Imaging Techniques for Aortic Aneurysms and Dissections in Mice: Comparisons of Ex Vivo, In Situ, and Ultrasound Approaches
Abstract
:1. Introduction
2. Preferred Approaches for Aortic Imaging in Mice
2.1. Imaging Approaches for Aortic Aneurysms
2.2. Imaging of Aortic Dissections
3. Imaging Approaches for Aortic Measurements in Mice
3.1. Ex Vivo Imaging
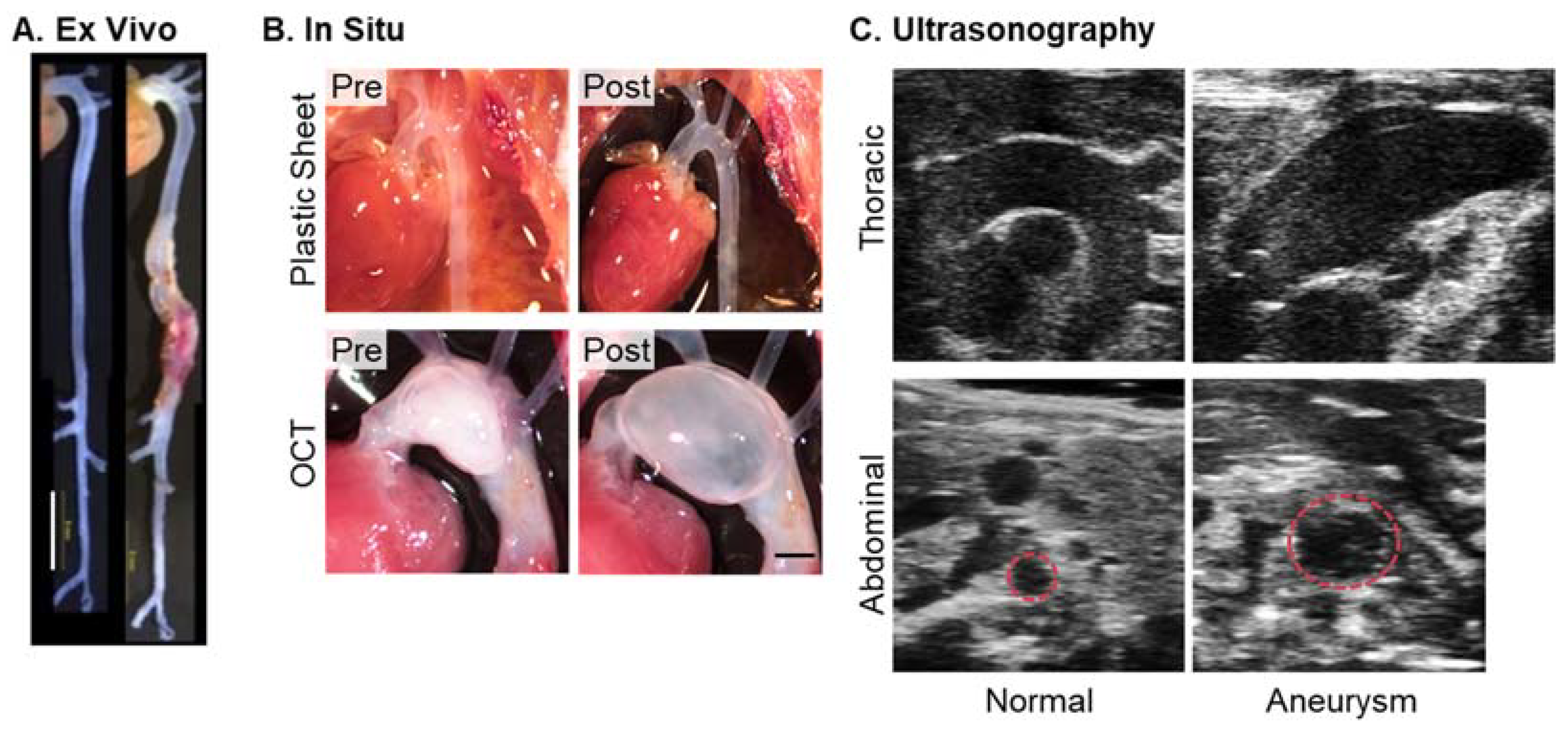
3.2. In Situ Imaging
3.3. Ultrasonography
3.3.1. Ultrasound System
3.3.2. Ultrasound View
3.3.3. Cardiac Cycle
3.3.4. Three-Dimensional Imaging
3.3.5. Doppler Ultrasonography
4. Comparisons of Ex Vivo, In Situ, and Ultrasound Approaches
5. Limitations
6. Summary
Funding
Conflicts of Interest
References
- Shen, Y.H.; LeMaire, S.A.; Webb, N.R.; Cassis, L.A.; Daugherty, A.; Lu, H.S. Aortic Aneurysms and Dissections Series. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Sakalihasan, N.; Michel, J.B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal aortic aneurysms. Nat. Rev. Dis. Primers 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Ramirez, F. Therapies for thoracic aortic aneurysms and acute aortic dissections. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nienaber, C.A.; Clough, R.E.; Sakalihasan, N.; Suzuki, T.; Gibbs, R.; Mussa, F.; Jenkins, M.P.; Thompson, M.M.; Evangelista, A.; Yeh, J.S.; et al. Aortic dissection. Nat. Rev. Dis. Primers 2016, 2, 16053. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhamidipati, C.M.; Mehta, G.S.; Lu, G.; Moehle, C.W.; Barbery, C.; DiMusto, P.D.; Laser, A.; Kron, I.L.; Upchurch, G.R., Jr.; Ailawadi, G. Development of a novel murine model of aortic aneurysms using peri-adventitial elastase. Surgery 2012, 152, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Kanematsu, Y.; Kanematsu, M.; Kurihara, C.; Tsou, T.L.; Nuki, Y.; Liang, E.I.; Makino, H.; Hashimoto, T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension 2010, 55, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Shimizu-Hirota, R.; Shimoda, M.; Adachi, T.; Shimizu, H.; Weiss, S.J.; Itoh, H.; Hori, S.; Aikawa, N.; Okada, Y. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation 2012, 126, 3070–3080. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Liu, Y.; Wang, X.; Jia, L.; Piao, C.; Lan, F.; Du, J. beta-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Sci. Rep. 2016, 6, 28149. [Google Scholar] [CrossRef] [Green Version]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [PubMed] [Green Version]
- Hong, H.; Yang, Y.; Liu, B.; Cai, W. Imaging of Abdominal Aortic Aneurysm: The present and the future. Curr. Vasc. Pharm. 2010, 8, 808–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, H.; Chen, J.Z.; Wright, B.C.; Moorleghen, J.J.; Lu, H.S.; Daugherty, A. Ultrasound imaging of the thoracic and abdominal aorta in mice to determine aneurysm dimensions. J. Vis. Exp. 2019, 145, e59013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, H.; Franklin, M.K.; Moorleghen, J.J.; Howatt, D.A.; Kukida, M.; Lu, H.S.; Daugherty, A. Ultrasound monitoring of descending aortic aneurysms and dissections in mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.R.; Clayton, N.P.; Carta, L.; Galatioto, J.; Chiu, E.; Smaldone, S.; Nelson, C.A.; Cheng, S.H.; Wentworth, B.M.; Ramirez, F. Dimorphic effects of transforming growth factor-beta signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emrich, F.C.; Okamura, H.; Dalal, A.R.; Penov, K.; Merk, D.R.; Raaz, U.; Hennigs, J.K.; Chin, J.T.; Miller, M.O.; Pedroza, A.J.; et al. Enhanced caspase activity contributes to aortic wall remodeling and early aneurysm development in a murine model of Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachet, B.; Piersigilli, A.; Fraga-Silva, R.A.; Aslanidou, L.; Sordet-Dessimoz, J.; Astolfo, A.; Stampanoni, M.F.; Segers, P.; Stergiopulos, N. Ascending aortic aneurysm in angiotensin II-infused mice: Formation, progression, and the role of focal dissections. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 673–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galatioto, J.; Caescu, C.I.; Hansen, J.; Cook, J.R.; Miramontes, I.; Iyengar, R.; Ramirez, F. Cell type-specific contributions of the angiotensin II type 1a receptor to aorta homeostasis and aneurysmal disease-brief report. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 588–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fava, M.; Barallobre-Barreiro, J.; Mayr, U.; Lu, R.; Didangelos, A.; Baig, F.; Lynch, M.; Catibog, N.; Joshi, A.; Barwari, T.; et al. Role of ADAMTS-5 in Aortic Dilatation and Extracellular Matrix Remodeling. