Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, Partly through SIRT1 Signaling
Abstract
:1. Introduction
2. Material and Methods
2.1. Isolation and Cultivation of BM-MSCs
2.2. Characterization of BM-MSCs
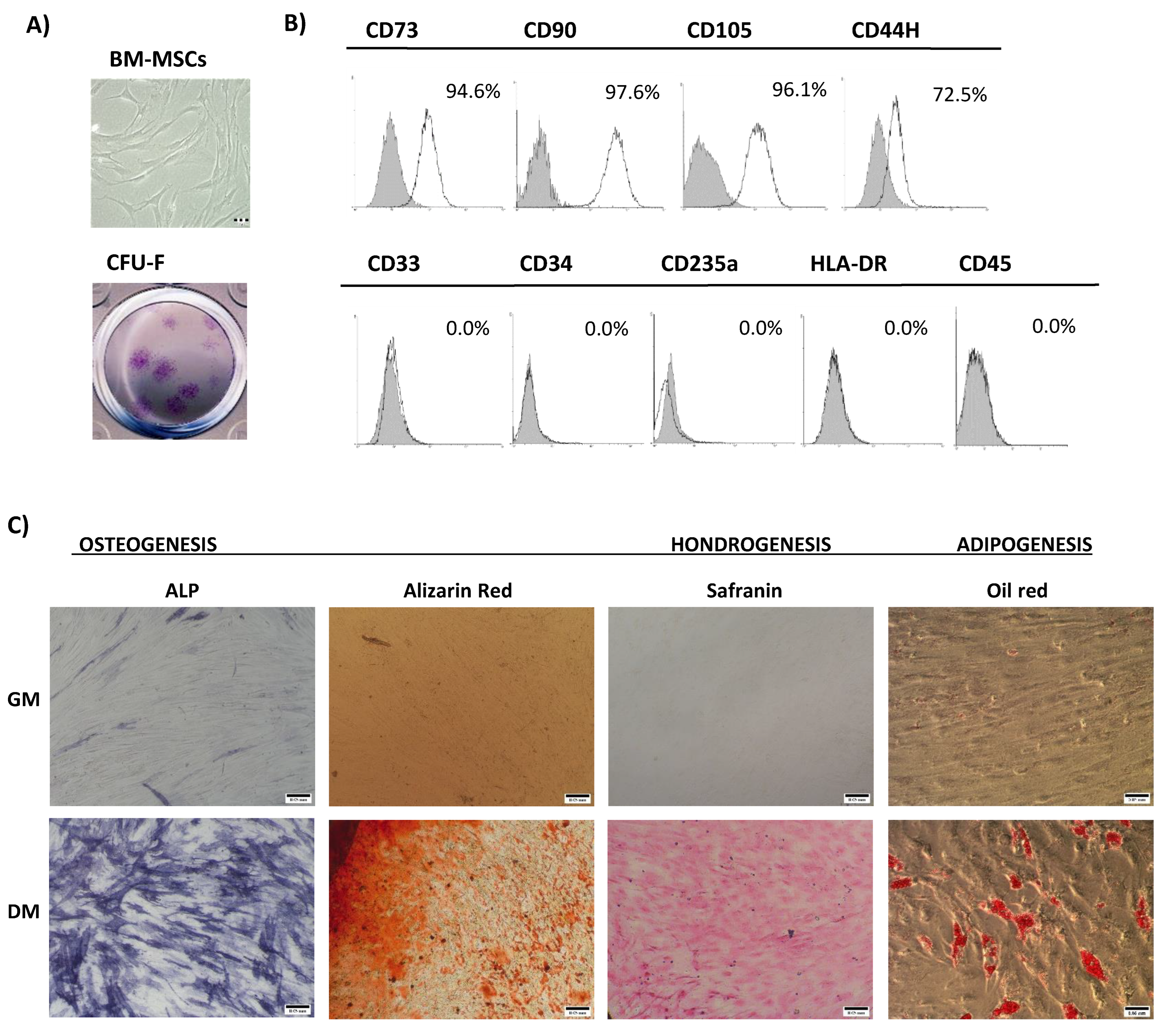
2.2.1. Immunophenotype Analysis
2.2.2. In Vitro Multilineage Differentiation
2.3. CFU–F (Colony Forming Unit–Fibroblast) Assay
2.4. Cellular Viability and Cell Cycle Progression
2.5. Osteogenic and Adipogenic Differentiation of BM-MSCs
2.6. Immunofluorescence Assay
2.7. β-Galactosidase Staining
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Bone Marrow Mesenchymal Stromal/Stem Cells’ Characterization
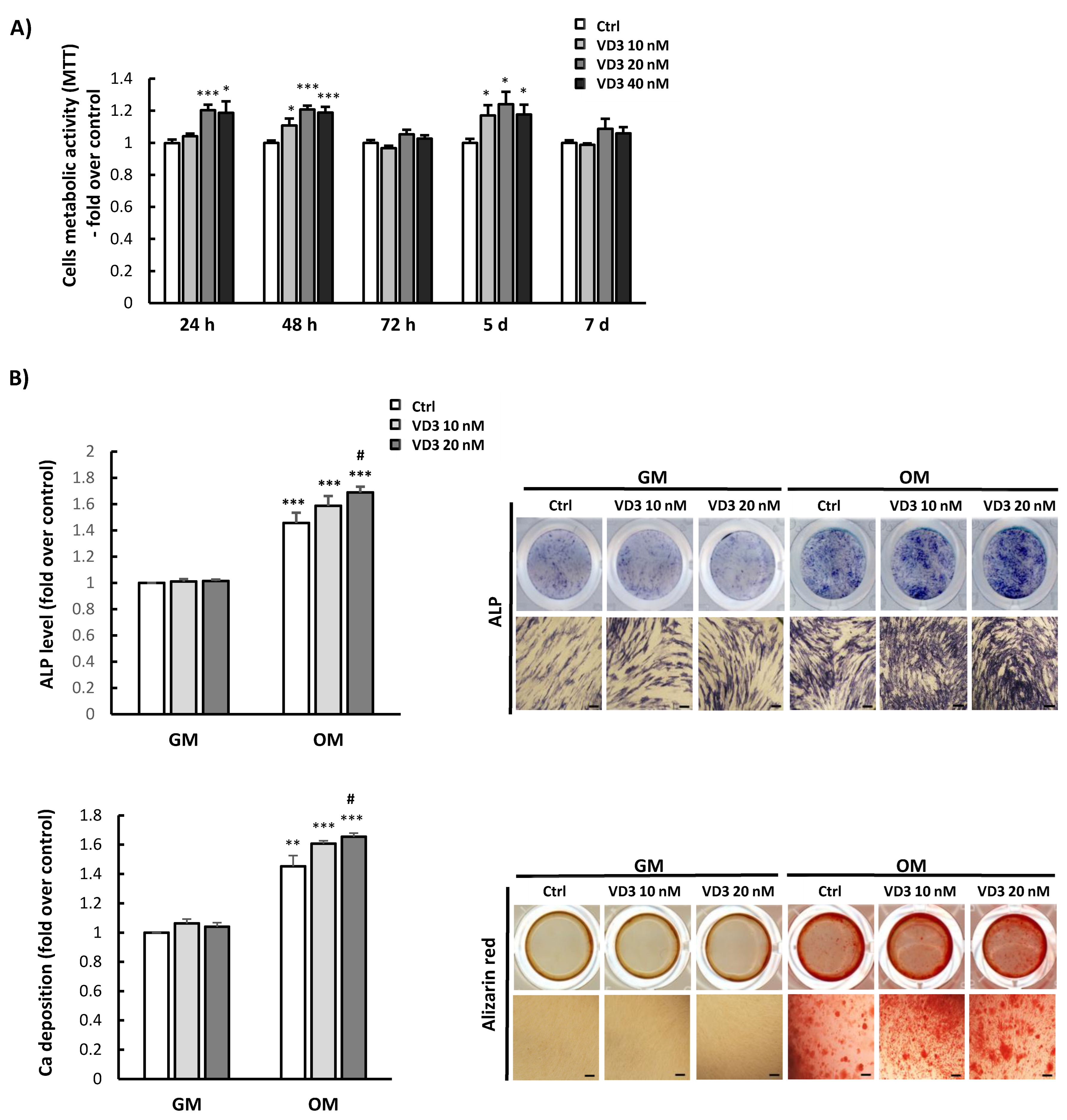
3.2. Influence of VD3 on BM-MSCs’ Proliferation and Differentiation
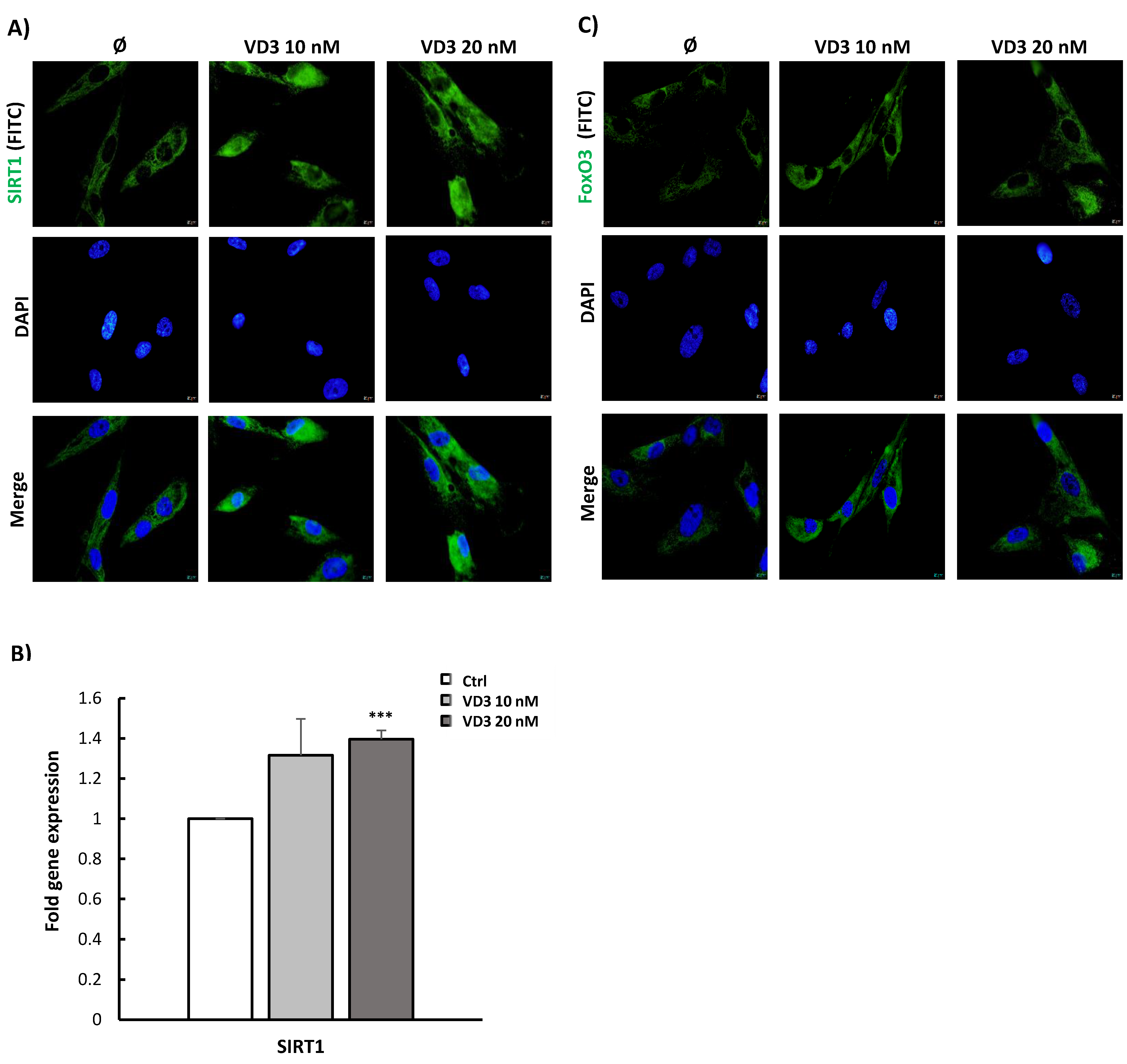
3.3. VD3 Activates SIRT1-FoxO3 Signaling Pathways in BM-MSCs
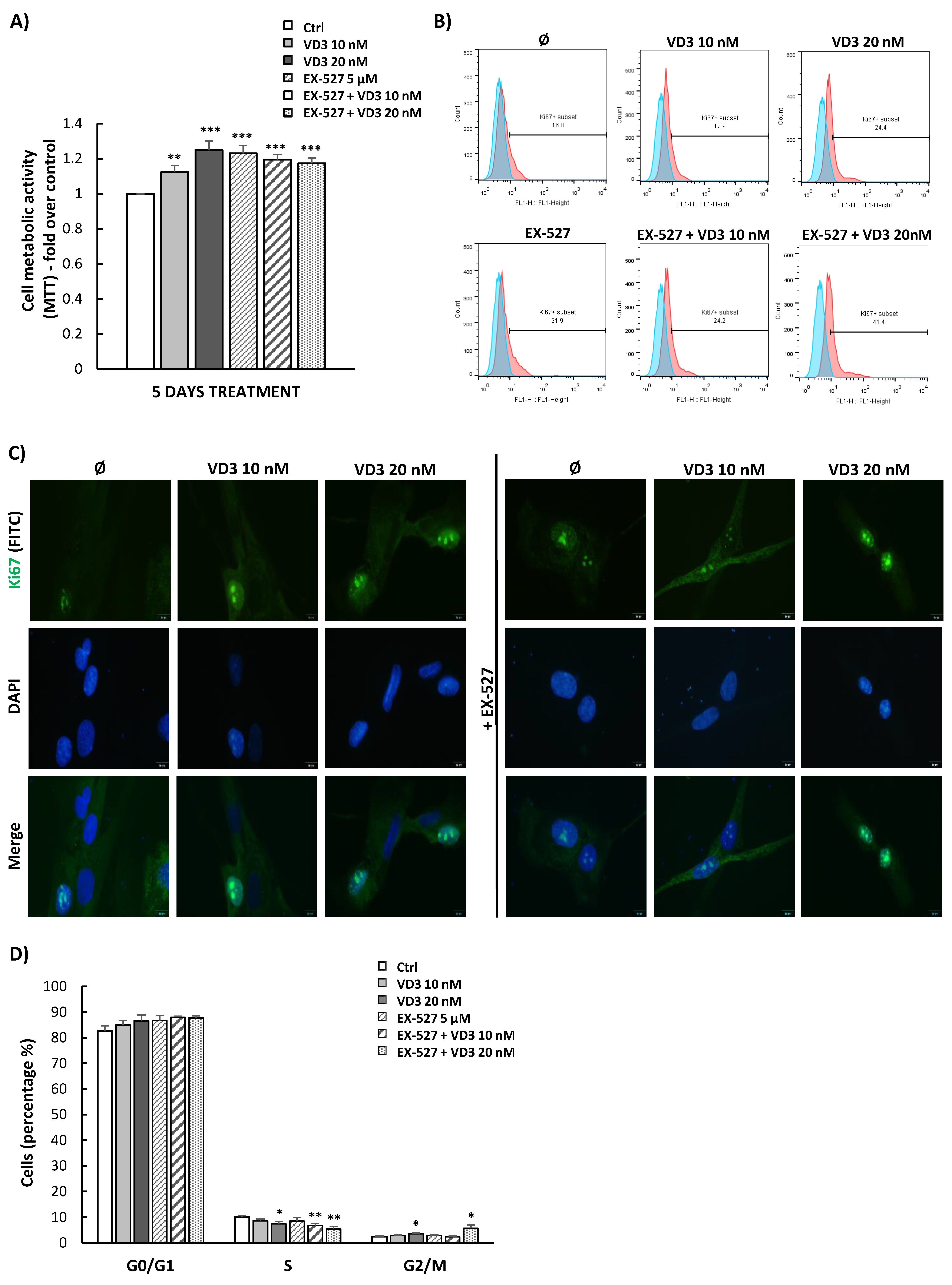
3.4. VD3 Treatment Modulates Proliferation and Cell Cycle Progression of BM-MSCs Independently of SIRT1 Signaling
3.5. VD3 Treatment Modulates the Expression of Pluripotency-Associated Markers in BM-MSCs: Involvement of SIRT1 Signaling
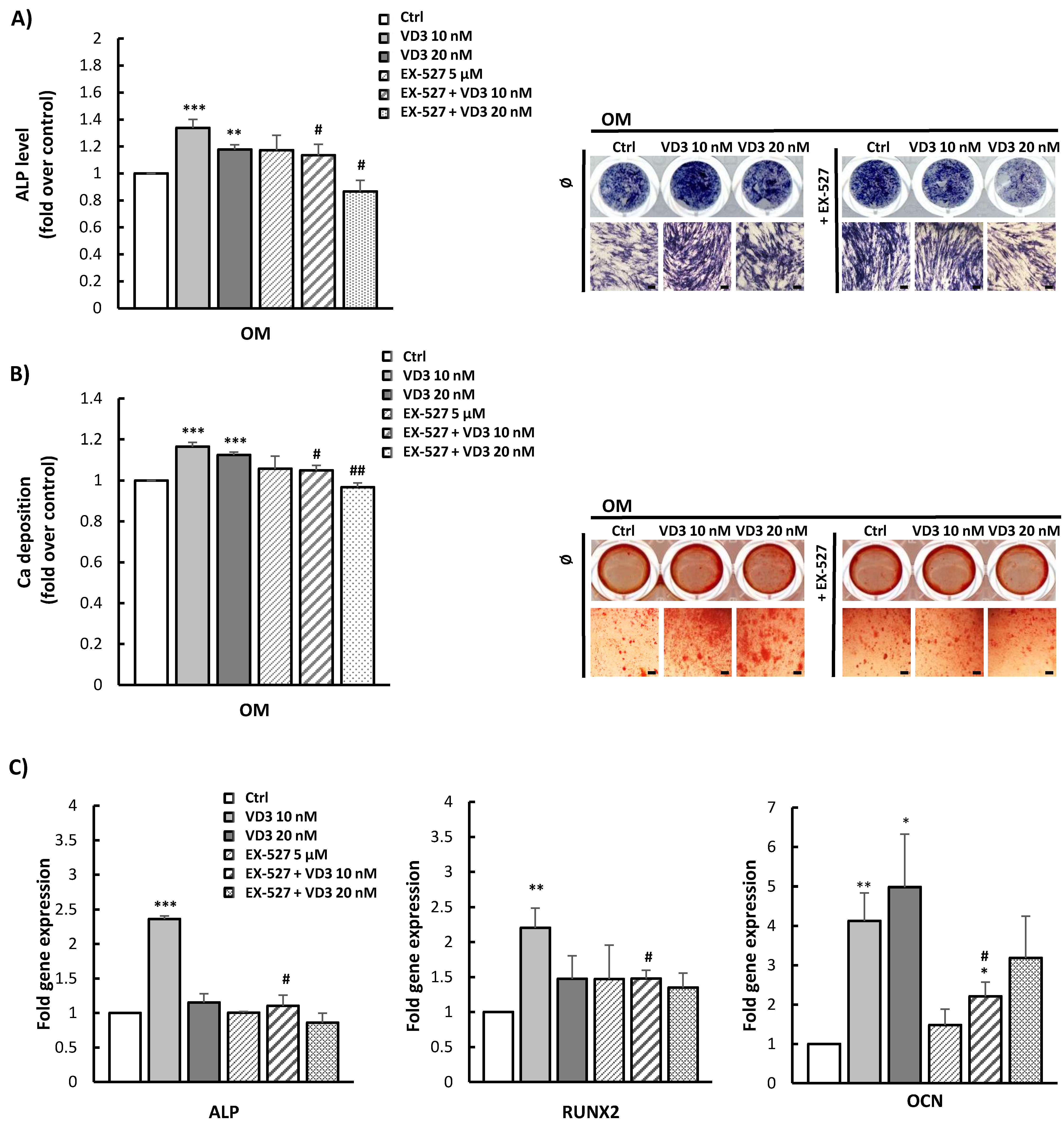
3.6. Modulated Differentiation Potential of VD3-Pretreated BM-MSCs: Involvement of SIRT1 Signaling
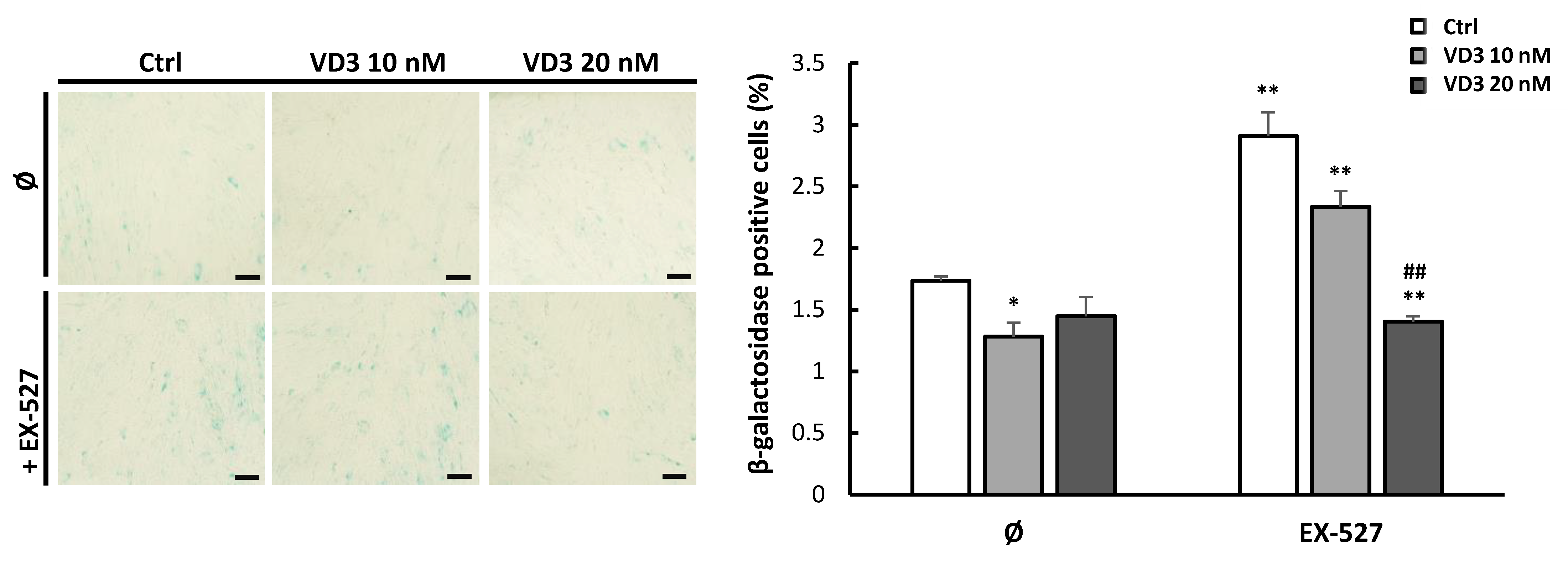
3.7. VD3 Treatment Reduces β-Galactosidase Expression in BM-MSCs: The Role of SIRT1 Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Merimi, M.; Lewalle, P.; Meuleman, N.; Agha, D.M.; El-Kehdy, H.; Bouhtit, F.; Ayoub, S.; Burny, A.; Fahmi, H.; Lagneaux, L.; et al. Mesenchymal Stem/Stromal Cell Therapeutic Features: The Bridge between the Bench and the Clinic. J. Clin. Med. 2021, 10, 905. [Google Scholar] [CrossRef]
- Galderisi, U.; Peluso, G.; Di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent Years? Stem Cell Rev. Rep. 2021, 18, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Stamnitz, S.; Klimczak, A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells 2021, 10, 1925. [Google Scholar] [CrossRef] [PubMed]
- Sattary, M.; Rafienia, M.; Kazemi, M.; Salehi, H.; Mahmoudzadeh, M. Promoting effect of nano hydroxyapatite and vitamin D3 on the osteogenic differentiation of human adipose-derived stem cells in polycaprolactone/gelatin scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, M.; Ding, Z.; Yan, Y. Vitamin D3-loaded calcium citrate/calcium sulfate composite cement with enhanced physicochemical properties, drug release, and cytocompatibility. J. Biomater. Appl. 2020, 34, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Belgheisi, G.; Haghbin-Nazarpak, M.; Omidi, M.; Khojasteh, A.; Solati-Hashjin, M. Bone tissue engineering gelatin-hydroxyapatite/graphene oxide scaffolds with the ability to release vitamin D: Fabrication, characterization, and in vitro study. J. Mater. Sci. Mater. Med. 2020, 31, 97. [Google Scholar] [CrossRef]
