine γ lyase expression to preserve endothelial redox balance and reduce membrane lipid peroxidation: Regulation of CSE by KLF2 and miR-27b. Redox Biol. 2020, 28, 101379. [Google Scholar] [CrossRef]
- Lagoutte, E.; Mimoun, S.; Andriamihaja, M.; Chaumontet, C.; Blachier, F.; Bouillaud, F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1500–1511. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Módis, K.; Ju, Y.J.; Ahmad, A.; Untereiner, A.A.; Altaany, Z.; Wu, L.; Szabo, C.; Wang, R. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016, 113, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Ransy, C.; Módis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 2014, 41, 120–130. [Google Scholar] [CrossRef]
- Gerő, D.; Torregrossa, R.; Perry, A.; Waters, A.; Le-Trionnaire, S.; Whatmore, J.L.; Wood, M.; Whiteman, M. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016, 113, 186–198. [Google Scholar] [CrossRef]
- Ahmad, A.; Olah, G.; Szczesny, B.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, A Mitochondrially Targeted Hydrogen Sulfide Donor, Exerts Protective Effects in Renal Epithelial Cells Subjected to Oxidative Stress in Vitro and in Acute Renal Injury in Vivo. Shock 2016, 45, 88–97. [Google Scholar] [CrossRef]
- Latorre, E.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Harries, L.W. Mitochondria-targeted hydrogen sulfide attenuates endothelial senescence by selective induction of splicing factors HNRNPD and SRSF2. Aging 2018, 10, 1666–1681. [Google Scholar] [CrossRef]
- Chatzianastasiou, A.; Bibli, S.-I.S.; Andreadou, I.; Efentakis, P.; Kaludercic, N.; Wood, M.E.; Whiteman, M.; Di Lisa, F.; Daiber, A.; Manolopoulos, V.G.; et al. Cardioprotection by H2S Donors: Nitric Oxide-Dependent and -Independent Mechanisms. J. Pharmacol. Exp. Ther. 2016, 358, 431–440. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef]
- Tomasova, L.; Pavlovicova, M.; Malekova, L.; Misak, A.; Kristek, F.; Grman, M.; Cacanyiova, S.; Tomasek, M.; Tomaskova, Z.; Perry, A.; et al. Effects of AP39, a novel triphenylphosphonium derivatised anethole dithiolethione hydrogen sulfide donor, on rat haemodynamic parameters and chloride and calcium Cav3 and RyR2 channels. Nitric Oxide 2015, 46, 131–144. [Google Scholar] [CrossRef]
- Le Trionnaire, S.; Perry, A.; Szczesny, B.; Szabo, C.; Winyard, P.G.; Whatmore, J.L.; Wood, M.E.; Whiteman, M. The synthesis and functional evaluation of a mitochondria-targeted hydrogen sulfide donor, (10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)decyl)triphenylphosphonium bromide (AP39). Medchemcomm 2014, 5, 728–736. [Google Scholar] [CrossRef]
- Coavoy-Sánchez, S.A.; Rodrigues, L.; Teixeira, S.A.; Soares, A.G.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Costa, S.K.P.; Muscará, M.N. Hydrogen sulfide donors alleviate itch secondary to the activation of type-2 protease activated receptors (PAR-2) in mice. Pharmacol. Res. 2016, 113, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Campi, P.; Herrera, B.S.; de Jesus, F.N.; Napolitano, M.; Teixeira, S.A.; Maia-Dantas, A.; Spolidorio, L.C.; Akamine, E.H.; Mayer, M.P.A.; de Carvalho, M.H.C.; et al. Endothelial dysfunction in rats with ligature-induced periodontitis: Participation of nitric oxide and cycloxygenase-2-derived products. Arch. Oral Biol. 2016, 63, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Treviño-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous Hydrogen Sulfide Production Is Essential for Dietary Restriction Benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Morales-Loredo, H.; Barrera, A.; Garcia, J.M.; Pace, C.E.; Naik, J.S.; Gonzalez Bosc, L.V.; Kanagy, N.L. Hydrogen sulfide regulation of renal and mesenteric blood flow. Am. J. Physiol. Circ. Physiol. 2019, 317, H1157–H1165. [Google Scholar] [CrossRef]