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1537–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Yue, Z.; Wu, J.; Chen, J.; Wang, S.; Wu, J.; Ren, L.; Zhang, A.; Deng, P.; Wang, K.; et al. MicroRNA-21 Knockout Exacerbates Angiotensin II-Induced Thoracic Aortic Aneurysm and Dissection in Mice With Abnormal Transforming Growth Factor-beta-SMAD3 Signaling. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1086–1101. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Peters, A.M.; Wang, S.; Janda, A.; Chen, J.; Zhou, P.; Arthur, E.; Kwartler, C.S.; Milewicz, D.M. Reversal of Aortic Enlargement Induced by Increased Biomechanical Forces Requires AT1R Inhibition in Conjunction With AT2R Activation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroza, A.J.; Tashima, Y.; Shad, R.; Cheng, P.; Wirka, R.; Churovich, S.; Nakamura, K.; Yokoyama, N.; Cui, J.Z.; Iosef, C.; et al. Single-cell transcriptomic profiling of vascular smooth muscle cell phenotype modulation in Marfan syndrome aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2195–2211. [Google Scholar] [CrossRef]
- Hibender, S.; Franken, R.; van Roomen, C.; Ter Braake, A.; van der Made, I.; Schermer, E.E.; Gunst, Q.; van den Hoff, M.J.; Lutgens, E.; Pinto, Y.M.; et al. Resveratrol inhibits aortic root dilatation in the Fbn1C1039G/+ Marfan mouse model. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1618–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Lee, J.; Basu, R.; Sakamuri, S.S.V.P.; Wang, X.; Fan, D.; Kassiri, Z. Divergent role of matrix metalloproteinase 2 in pathogenesis of thoracic aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 888–898. [Google Scholar] [CrossRef] [Green Version]
- Lutshumba, J.; Liu, S.; Zhong, Y.; Hou, T.; Daugherty, A.; Lu, H.; Guo, Z.; Gong, M.C. Deletion of BMAL1 in smooth muscle cells protects mice from abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1063–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlSiraj, Y.; Thatcher, S.E.; Blalock, E.; Saintilnord, W.N.; Daugherty, A.; Lu, H.S.; Luo, W.; Shen, Y.H.; LeMaire, S.A.; Arnold, A.P.; et al. Monosomy X in female mice influences the regional formation and augments the severity of angiotensin II-induced aortopathies. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 269–283. [Google Scholar] [CrossRef]
- Davis, F.M.; Rateri, D.L.; Balakrishnan, A.; Howatt, D.A.; Strickland, D.K.; Muratoglu, S.C.; Haggerty, C.M.; Fornwalt, B.K.; Cassis, L.A.; Daugherty, A. Smooth muscle cell deletion of low-density lipoprotein receptor-related protein 1 augments angiotensin II-induced superior mesenteric arterial and ascending aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.M.; Seto, S.W.; Jose, R.J.; Li, J.; Morton, S.K.; Biros, E.; Wang, Y.; Nsengiyumva, V.; Lindeman, J.H.; Loots, G.G.; et al. Wnt Signaling Pathway Inhibitor Sclerostin Inhibits Angiotensin II-Induced Aortic Aneurysm and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 553–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelov, S.N.; Hu, J.H.; Wei, H.; Airhart, N.; Shi, M.; Dichek, D.A. TGF-beta (Transforming Growth Factor-beta) Signaling Protects the Thoracic and Abdominal Aorta From Angiotensin II-Induced Pathology by Distinct Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2102–2113. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Ren, P.; Zheng, Y.; Zhang, L.; Xu, G.; Xie, W.; Lloyd, E.E.; Zhang, S.; Zhang, Q.; Curci, J.A.; et al. NLRP3 (Nucleotide Oligomerization Domain-Like Receptor Family, Pyrin Domain Containing 3)-Caspase-1 Inflammasome Degrades Contractile Proteins: Implications for Aortic Biomechanical Dysfunction and Aneurysm and Dissection Formation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 694–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.H.; Wei, H.; Jaffe, M.; Airhart, N.; Du, L.; Angelov, S.N.; Yan, J.; Allen, J.K.; Kang, I.; Wight, T.N.; et al. Postnatal deletion of the type II transforming growth factor-beta receptor in smooth muscle cells causes severe aortopathy in mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2647–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeill, E.; Iqbal, A.J.; Jones, D.; Patel, J.; Coutinho, P.; Taylor, L.; Greaves, D.R.; Channon, K.M. Tracking Monocyte Recruitment and Macrophage Accumulation in Atherosclerotic Plaque Progression Using a Novel hCD68GFP/ApoE-/- Reporter Mouse-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 258–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.J.; Hang, H.T.; Thang, B.Q.; Shimoda, T.; Sakamoto, H.; Osaka, M.; Hiramatsu, Y.; Yamashiro, Y.; Yanagisawa, H. Role of PAR1-Egr1 in the Initiation of Thoracic Aortic Aneurysm in Fbln4-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1905–1917. [Google Scholar] [CrossRef]