- Vu, A.A.; Bose, S. Vitamin D(3) Release from Traditionally and Additively Manufactured Tricalcium Phosphate Bone Tissue Engineering Scaffolds. Ann. Biomed. Eng. 2020, 48, 1025–1033. [Google Scholar] [CrossRef]
- Van Driel, M.; van Leeuwen, J. Vitamin D endocrinology of bone mineralization. Mol. Cell. Endocrinol. 2017, 453, 46–51. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Liu, Y.; Peng, X.; Porta, A. New insights into the mechanisms of vitamin D action. J. Cell. Biochem. 2003, 88, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Zhou, S.; Bi, Z.; Glowacki, J. Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells. Metab. Clin. Exp. 2013, 62, 768–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; LeBoff, M.S.; Glowacki, J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology 2010, 151, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Zhou, S.; Glowacki, J. Effects of 25-hydroxyvitamin D(3) on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase. J. Bone Miner. Res. 2011, 26, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, B.; Padwa, B.L.; Christoph, K.M.; Zhou, S.; Glowacki, J. Vitamin D metabolism and regulation in pediatric MSCs. J. Steroid Biochem. Mol. Biol. 2016, 164, 287–291. [Google Scholar] [CrossRef]
- Doering, L.; Khatri, R.; Petry, S.F.; Sauer, H.; Howaldt, H.P.; Linn, T. Regulation of somatostatin expression by vitamin D3 and valproic acid in human adipose-derived mesenchymal stem cells. Stem Cell Res. Ther. 2019, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D(3) induces osteogenic differentiation of human mesenchymal stem cells. Sci. Rep. 2017, 7, 42816. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Song, J.; Ma, H. An in vitro Experimental Insight into the Osteoblast Responses to Vitamin D3 and Its Metabolites. Pharmacology 2018, 101, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.; Baripoglu, Y.E.; Alenezi, H.; Arikan, A.; Babazade, R.; Unal, S.; Duruksu, G.; Alfares, F.S.; Yazir, Y.; Oktar, F.N.; et al. Vitamin D(3)/vitamin K(2)/magnesium-loaded polylactic acid/tricalcium phosphate/polycaprolactone composite nanofibers demonstrated osteoinductive effect by increasing Runx2 via Wnt/β-catenin pathway. Int. J. Biol. Macromol. 2021, 190, 244–258. [Google Scholar] [CrossRef]