- Kimura, Y.; Koike, S.; Shibuya, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine- and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Sci. Rep. 2017, 7, 10459. [Google Scholar] [CrossRef]
- Barrera, A.; Naik, J.; Gonzalez Bosc, L.V.; Mendiola, P.; Kanagy, N.L. Effects of Hydrogen Sulfide on Mesenteric Blood Flow. FASEB J. 2017, 31, 1012.21. [Google Scholar] [CrossRef]
- Kuo, M.M.; Kim, D.H.; Jandu, S.; Bergman, Y.; Tan, S.; Wang, H.; Pandey, D.R.; Abraham, T.P.; Shoukas, A.A.; Berkowitz, D.E.; et al. MPST but not CSE is the primary regulator of hydrogen sulfide production and function in the coronary artery. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H71–H79. [Google Scholar] [CrossRef]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular Endothelium Expresses 3-Mercaptopyruvate Sulfurtransferase and Produces Hydrogen Sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Albertini, E.; Kozieł, R.; Dürr, A.; Neuhaus, M.; Jansen-Dürr, P. Cystathionine beta synthase modulates senescence of human endothelial cells. Aging 2012, 4, 664–673. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saha, S.; Chakraborty, P.K.; Xiong, X.; Dwivedi, S.K.D.; Mustafi, S.B.; Leigh, N.R.; Ramchandran, R.; Mukherjee, P.; Bhattacharya, R. Cystathionine β-synthase regulates endothelial function via protein S-sulfhydration. FASEB J. 2016, 30, 441–456. [Google Scholar] [CrossRef]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen Sulfide Is an Endogenous Inhibitor of Phosphodiesterase Activity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Katsouda, A.; Bibli, S.-I.; Pyriochou, A.; Szabo, C.; Papapetropoulos, A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol. Res. 2016, 113, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulou, A.; Panopoulos, P.; Chasapis, C.T.; Coletta, C.; Zhou, Z.; Cirino, G.; Giannis, A.; Szabo, C.; Spyroulias, G.A.; Papapetropoulos, A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol. 2013, 169, 922–932. [Google Scholar] [CrossRef]
- Trnka, J.; Elkalaf, M.; Anděl, M. Lipophilic Triphenylphosphonium Cations Inhibit Mitochondrial Electron Transport Chain and Induce Mitochondrial Proton Leak. PLoS ONE 2015, 10, e0121837. [Google Scholar] [CrossRef]
- Olson, K.R. H2S and polysulfide metabolism: Conventional and unconventional pathways. Biochem. Pharmacol. 2018, 149, 77–90. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide Biol. Chem. 2013, 35, 5–20. [Google Scholar] [CrossRef]
- Mendiola, P.; Gonzalez Bosc, L.V.; Rios, L.; Naik, J.; Kanagy, N. Acetylcholine Activates Cystathionine γ-Lyase Production of H2S in Aortic Endothelial Cells. FASEB J. 2017, 31, 837.17. [Google Scholar] [CrossRef]