- Mellak, S.; Ait-Oufella, H.; Esposito, B.; Loyer, X.; Poirier, M.; Tedder, T.F.; Tedgui, A.; Mallat, Z.; Potteaux, S. Angiotensin II Mobilizes Spleen Monocytes to Promote the Development of Abdominal Aortic Aneurysm in Apoe-/- Mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.W.; Jia, L.X.; Ni, X.Q.; Zhao, L.; Chang, J.R.; Zhang, J.S.; Hou, Y.L.; Zhu, Y.; Guan, Y.F.; Yu, Y.R.; et al. Intermedin1-53 Attenuates Abdominal Aortic Aneurysm by Inhibiting Oxidative Stress. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2176–2190. [Google Scholar] [CrossRef] [Green Version]
- Alsiraj, Y.; Thatcher, S.E.; Blalock, E.; Fleenor, B.; Daugherty, A.; Cassis, L.A. Sex chromosome complement defines diffuse versus focal angiotensin II-induced aortic pathology. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Takei, Y.; Tanaka, T.; Kent, K.C.; Yamanouchi, D. Osteoclastogenic Differentiation of Macrophages in the Development of Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1962–1971. [Google Scholar] [CrossRef] [Green Version]
- Moran, C.S.; Rush, C.M.; Dougan, T.; Jose, R.J.; Biros, E.; Norman, P.E.; Gera, L.; Golledge, J. Modulation of Kinin B2 Receptor Signaling Controls Aortic Dilatation and Rupture in the Angiotensin II-Infused Apolipoprotein E-Deficient Mouse. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.C.; Li, Y.H.; Shi, G.Y.; Tsai, H.W.; Luo, C.Y.; Cheng, M.H.; Ma, C.Y.; Hsu, Y.Y.; Cheng, T.L.; Chang, B.I.; et al. Membrane-Bound Thrombomodulin Regulates Macrophage Inflammation in Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2412–2422. [Google Scholar] [CrossRef] [Green Version]
- Sawada, H.; Hao, H.; Naito, Y.; Oboshi, M.; Hirotani, S.; Mitsuno, M.; Miyamoto, Y.; Hirota, S.; Masuyama, T. Aortic iron overload with oxidative stress and inflammation in human and murine abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Usui, F.; Shirasuna, K.; Kimura, H.; Tatsumi, K.; Kawashima, A.; Karasawa, T.; Yoshimura, K.; Aoki, H.; Tsutsui, H.; Noda, T.; et al. Inflammasome Activation by Mitochondrial Oxidative Stress in Macrophages Leads to the Development of Angiotensin II-Induced Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lu, H.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Sorci-Thomas, M.; Cassis, L.A.; Daugherty, A. Associations of apoAI and apoB-containing lipoproteins with AngII-induced abdominal aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1826–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakita, D.; Kurashima, Y.; Crother, T.R.; Noval Rivas, M.; Lee, Y.; Chen, S.; Fury, W.; Bai, Y.; Wagner, S.; Li, D.; et al. Role of Interleukin-1 Signaling in a Mouse Model of Kawasaki Disease-Associated Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 886–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Howatt, D.A.; Balakrishnan, A.; Graham, M.J.; Mullick, A.E.; Daugherty, A. Hypercholesterolemia induced by a PCSK9 gain-of-function mutation augments angiotensin II-induced abdominal aortic aneurysms in C57BL/6 mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1753–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaheen, B.; Downs, E.A.; Serbulea, V.; Almenara, C.C.; Spinosa, M.; Su, G.; Zhao, Y.; Srikakulapu, P.; Butts, C.; McNamara, C.A.; et al. B-cell depletion promotes aortic infiltration of immunosuppressive cells and is protective of experimental aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2191–2202. [Google Scholar] [CrossRef] [Green Version]
- Imanishi, M.; Chiba, Y.; Tomita, N.; Matsunaga, S.; Nakagawa, T.; Ueno, M.; Yamamoto, K.; Tamaki, T.; Tomita, S. Hypoxia-inducible factor-1alpha in smooth muscle cells protects against aortic aneurysms-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2158–2162. [Google Scholar] [CrossRef] [Green Version]
- Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Muniappan, L.; Rateri, D.L.; Uchida, H.A.; Takano, J.; Saido, T.C.; Chishti, A.H.; Baud, L.; et al. Leukocyte Calpain Deficiency Reduces Angiotensin II-Induced Inflammation and Atherosclerosis But Not Abdominal Aortic Aneurysms in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Salmon, M.D.; Lu, G.; Su, G.; Pope, N.H.; Smith, J.R.; Weiss, M.L.; Upchurch, G.R.J. Mesenchymal Stem Cells Attenuate NADPH Oxidase-Dependent High Mobility Group Box 1 Production and Inhibit Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.L.; Wang, Y.; Liao, M.; Wemmelund, H.; Ren, J.; Fernandes, C.; Zhou, Y.; Sukhova, G.K.; Lindholt, J.S.; Johnsen, S.P.; et al. Allergic Lung Inflammation Aggravates Angiotensin II-Induced Abdominal Aortic Aneurysms