- Sumathra, M.; Munusamy, M.A.; Alarfaj, A.A.; Rajan, M. Osteoblast response to Vitamin D3 loaded cellulose enriched hydroxyapatite Mesoporous silica nanoparticles composite. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 103, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Zainabadi, K. The variable role of SIRT1 in the maintenance and differentiation of mesenchymal stem cells. Regen. Med. 2018, 13, 343–356. [Google Scholar] [CrossRef]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Kukolj, T.; Trivanović, D.; Djordjević, I.O.; Mojsilović, S.; Krstić, J.; Obradović, H.; Janković, S.; Santibanez, J.F.; Jauković, A.; Bugarski, D. Lipopolysaccharide can modify differentiation and immunomodulatory potential of periodontal ligament stem cells via ERK1,2 signaling. J. Cell. Physiol. 2018, 233, 447–462. [Google Scholar] [CrossRef]
- Jauković, A.; Kukolj, T.; Trivanović, D.; Okić-Đorđević, I.; Obradović, H.; Miletić, M.; Petrović, V.; Mojsilović, S.; Bugarski, D. Modulating stemness of mesenchymal stem cells from exfoliated deciduous and permanent teeth by IL-17 and bFGF. J. Cell. Physiol. 2021, 236, 7322–7341. [Google Scholar] [CrossRef]
- Fathi, E.; Farahzadi, R.; Vietor, I.; Javanmardi, S. Cardiac differentiation of bone-marrow-resident c-kit(+) stem cells by L-carnitine increases through secretion of VEGF, IL6, IGF-1, and TGF- β as clinical agents in cardiac regeneration. J. Biosci. 2020, 45, 92. [Google Scholar] [CrossRef]
- Guo, Y.; Chi, X.; Wang, Y.; Heng, B.C.; Wei, Y.; Zhang, X.; Zhao, H.; Yin, Y.; Deng, X. Mitochondria transfer enhances proliferation, migration, and osteogenic differentiation of bone marrow mesenchymal stem cell and promotes bone defect healing. Stem Cell Res. Ther. 2020, 11, 245. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Adibkia, K.; Ehsani, A.; Jodaei, A.; Fathi, E.; Farahzadi, R.; Barzegar-Jalali, M. Silver nanoparticles induce the cardiomyogenic differentiation of bone marrow derived mesenchymal stem cells via telomere length extension. Beilstein J. Nanotechnol. 2021, 12, 786–797. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [Green Version]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Weng, Y.; Lin, S.; Lin, J.; Huang, Z.; Huang, W.; Cai, X. 1,25(OH)(2)D(3) Strengthens the Vasculogenesis of Multipotent Mesenchymal Stromal Cells from Rat Bone Marrow by Regulating the PI3K/AKT Pathway. Drug Des. Dev. Ther. 2020, 14, 1157–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Huang, B.; Yang, H.; Li, G.; Zhang, C.; Yang, G.; Lin, F.; Lin, G. TGF-β1 is Involved in Vitamin D-Induced Chondrogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells