The Role of Anti-Viral Effector Molecules in Mollusc Hemolymph
Abstract
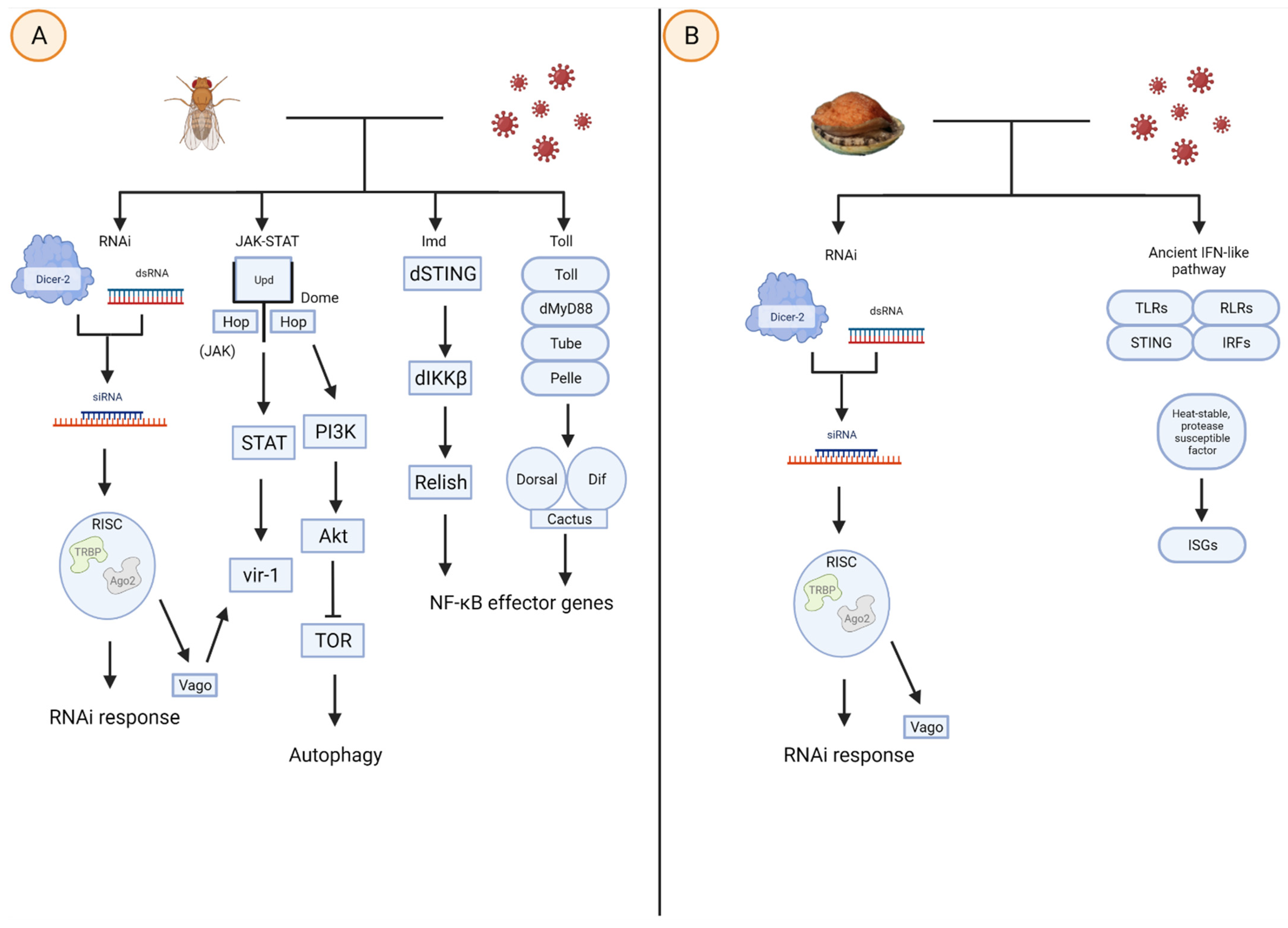
1. Introduction
2. Interferon and Innate Immunity Signaling Pathways
3. Antiviral and Antimicrobial Peptides
3.1. Defensins and Big Defensins
3.2. Mytilus AMPs
3.3. Hemocyanins
4. Antiviral Lectins
4.1. C-Type Lectins
4.2. Galectins
4.3. Other Lectins
5. Future Directions and Applications of Mollusc Proteome-Encoded Effector Molecules
Author Contributions
Funding
Conflicts of Interest
References
- Kurtz, J. Memory in the Innate and Adaptive Immune Systems. Microbes Infect. 2004, 6, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.; Tobe, S.S. Hemolymph Clotting in Crustaceans: Implications for Neuropeptide Extraction from Invertebrate Hemolymph. Peptides 2006, 27, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, D.; Ottaviani, E. Cross-Talk among Immune and Neuroendocrine Systems in Molluscs and Other Invertebrate Models. Horm. Behav. 2017, 88, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.; Dishongh, R.; Moore, S.C.; Whitt, M.A.; Chow, M.; Machaca, K. RNA Interference Is an Antiviral Defence Mechanism in Caenorhabditis Elegans. Nature 2005, 436, 1044–1047. [Google Scholar] [CrossRef]
- Lamiable, O.; Imler, J.-L. Induced Antiviral Innate Immunity in Drosophila. Curr. Opin. Microbiol. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Gerdol, M.; De Moro, G.; Manfrin, C.; Venier, P.; Pallavicini, A. Big Defensins and Mytimacins, New AMP Families of the Mediterranean Mussel Mytilus Galloprovincialis. Dev. Comp. Immunol. 2012, 36, 390–399. [Google Scholar] [CrossRef]
- Rosani, U.; Varotto, L.; Domeneghetti, S.; Arcangeli, G.; Pallavicini, A.; Venier, P. Dual Analysis of Host and Pathogen Transcriptomes in Ostreid Herpesvirus 1-Positive Crassostrea gigas. Environ. Microbiol. 2015, 17, 4200–4212. [Google Scholar] [CrossRef]
- Liu, F.; Li, Y.; Yu, H.; Zhang, L.; Hu, J.; Bao, Z.; Wang, S. MolluscDB: An Integrated Functional and Evolutionary Genomics Database for the Hyper-Diverse Animal Phylum Mollusca. Nucleic Acids Res. 2021, 49, D988–D997. [Google Scholar] [CrossRef]