in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Martorell, S.; Hueso, L.; Gonzalez-Navarro, H.; Collado, A.; Sanz, M.J.; Piqueras, L. Vitamin D Receptor Activation Reduces Angiotensin-II-Induced Dissecting Abdominal Aortic Aneurysm in Apolipoprotein E-Knockout Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1587–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.F.; Pei, J.F.; Zhang, Y.; Zhang, R.; Wang, F.; Gao, P.; Zhang, Z.Q.; Wang, T.T.; She, Z.G.; Chen, H.Z.; et al. The Paraoxonase Gene Cluster Protects Against Abdominal Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, T.; Yoshimura, K.; Yamashita, O.; Ueda, K.; Morikage, N.; Sawada, Y.; Hamano, K. Focal Adhesion Kinase Promotes the Progression of Aortic Aneurysm by Modulating Macrophage Behavior. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, T.; Horie, T.; Baba, O.; Nishiga, M.; Nishino, T.; Izuhara, M.; Kuwabara, Y.; Nishi, H.; Usami, S.; Nakazeki, F.; et al. Genetic Ablation of MicroRNA-33 Attenuates Inflammation and Abdominal Aortic Aneurysm Formation via Several Anti-Inflammatory Pathways. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2161–2170. [Google Scholar] [CrossRef] [Green Version]
- Batra, R.; Suh, M.K.; Carson, J.S.; Dale, M.A.; Meisinger, T.M.; Fitzgerald, M.; Opperman, P.J.; Luo, J.; Pipinos, I.I.; Xiong, W.; et al. IL-1beta (Interleukin-1beta) and TNF-alpha (Tumor Necrosis Factor-alpha) Impact Abdominal Aortic Aneurysm Formation by Differential Effects on Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.K.; Spinosa, M.; Davis, J.P.; Pope, N.; Laubach, V.E.; Su, G.; Serbulea, V.; Leitinger, N.; Ailawadi, G.; Upchurch, G.R., Jr. Novel Role of IL (Interleukin)-1beta in Neutrophil Extracellular Trap Formation and Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 843–853. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Fu, Y.; Deng, J.; Shen, Y.; Wang, Y.; Yu, F.; Xie, N.; Chen, Z.; Hong, T.; Peng, X.; et al. Deficiency of FAM3D (Family With Sequence Similarity 3, Member D), A Novel Chemokine, Attenuates Neutrophil Recruitment and Ameliorates Abdominal Aortic Aneurysm Development. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1616–1631. [Google Scholar] [CrossRef]
- Umebayashi, R.; Uchida, H.A.; Kakio, Y.; Subramanian, V.; Daugherty, A.; Wada, J. Cilostazol attenuates angiotensin II-induced abdominal aortic aneurysms but not atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xia, N.; Wen, S.; Li, D.; Lu, Y.; Gu, M.; Tang, T.; Jiao, J.; Lv, B.; Nie, S.; et al. IL (Interleukin)-33 Suppresses Abdominal Aortic Aneurysm by Enhancing Regulatory T-Cell Expansion and Activity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 446–458. [Google Scholar] [CrossRef]
- Salmon, M.; Schaheen, B.; Spinosa, M.; Montgomery, W.; Pope, N.H.; Davis, J.P.; Johnston, W.F.; Sharma, A.K.; Owens, G.K.; Merchant, J.L.; et al. ZFP148 (Zinc-Finger Protein 148) binds cooperatively with NF-1 (Neurofibromin 1) to inhibit smooth muscle marker gene expression during abdominal aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Moran, C.S.; Biros, E.; Krishna, S.M.; Wang, Y.; Tikellis, C.; Morton, S.K.; Moxon, J.V.; Cooper, M.E.; Norman, P.E.; Burrell, L.M.; et al. Resveratrol inhibits growth of experimental abdominal aortic aneurysm associated with upregulation of angiotensin-converting enzyme 2. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2195–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, R.; Collado, A.; Selles, F.; Gonzalez-Navarro, H.; Sanz, M.J.; Real, J.T.; Piqueras, L. SGLT-2 (Sodium-Glucose Cotransporter 2) inhibition reduces Ang II (angiotensin II)-induced dissecting abdominal aortic aneurysm in apoE (apolipoprotein E) knockout mice. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1614–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhahar, V.; Das, A.; Horimatsu, T.; Ash, D.; Leanhart, S.; Antipova, O.; Vogt, S.; Singla, B.; Csanyi, G.; White, J.; et al. Copper Transporter ATP7A (Copper-Transporting P-Type ATPase/Menkes ATPase) Limits Vascular Inflammation and Aortic Aneurysm Development: Role of MicroRNA-125b. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2320–2337. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, J.; Jiang, T.; Monslow, J.; Tang, S.Y.; Todd, L.; Pure, E.; Chen, L.; FitzGerald, G.A. Bmal1 Deletion in Myeloid Cells Attenuates Atherosclerotic Lesion Development and Restrains Abdominal Aortic Aneurysm Formation in Hyperlipidemic Mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1523–1532. [Google Scholar] [CrossRef]