by Regulating the ERK/JNK Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 42, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qiao, W.; Cui, M.; Yang, C.; Wang, R.; Goltzman, D.; Jin, J.; Miao, D. The Polycomb Protein Bmi1 Plays a Crucial Role in the Prevention of 1,25(OH)(2) D Deficiency-Induced Bone Loss. J. Bone Miner. Res. 2020, 35, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, J.; Zhang, J.; Qin, R.; Wang, R.; Qiu, Y.; Mao, Z.; Goltzman, D.; Miao, D. 1,25-Dihydroxyvitamin D protects against age-related osteoporosis by a novel VDR-Ezh2-p16 signal axis. Aging Cell 2020, 19, e13095. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Z.; Wu, J.; Mei, Y.; Zhang, Q.; Zhang, H.; Miao, D.; Sun, W. Sirt1 Promotes Osteogenic Differentiation and Increases Alveolar Bone Mass via Bmi1 Activation in Mice. J. Bone Miner. Res. 2019, 34, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Artaza, J.N.; Sirad, F.; Ferrini, M.G.; Norris, K.C. 1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J. Steroid Biochem. Mol. Biol. 2010, 119, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Klotz, B.; Mentrup, B.; Regensburger, M.; Zeck, S.; Schneidereit, J.; Schupp, N.; Linden, C.; Merz, C.; Ebert, R.; Jakob, F. 1,25-dihydroxyvitamin D3 treatment delays cellular aging in human mesenchymal stem cells while maintaining their multipotent capacity. PLoS ONE 2012, 7, e29959. [Google Scholar] [CrossRef]
- Tang, X.; Meng, H. Osteogenic induction and 1,25-dihydroxyvitamin D3 oppositely regulate the proliferation and expression of RANKL and the vitamin D receptor of human periodontal ligament cells. Arch. Oral Biol. 2009, 54, 625–633. [Google Scholar] [CrossRef]
- Zhou, S.; Glowacki, J.; Kim, S.W.; Hahne, J.; Geng, S.; Mueller, S.M.; Shen, L.; Bleiberg, I.; LeBoff, M.S. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D(3) in human marrow stromal cells. J. Bone Miner. Res. 2012, 27, 1992–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Qiao, W.; Zhou, B.; Hu, Z.; Yan, Q.; Wu, J.; Wang, R.; Zhang, Q.; Miao, D. Overexpression of Sirt1 in mesenchymal stem cells protects against bone loss in mice by FOXO3a deacetylation and oxidative stress inhibition. Metab. Clin. Exp. 2018, 88, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, X.; Yang, R.; Wu, G.; Tan, Q.; Goltzman, D.; Miao, D. SIRT1/FOXO3a axis plays an important role in the prevention of mandibular bone loss induced by 1,25(OH)(2)D deficiency. Int. J. Biol. Sci. 2020, 16, 2712–2726. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, F.; Liu, H.; Li, J.; Che, H.; Shen, J.; Luo, E. SIRT1, a promising regulator of bone homeostasis. Life Sci. 2021, 269, 119041. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, F.; Ma, Y.; Wei, F. Vitamin D3 contributes to enhanced osteogenic differentiation of MSCs under oxidative stress condition via activating the endogenous antioxidant system. Osteoporos. Int. 2018, 29, 1917–1926. [Google Scholar] [CrossRef]