- Szijártó, I.A.; Markó, L.; Filipovic, M.R.; Miljkovic, J.L.; Tabeling, C.; Tsvetkov, D.; Wang, N.; Rabelo, L.A.; Witzenrath, M.; Diedrich, A.; et al. Cystathionine γ-Lyase–Produced Hydrogen Sulfide Controls Endothelial NO Bioavailability and Blood Pressure. Hypertension 2018, 71, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Marino, A.; Piano, I.; Brancaleone, V.; Tomita, K.; Di Cesare Mannelli, L.; Martelli, A.; Citi, V.; Breschi, M.C.; Levi, R.; et al. The novel H(2)S-donor 4-carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia/reperfusion injury through activation of mitoK(ATP) channels and reduction of oxidative stress. Pharmacol. Res. 2016, 113, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Katakam, P.V.G.; Wappler, E.A.; Katz, P.S.; Rutkai, I.; Institoris, A.; Domoki, F.; Gáspár, T.; Grovenburg, S.M.; Snipes, J.A.; Busija, D.W. Depolarization of Mitochondria in Endothelial Cells Promotes Cerebral Artery Vasodilation by Activation of Nitric Oxide Synthase. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Ardehali, H.; O’Rourke, B. Mitochondrial KATP channels in cell survival and death. J. Mol. Cell. Cardiol. 2005, 39, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Bolotina, V.M.; Najibi, S.; Palacino, J.J.; Pagano, P.J.; Cohen, R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 1994, 368, 850–853. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Durante, W. Hydrogen Sulfide Therapy in Diabetes-Accelerated Atherosclerosis: A Whiff of Success. Diabetes 2016, 65, 2832–2834. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, S.; Teng, X.; Duan, X.; Chen, Y.; Wu, Y. Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide Biol. Chem. 2017, 67, 10–25. [Google Scholar] [CrossRef]
- Wu, D.; Luo, N.; Wang, L.; Zhao, Z.; Bu, H.; Xu, G.; Yan, Y.; Che, X.; Jiao, Z.; Zhao, T.; et al. Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κB signaling pathways. Sci. Rep. 2017, 7, 455. [Google Scholar] [CrossRef]

| Protocol | Emax (%) | pA2 | n |
|---|---|---|---|
| E+ | 72.5 ± 4.6 | 12.2 ± 0.4 | 7 |
| E− | 34.6 ± 3.1 ** | 10.0 ± 0.6 ** | 5 |
| 10 µM indomethacin | 57.1 ± 6.3 | 11.6 ± 0.4 | 7 |
| 100 µM L-NAME | 23.9 ± 5.1 ** | 10.5 ± 0.7 * | 5 |
| 10 µM ODQ | 22.9 ± 3.3 ** | 11.3 ± 0.2 | 7 |
| 5 nM sildenafil | 81.0 ± 5.7 | 11.7 ± 0.3 | 7 |
| 10 mM AOAA | 72.8 ± 6.4 | 11.0 ± 0.3 * | 7 |
| 3 mM TEA | 38.6 ± 5.2 ** | 11.5 ± 0.3 | 7 |
| 10 µM glibenclamide | 72.6 ± 4.2 | 11.2 ± 0.6 | 5 |
| 5 µM apamin | 52.0 ± 4.9 * | 11.5 ± 0.4 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa Marques, L.A.; Teixeira, S.A.; de Jesus, F.N.; Wood, M.E.; Torregrossa, R.; Whiteman, M.; Costa, S.K.P.; Muscará, M.N. Vasorelaxant Activity of AP39, a Mitochondria-Targeted H2S Donor, on Mouse Mesenteric Artery Rings In Vitro. Biomolecules 2022, 12, 280. https://doi.org/10.3390/biom12020280
da Costa Marques LA, Teixeira SA, de Jesus FN, Wood ME, Torregrossa R, Whiteman M, Costa SKP, Muscará MN. Vasorelaxant Activity of AP39, a Mitochondria-Targeted H2S Donor, on Mouse Mesenteric Artery Rings In Vitro. Biomolecules. 2022; 12(2):280. https://doi.org/10.3390/biom12020280
Chicago/Turabian Styleda Costa Marques, Leonardo A., Simone A. Teixeira, Flávia N. de Jesus, Mark E. Wood, Roberta Torregrossa, Matthew Whiteman, Soraia K. P. Costa, and Marcelo N. Muscará. 2022. "Vasorelaxant Activity of AP39, a Mitochondria-Targeted H2S Donor, on Mouse Mesenteric Artery Rings In Vitro" Biomolecules 12, no. 2: 280. https://doi.org/10.3390/biom12020280
APA Styleda Costa Marques, L. A., Teixeira, S. A., de Jesus, F. N., Wood, M. E., Torregrossa, R., Whiteman, M., Costa, S. K. P., & Muscará, M. N. (2022). Vasorelaxant Activity of AP39, a Mitochondria-Targeted H2S Donor, on Mouse Mesenteric Artery Rings In Vitro. Biomolecules, 12(2), 280. https://doi.org/10.3390/biom12020280