- Hiromi, T.; Yokoyama, U.; Kurotaki, D.; Mamun, A.; Ishiwata, R.; Ichikawa, Y.; Nishihara, H.; Umemura, M.; Fujita, T.; Yasuda, S.; et al. Excessive EP4 Signaling in Smooth Muscle Cells Induces Abdominal Aortic Aneurysm by Amplifying Inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1559–1573. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.; Zhao, G.; Zhao, Y.; Lu, H.; Xiong, W.; Liang, W.; Sun, J.; Wang, H.; Zhu, T.; Rom, O.; et al. BAF60a Deficiency in Vascular Smooth Muscle Cells Prevents Abdominal Aortic Aneurysm by Reducing Inflammation and Extracellular Matrix Degradation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2494–2507. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, T.; Sorenson, C.M.; Sheibani, N.; Liu, B. Myeloid-derived TSP1 (Thrombospondin-1) contributes to abdominal aortic aneurysm through suppressing tissue inhibitor of metalloproteinases-1. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e350–e366. [Google Scholar] [CrossRef]
- Yu, B.; Liu, Z.; Fu, Y.; Wang, Y.; Zhang, L.; Cai, Z.; Yu, F.; Wang, X.; Zhou, J.; Kong, W. CYLD Deubiquitinates Nicotinamide Adenine Dinucleotide Phosphate Oxidase 4 Contributing to Adventitial Remodeling. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1698–1709. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Xing, L.; Zeng, C.; Wu, T.; Gui, Y.; Li, W.; Lan, T.; Yang, Y.; Gu, Q.; Qi, C.; et al. Inactivation of PI3Kdelta induces vascular injury and promotes aneurysm development by upregulating the AP-1/MMP-12 pathway in macrophages. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 368–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, S.M.; Seto, S.W.; Jose, R.J.; Biros, E.; Moran, C.S.; Wang, Y.; Clancy, P.; Golledge, J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, N.R.; De Beer, M.C.; Wroblewski, J.M.; Ji, A.; Bailey, W.; Shridas, P.; Charnigo, R.J.; Noffsinger, V.P.; Witta, J.; Howatt, D.A.; et al. Deficiency of Endogenous Acute-Phase Serum Amyloid A Protects apoE-/- Mice from Angiotensin II-Induced Abdominal Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1156–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, A.P.r.; Edwards, T.L.; Antoniak, S.; Geddings, J.E.; Jahangir, E.; Wei, W.Q.; Denny, J.C.; Boulaftali, Y.; Bergmeier, W.; Daugherty, A.; et al. Platelet Inhibitors Reduce Rupture in a Mouse Model of Established Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2032–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ijaz, T.; Sun, H.; Pinchuk, I.V.; Milewicz, D.M.; Tilton, R.G.; Brasier, A.R. Deletion of NF-kappaB/RelA in angiotensin II-sensitive mesenchymal cells blocks aortic vascular inflammation and abdominal aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1881–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lareyre, F.; Clement, M.; Raffort, J.; Pohlod, S.; Patel, M.; Esposito, B.; Master, L.; Finigan, A.; Vandestienne, M.; Stergiopulos, N.; et al. TGFbeta (Transforming Growth Factor-beta) Blockade Induces a Human-Like Disease in a Nondissecting Mouse Model of Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2171–2181. [Google Scholar] [CrossRef] [Green Version]
- Adam, M.; Kooreman, N.; Jagger, A.; Wagenhaeuser, M.U.; Mehrkens, D.; Wang, Y.; Kayama, Y.; Toyama, K.; Raaz, U.; Schellinger, I.N.; et al. Systemic upregulation of IL-10 (Interleukin-10) using a nonimmunogenic vector reduces growth and rate of dissecting abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1796–1805. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; He, X.; Si, X.; Wang, H.; Li, B.; Hu, Y.; Li, M.; Chen, X.; Liao, W.; Liao, Y.; et al. SM22alpha (Smooth Muscle 22alpha) Prevents Aortic Aneurysm Formation by Inhibiting Smooth Muscle Cell Phenotypic Switching through Suppressing Reactive Oxygen Species/NF-kappaB (Nuclear Factor-kappaB). Arterioscler. Thromb. Vasc. Biol. 2019, 39, e10–e25. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Dev, R.; Belenchia, A.M.; Aroor, A.R.; Whaley-Connell, A.; Pulakat, L.; Hans, C.P. Deficiency of IL12p40 (Interleukin 12 p40) Promotes Ang II (Angiotensin II)-Induced Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 212–223. [Google Scholar] [CrossRef]
- Xu, B.; Iida, Y.; Glover, K.J.; Ge, Y.; Wang, Y.; Xuan, H.; Hu, X.; Tanaka, H.; Wang, W.; Fujimura, N.; et al. Inhibition of VEGF (Vascular Endothelial Growth Factor)-A or its Receptor Activity Suppresses Experimental Aneurysm Progression in the Aortic Elastase Infusion Model. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1652–1666. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, Z.; Cai, Z.; Sun, Y.; Li, L.; Yao, F.; Yang, L.; Zhou, Y.; Zhu, H.; Fu, Y.; et al. Runx2 (Runt-Related Transcription Factor 2)-Mediated Microcalcification Is a Novel Pathological Characteristic and Potential Mediator of Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1352–1369. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Chute, M.; Hu, M.; Winkelaar, G.; Owen, C.A.; Oudit, G.Y.; Kassiri, Z. ADAM (a Disintegrin and Metalloproteinase) 15 Deficiency Exacerbates Ang II (Angiotensin II)-Induced Aortic Remodeling Leading to Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1918–1934. [Google Scholar] [CrossRef]