- Tanno, M.; Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 2007, 282, 6823–6832. [Google Scholar] [CrossRef] [Green Version]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Boiani, M.; Schöler, H.R. Regulatory networks in embryo-derived pluripotent stem cells. Nat. Rev. Mol. Cell Biol. 2005, 6, 872–884. [Google Scholar] [CrossRef]
- Seo, E.; Basu-Roy, U.; Gunaratne, P.H.; Coarfa, C.; Lim, D.S.; Basilico, C.; Mansukhani, A. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013, 3, 2075–2087. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.C.; Su, P.F.; Huang, Y.F.; Yew, T.L.; Hung, S.C. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol. Cell 2012, 47, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Malvicini, R.; Santa-Cruz, D.; Pacienza, N.; Yannarelli, G. OCT4 Silencing Triggers Its Epigenetic Repression and Impairs the Osteogenic and Adipogenic Differentiation of Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2019, 20, 3268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierantozzi, E.; Gava, B.; Manini, I.; Roviello, F.; Marotta, G.; Chiavarelli, M.; Sorrentino, V. Pluripotency regulators in human mesenchymal stem cells: Expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011, 20, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Riekstina, U.; Cakstina, I.; Parfejevs, V.; Hoogduijn, M.; Jankovskis, G.; Muiznieks, I.; Muceniece, R.; Ancans, J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. Rep. 2009, 5, 378–386. [Google Scholar] [CrossRef]
- Chen, L.; Samanta, A.; Zhao, L.; Dudley, N.R.; Buehler, T.; Vincent, R.J.; Hauptman, J.; Girgis, M.; Dawn, B. Vitamin D3 induces mesenchymal-to-endothelial transition and promotes a proangiogenic niche through IGF-1 signaling. iScience 2021, 24, 102272. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Wu, Y.N.; Guo, X.M.; Hui, J.H.; Lee, E.H.; Lim, B. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev. 2009, 18, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Go, M.J.; Takenaka, C.; Ohgushi, H. Forced expression of Sox2 or Nanog in human bone marrow derived mesenchymal stem cells maintains their expansion and differentiation capabilities. Exp. Cell Res. 2008, 314, 1147–1154. [Google Scholar] [CrossRef]
- Piccinato, C.A.; Sertie, A.L.; Torres, N.; Ferretti, M.; Antonioli, E. High OCT4 and Low p16(INK4A) Expressions Determine In Vitro Lifespan of Mesenchymal Stem Cells. Stem Cells Int. 2015, 2015, 369828. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.S.; Choi, Y.; Jang, Y.; Lee, M.; Choi, W.J.; Kim, S.H.; Lee, J.W. SIRT1 directly regulates SOX2 to maintain self-renewal and multipotency in bone marrow-derived mesenchymal stem cells. Stem Cells 2014, 32, 3219–3231. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, C.H.; Chang, G.E.; Cheong, E.; Shin, I. A potent and selective small molecule inhibitor of sirtuin 1 promotes differentiation of pluripotent P19 cells into functional neurons. Sci. Rep. 2016, 6, 34324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, F.X.; Wang, Z.M.; Wang, Q.; Wang, H.F.; Ren, Y.; Tai, D.P.; Liang, H.; Liu, D.J. Histone H3K9 acetylation influences growth characteristics of goat adipose-derived stem cells in vitro. Genet. Mol. Res. GMR 2016, 15, gmr15048954. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, D.; Li, K.; Pedersen-White, J.; Stallmann-Jorgensen, I.S.; Huang, Y.; Parikh, S.; Liu, K.; Dong, Y. Increased telomerase activity and vitamin D supplementation in overweight African Americans. Int. J. Obes. 2012, 36, 805–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, M.J. Vitamin D deficiency accelerates ageing and age-related diseases: A novel hypothesis. J. Physiol. 2017, 595, 6825–6836. [Google Scholar] [CrossRef] [PubMed]
- Bima, A.I.; Mahdi, A.S.; Al Fayez, F.F.; Khawaja, T.M.; Abo El-Khair, S.M.; Elsamanoudy, A.Z. Cellular Senescence and Vitamin D Deficiency Play a Role in the Pathogenesis of Obesity-Associated Subclinical Atherosclerosis: Study of the Potential Protective Role of Vitamin D Supplementation. Cells 2021, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, R.; Qiao, W.; Zhang, W.; Chen, J.; Mao, L.; Goltzman, D.; Miao, D. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell 2019, 18, e12951. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Zhu, W.; Chen, H.; Hu, X.; Jiang, Z.; Xu, Y.; Wang, L.; Zhou, Y.; Chen, P.; et al. SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front. Aging Neurosci. 2014, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chen, X.; Liu, T.; Gong, Y.; Chen, S.; Pan, G.; Cui, W.; Luo, Z.P.; Pei, M.; Yang, H.; et al. Melatonin reverses H2O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J. Pineal Res. 2015, 59, 190–205. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Pi, C.; Yang, Y.; Lin, L.; Shi, Y.; Li, Y.; Li, Y.; He, X. Nampt Expression Decreases Age-Related Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Sirt1. PLoS ONE 2017, 12, e0170930. [Google Scholar] [CrossRef] [Green Version]