- Chiang, M.T.; Chen, I.M.; Hsu, F.F.; Chen, Y.H.; Tsai, M.S.; Hsu, Y.W.; Leu, H.B.; Huang, P.H.; Chen, J.W.; Liu, F.T.; et al. Gal-1 (Galectin-1) Upregulation Contributes to Abdominal Aortic Aneurysm Progression by Enhancing Vascular Inflammation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Cassis, L.A. Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 429–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Krishna, S.; Golledge, J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis 2013, 226, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Rateri, D.L.; Davis, F.M.; Balakrishnan, A.; Howatt, D.A.; Moorleghen, J.J.; O’Connor, W.N.; Charnigo, R.; Cassis, L.A.; Daugherty, A. Angiotensin II induces region-specific medial disruption during evolution of ascending aortic aneurysms. Am. J. Pathol. 2014, 184, 2586–2595. [Google Scholar] [CrossRef] [Green Version]
- Sawada, H.; Lu, H.S.; Cassis, L.A.; Daugherty, A. Twenty Years of Studying AngII (Angiotensin II)-Induced Abdominal Aortic Pathologies in Mice: Continuing Questions and Challenges to Provide Insight into the Human Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42. [Google Scholar] [CrossRef]
- Bersi, M.R.; Acosta Santamaria, V.A.; Marback, K.; Di Achille, P.; Phillips, E.H.; Goergen, C.J.; Humphrey, J.D.; Avril, S. Multimodality Imaging-Based Characterization of Regional Material Properties in a Murine Model of Aortic Dissection. Sci. Rep. 2020, 10, 9244. [Google Scholar] [CrossRef]
- Brangsch, J.; Reimann, C.; Kaufmann, J.O.; Adams, L.C.; Onthank, D.C.; Thone-Reineke, C.; Robinson, S.P.; Buchholz, R.; Karst, U.; Botnar, R.M.; et al. Concurrent Molecular Magnetic Resonance Imaging of Inflammatory Activity and Extracellular Matrix Degradation for the Prediction of Aneurysm Rupture. Circ. Cardiovasc. Imaging 2019, 12, e008707. [Google Scholar] [CrossRef] [Green Version]
- du Plessis, A.; Broeckhoven, C.; Guelpa, A.; le Roux, S.G. Laboratory x-ray micro-computed tomography: A user guideline for biological samples. Gigascience 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Willekens, I.; Buls, N.; Lahoutte, T.; Baeyens, L.; Vanhove, C.; Caveliers, V.; Deklerck, R.; Bossuyt, A.; de Mey, J. Evaluation of the radiation dose in micro-CT with optimization of the scan protocol. Contrast Media Mol. Imaging 2010, 5, 201–207. [Google Scholar] [CrossRef]
- Klink, A.; Heynens, J.; Herranz, B.; Lobatto, M.E.; Arias, T.; Sanders, H.M.; Strijkers, G.J.; Merkx, M.; Nicolay, K.; Fuster, V.; et al. In vivo characterization of a new abdominal aortic aneurysm mouse model with conventional and molecular magnetic resonance imaging. J. Am. Coll. Cardiol. 2011, 58, 2522–2530. [Google Scholar] [CrossRef] [Green Version]
- Turner, G.H.; Olzinski, A.R.; Bernard, R.E.; Aravindhan, K.; Karr, H.W.; Mirabile, R.C.; Willette, R.N.; Gough, P.J.; Jucker, B.M. In vivo serial assessment of aortic aneurysm formation in apolipoprotein E-deficient mice via MRI. Circ. Cardiovasc. Imaging 2008, 1, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferruzzi, J.; Murtada, S.I.; Li, G.; Jiao, Y.; Uman, S.; Ting, M.Y.; Tellides, G.; Humphrey, J.D. Pharmacologically Improved Contractility Protects Against Aortic Dissection in Mice with Disrupted Transforming Growth Factor-beta Signaling Despite Compromised Extracellular Matrix Properties. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 919–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clement, M.; Chappell, J.; Raffort, J.; Lareyre, F.; Vandestienne, M.; Taylor, A.L.; Finigan, A.; Harrison, J.; Bennett, M.R.; Bruneval, P.; et al. Vascular Smooth Muscle Cell Plasticity and Autophagy in Dissecting Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lin, Z.; Tang, X.; Tian, J.; Zheng, Q.; Jing, J.; Xie, L.; Chen, H.; Lu, Q.; Wang, H.; et al. S-Nitrosylation of Plastin-3 Exacerbates Thoracic Aortic Dissection Formation via Endothelial Barrier Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 175–188. [Google Scholar] [CrossRef]