- Raj, V.; Natarajan, S.; Marimuthu, C.; Chatterjee, S.; Ramasamy, M.; Ramanujam, G.M.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Arockiaraj, J.; et al. Cholecalciferol and metformin protect against lipopolysaccharide-induced endothelial dysfunction and senescence by modulating sirtuin-1 and protein arginine methyltransferase-1. Eur. J. Pharmacol. 2021, 912, 174531. [Google Scholar] [CrossRef]
- Chen, L.; Holder, R.; Porter, C.; Shah, Z. Vitamin D3 attenuates doxorubicin-induced senescence of human aortic endothelial cells by upregulation of IL-10 via the pAMPKα/Sirt1/Foxo3a signaling pathway. PLoS ONE 2021, 16, e0252816. [Google Scholar] [CrossRef]
- Yoon, J.K.; Kang, M.L.; Park, J.H.; Lee, K.M.; Shin, Y.M.; Lee, J.W.; Kim, H.O.; Sung, H.J. Direct Control of Stem Cell Behavior Using Biomaterials and Genetic Factors. Stem Cells Int. 2018, 2018, 8642989. [Google Scholar] [CrossRef]
- Viereck, V.; Siggelkow, H.; Tauber, S.; Raddatz, D.; Schutze, N.; Hüfner, M. Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J. Cell. Biochem. 2002, 86, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Song, Y.M.; Baek, S.; Park, Y.H.; Park, J.B. Vitamin D Enhanced the Osteogenic Differentiation of Cell Spheroids Composed of Bone Marrow Stem Cells. Medicina 2021, 57, 1271. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.A.; Kheur, S.; Badhe, R.V.; Raj, A.T.; Bhonde, R.; Jaisinghani, A.; Vyas, N.; Patil, V.R.; Alhazmi, Y.A.; Parveen, S.; et al. Assessing the potential use of chitosan scaffolds for the sustained localized delivery of vitamin D. Saudi J. Biol. Sci. 2021, 28, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, X.; Chen, H.; Cao, J.; Zhang, L.; Hu, X.; Wang, J. Role of SIRT1 and AMPK in mesenchymal stem cells differentiation. Ageing Res. Rev. 2014, 13, 55–64. [Google Scholar] [CrossRef]
- Baur, J.A. Biochemical effects of SIRT1 activators. Biochim. Biophys. Acta 2010, 1804, 1626–1634. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Meng, H.; Wang, X.; Zhao, C.; Peng, J.; Wang, Y. Differentiation of Bone Marrow Mesenchymal Stem Cells in Osteoblasts and Adipocytes and its Role in Treatment of Osteoporosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 226–233. [Google Scholar] [CrossRef]
- Kelly, K.A.; Gimble, J.M. 1,25-Dihydroxy vitamin D3 inhibits adipocyte differentiation and gene expression in murine bone marrow stromal cell clones and primary cultures. Endocrinology 1998, 139, 2622–2628. [Google Scholar] [CrossRef]