- Nishida, N.; Aoki, H.; Ohno-Urabe, S.; Nishihara, M.; Furusho, A.; Hirakata, S.; Hayashi, M.; Ito, S.; Yamada, H.; Hirata, Y.; et al. High salt intake worsens aortic dissection in mice: Involvement of IL (interleukin)-17A-dependent ECM (extracellular matrix) metabolism. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Trachet, B.; Fraga-Silva, R.A.; Jacquet, P.A.; Stergiopulos, N.; Segers, P. Incidence, severity, mortality, and confounding factors for dissecting AAA detection in angiotensin II-infused mice: A meta-analysis. Cardiovasc. Res. 2015, 108, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Hashimoto, Y.; Majima, R.; Nakao, E.; Aoki, H.; Nishihara, M.; Ohno-Urabe, S.; Furusho, A.; Hirakata, S.; Nishida, N.; et al. MRTF-A promotes angiotensin II-induced inflammatory response and aortic dissection in mice. PLoS ONE 2020, 15, e0229888. [Google Scholar] [CrossRef] [Green Version]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole animal perfusion fixation for rodents. J. Vis. Exp. 2012, 65, 3564. [Google Scholar] [CrossRef] [Green Version]
- Gallo, E.M.; Loch, D.C.; Habashi, J.P.; Calderon, J.F.; Chen, Y.; Bedja, D.; van Erp, C.; Gerber, E.E.; Parker, S.J.; Sauls, K.; et al. Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J. Clin. Investig. 2014, 124, 448–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.J.; Staiculescu, M.C.; Hawes, J.Z.; Cocciolone, A.J.; Hunkins, B.M.; Roth, R.A.; Lin, C.Y.; Mecham, R.P.; Wagenseil, J.E. Heterogeneous Cellular Contributions to Elastic Laminae Formation in Arterial Wall Development. Circ. Res. 2019, 125, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Trachet, B.; Aslanidou, L.; Piersigilli, A.; Fraga-Silva, R.A.; Sordet-Dessimoz, J.; Villanueva-Perez, P.; Stampanoni, M.F.M.; Stergiopulos, N.; Segers, P. Angiotensin II infusion into ApoE-/- mice: A model for aortic dissection rather than abdominal aortic aneurysm? Cardiovasc. Res. 2017, 113, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Trachet, B.; Fraga-Silva, R.A.; Piersigilli, A.; Tedgui, A.; Sordet-Dessimoz, J.; Astolfo, A.; Van der Donckt, C.; Modregger, P.; Stampanoni, M.F.; Segers, P.; et al. Dissecting abdominal aortic aneurysm in Ang II-infused mice: Suprarenal branch ruptures and apparent luminal dilatation. Cardiovasc. Res. 2015, 105, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bersi, M.R.; Bellini, C.; Humphrey, J.D.; Avril, S. Local variations in material and structural properties characterize murine thoracic aortic aneurysm mechanics. Biomech. Model. Mechanobiol. 2019, 18, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Urabe, S.; Kukida, M.; Franklin, M.K.; Katsumata, Y.; Su, W.; Gong, M.C.; Lu, H.S.; Daugherty, A.; Sawada, H. Authentication of in situ measurements for thoracic aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2117–2119. [Google Scholar] [CrossRef]
- He, C.; Jiang, B.; Wang, M.; Ren, P.; Murtada, S.I.; Caulk, A.W.; Li, G.; Qin, L.; Assi, R.; Lovoulos, C.J.; et al. mTOR inhibition prevents angiotensin II-induced aortic rupture and pseudoaneurysm but promotes dissection in Apoe-deficient mice. JCI Insight 2022, 7, e155815. [Google Scholar] [CrossRef]
- Trachet, B.; Fraga-Silva, R.A.; Londono, F.J.; Swillens, A.; Stergiopulos, N.; Segers, P. Performance comparison of ultrasound-based methods to assess aortic diameter and stiffness in normal and aneurysmal mice. PLoS ONE 2015, 10, e0129007. [Google Scholar] [CrossRef] [Green Version]
- Waduud, M.A.; Kandavelu, P.; Reay, M.; Paradine, K.; Scott, D.J.A.; Bailey, M.A. High-Frequency Three-Dimensional Lumen Volume Ultrasound Is a Sensitive Method to Detect Early Aneurysmal Change in Elastase-Induced Murine Abdominal Aortic Aneurysm. Aorta 2021, 9, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Lee, S.Y.; Gayraud, B.; Andrikopoulos, K.; Shapiro, S.D.; Bunton, T.; Biery, N.J.; Dietz, H.C.; Sakai, L.Y.; Ramirez, F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA 1999, 96, 3819–3823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Schmit, B.M.; Fu, C.; DeSart, K.; Oh, S.P.; Berceli, S.A.; Jiang, Z. Smooth muscle cell-specific Tgfbr1 deficiency promotes aortic aneurysm formation by stimulating multiple signaling events. Sci. Rep. 2016, 6, 35444. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, Q.; Jiao, Y.; Qin, L.; Ali, R.; Zhou, J.; Ferruzzi, J.; Kim, R.W.; Geirsson, A.; Dietz, H.C.; et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J. Clin. Investig. 2014, 124, 755–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Z.; Sawada, H.; Moorleghen, J.J.; Weiland, M.; Daugherty, A.; Sheppard, M.B. Aortic strain correlates with elastin fragmentation in Fibrillin-1 hypomorphic mice. Circ. Rep. 2019, 1, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adelsperger, A.R.; Phillips, E.H.; Ibriga, H.S.; Craig, B.A.; Green, L.A.; Murphy, M.P.; Goergen, C.J. Development and growth trends in angiotensin II-induced murine dissecting abdominal aortic aneurysms. Physiol. Rep. 2018, 6, e13668. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Pakkiri, P.; Dai, E.; Lucas, A.; Fenster, A. Measurements of aneurysm morphology determined by 3-d micro-ultrasound imaging as potential quantitative biomarkers in a mouse aneurysm model. Ultrasound Med. Biol. 2007, 33, 1552–1560. [Google Scholar] [CrossRef]
- Soepriatna, A.H.; Damen, F.W.; Vlachos, P.P.; Goergen, C.J. Cardiac and respiratory-gated volumetric murine ultrasound. Int. J. Cardiovasc. Imaging 2018, 34, 713–724. [Google Scholar] [CrossRef]
- Yrineo, A.A.; Adelsperger, A.R.; Durkes, A.C.; Distasi, M.R.; Voytik-Harbin, S.L.; Murphy, M.P.; Goergen, C.J. Murine ultrasound-guided transabdominal para-aortic injections of self-assembling type I collagen oligomers. J. Control. Release 2017, 249, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Cebull, H.; Soepriatna, A.; Boyle, J.; Rothenberger, S.; Goergen, C. Strain mapping from 4D ultrasound reveals complex remodeling in dissecting murine abdominal aortic aneurysms. J. Biomech. Eng. 2019, 141, 060907. [Google Scholar] [CrossRef]
- Cao, R.Y.; Amand, T.; Ford, M.D.; Piomelli, U.; Funk, C.D. The Murine Angiotensin II-Induced Abdominal Aortic Aneurysm Model: Rupture Risk and Inflammatory Progression Patterns. Front. Pharm. 2010, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Ford, M.D.; Black, A.T.; Cao, R.Y.; Funk, C.D.; Piomelli, U. Hemodynamics of the mouse abdominal aortic aneurysm. J. Biomech. Eng. 2011, 133, 121008. [Google Scholar] [CrossRef]
- Lee, L.; Cui, J.Z.; Cua, M.; Esfandiarei, M.; Sheng, X.; Chui, W.A.; Xu, M.H.; Sarunic, M.V.; Beg, M.F.; van Breemen, C.; et al. Aortic and cardiac structure and function using high-resolution echocardiography and optical coherence tomography in a mouse model of Marfan syndrome. PLoS ONE 2016, 11, e0164778. [Google Scholar] [CrossRef]
- Lange, C.; Sommerfeld, M.; Namsolleck, P.; Kintscher, U.; Unger, T.; Kaschina, E. AT2R (Angiotensin AT2 Receptor) Agonist, Compound 21, Prevents Abdominal Aortic Aneurysm Progression in the Rat. Hypertension 2018, 72, e20–e29. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.H.; Yrineo, A.A.; Schroeder, H.D.; Wilson, K.E.; Cheng, J.X.; Goergen, C.J. Morphological and Biomechanical Differences in the Elastase and AngII apoE (-/-) Rodent Models of Abdominal Aortic Aneurysms. Biomed. Res. Int. 2015, 2015, 413189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lascio, N.; Kusmic, C.; Stea, F.; Faita, F. Ultrasound-based Pulse Wave Velocity Evaluation in Mice. J. Vis. Exp. 2017, 120, 54362. [Google Scholar] [CrossRef] [PubMed]
- Di Lascio, N.; Stea, F.; Kusmic, C.; Sicari, R.; Faita, F. Non-invasive assessment of pulse wave velocity in mice by means of ultrasound images. Atherosclerosis 2014, 237, 31–37. [Google Scholar] [CrossRef]
- Luo, J.; Fujikura, K.; Tyrie, L.S.; Tilson, M.D.; Konofagou, E.E. Pulse wave imaging of normal and aneurysmal abdominal aortas in vivo. IEEE Trans. Med. Imaging 2009, 28, 477–486. [Google Scholar]
- Nandlall, S.D.; Goldklang, M.P.; Kalashian, A.; Dangra, N.A.; D’Armiento, J.M.; Konofagou, E.E. Monitoring and staging abdominal aortic aneurysm disease with pulse wave imaging. Ultrasound Med. Biol. 2014, 40, 2404–2414. [Google Scholar] [CrossRef] [Green Version]


| System | Transducer | Frequency | View | Cardiac Cycle | |
|---|---|---|---|---|---|
| Number of Articles (%) | 32 (94) | 13 (38) | 21 (62) | 4 (12) | 12 (35) |
| Approach | Device Cost | Aortic Position | Sequential Imaging | Visible Region |
|---|---|---|---|---|
| Ex vivo | Low | Artificial | Not feasible | Unlimited |
| In situ | Low | Physiological | Not feasible | Unlimited |
| Ultrasound | High | Physiological | Feasible | Limited |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, S.; Lu, H.S.; Daugherty, A.; Sawada, H. Imaging Techniques for Aortic Aneurysms and Dissections in Mice: Comparisons of Ex Vivo, In Situ, and Ultrasound Approaches. Biomolecules 2022, 12, 339. https://doi.org/10.3390/biom12020339
Ito S, Lu HS, Daugherty A, Sawada H. Imaging Techniques for Aortic Aneurysms and Dissections in Mice: Comparisons of Ex Vivo, In Situ, and Ultrasound Approaches. Biomolecules. 2022; 12(2):339. https://doi.org/10.3390/biom12020339
Chicago/Turabian StyleIto, Sohei, Hong S. Lu, Alan Daugherty, and Hisashi Sawada. 2022. "Imaging Techniques for Aortic Aneurysms and Dissections in Mice: Comparisons of Ex Vivo, In Situ, and Ultrasound Approaches" Biomolecules 12, no. 2: 339. https://doi.org/10.3390/biom12020339