- Kong, J.; Li, Y.C. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am. J. Physiology. Endocrinol. Metab. 2006, 290, E916–E924. [Google Scholar] [CrossRef] [Green Version]
- Salehpour, A.; Hedayati, M.; Shidfar, F.; Neshatbini Tehrani, A.; Farshad, A.A.; Mohammadi, S. 1,25-Dihydroxyvitamin D3 modulates adipogenesis of human adipose-derived mesenchymal stem cells dose-dependently. Nutr. Metab. 2021, 18, 29. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef]
- Bäckesjö, C.M.; Li, Y.; Lindgren, U.; Haldosén, L.A. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. Cells Tissues Organs 2009, 189, 93–97. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence (5′-3′) |
|---|---|
| GAPDH | F: GAAGGTGAAGGTCGGAGT |
| R: GAAGATGGTGATGGGATTTC | |
| ALP | F: CACCCACGTCGATTGCATCT |
| R: TAGCCACGTTGGTGTTGAGC | |
| OCN | F: GGCGCTACCTGTATCAATGG |
| R: TCAGCCAACTCGTCACAGTC | |
| RUNX2 | F: GCCTAGGCGCATTTCAGA |
| R: CTGAGAGTGGAAGGCCAGAG | |
| NANOG | F: GATGCCTCACACGGAGACTG |
| R: GCAGAAGTGGGTTGTTTGCC | |
| Oct4 | F: TGAGTAGTCCCTTCGCAAGC |
| R. GCTTCGGATTTCGCCTTCTC | |
| SOX2 | F: GACAGTTACGCGCACATGAA |
| R: TAGGTCTGCGAGCTGGTCAT | |
| SIRT1 | F: TGCTGGCCTAATAGAGTGGCA |
| R: CTCAGCGCCATGGAAAATGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borojević, A.; Jauković, A.; Kukolj, T.; Mojsilović, S.; Obradović, H.; Trivanović, D.; Živanović, M.; Zečević, Ž.; Simić, M.; Gobeljić, B.; et al. Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, Partly through SIRT1 Signaling. Biomolecules 2022, 12, 323. https://doi.org/10.3390/biom12020323
Borojević A, Jauković A, Kukolj T, Mojsilović S, Obradović H, Trivanović D, Živanović M, Zečević Ž, Simić M, Gobeljić B, et al. Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, Partly through SIRT1 Signaling. Biomolecules. 2022; 12(2):323. https://doi.org/10.3390/biom12020323
Chicago/Turabian StyleBorojević, Ana, Aleksandra Jauković, Tamara Kukolj, Slavko Mojsilović, Hristina Obradović, Drenka Trivanović, Milena Živanović, Željko Zečević, Marija Simić, Borko Gobeljić, and et al. 2022. "Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, Partly through SIRT1 Signaling" Biomolecules 12, no. 2: 323. https://doi.org/10.3390/biom12020323
APA StyleBorojević, A., Jauković, A., Kukolj, T., Mojsilović, S., Obradović, H., Trivanović, D., Živanović, M., Zečević, Ž., Simić, M., Gobeljić, B., Vujić, D., & Bugarski, D. (2022). Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, Partly through SIRT1 Signaling. Biomolecules, 12(2), 323. https://doi.org/10.3390/biom12020323