- Mobsby, D.; Steven, H.A.; Curtotti, R. Australian Fisheries and Aquaculture Outlook 2020; Department of Agriculture Wate: Canberra, Australia, 2020.
- Mobsby, D.; Bath, A.; Curtotti, R. Curtotti Robert Fisheries: Outlook to 2022–23. Agric. Commod. 2018, 8, 128–137. [Google Scholar]
- Mobsby, D.; Steven, A.; Curtotti, R.; Dylewski, M. Australian Fisheries and Aquaculture: Outlook to 2025–26; Australian Bureau of Agricultural and Resource: Canberra, Australia, 2021.
- Document Card. Available online: https://www.fao.org/documents/card/en/c/I9540EN/ (accessed on 21 January 2022).
- Mugimba, K.K.; Byarugaba, D.K.; Mutoloki, S.; Evensen, Ø.; Munang’andu, H.M. Challenges and Solutions to Viral Diseases of Finfish in Marine Aquaculture. Pathogens 2021, 10, 673. [Google Scholar] [CrossRef]
- Leong, J.C.; Fryer, J.L. Viral Vaccines for Aquaculture. Annu. Rev. Fish Dis. 1993, 3, 225–240. [Google Scholar] [CrossRef]
- Davison, A.J.; Trus, B.L.; Cheng, N.; Steven, A.C.; Watson, M.S.; Cunningham, C.; Deuff, R.-M.L.; Renault, T. A Novel Class of Herpesvirus with Bivalve Hosts. J. Gen. Virol. 2005, 86, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Hardy-Smith, P.; Handlinger, J. Ganglioneuritis Causing High Mortalities in Farmed Australian Abalone (Haliotis laevigata and Haliotis rubra). Aust. Vet. J. 2007, 85, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, S. Abalone Viral Ganglioneuritis. Pathogens 2020, 9, 720. [Google Scholar] [CrossRef]
- Verley, A. Agriculture Victoria; ABC News Deadly Abalone Disease AVG Re-Emerges off Victoria’s South-West Coast. ABC News, 4 May 2021. [Google Scholar]
- Schoggins, J.W.; Rice, C.M. Interferon-Stimulated Genes and Their Antiviral Effector Functions. Curr. Opin. Virol. 2011, 1, 519–525. [Google Scholar] [CrossRef]
- Feng, D.; Li, Q.; Yu, H. RNA Interference by Ingested DsRNA-Expressing Bacteria to Study Shell Biosynthesis and Pigmentation in Crassostrea gigas. Mar. Biotechnol. 2019, 21, 526–536. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, K.S.; Eom, J.; Lee, J.B.; Seo, J.-Y. Viperin Differentially Induces Interferon-Stimulated Genes in Distinct Cell Types. Immune Netw. 2019, 19, e33. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Chen, Y.; Chen, Y.; Wang, S.; Weng, S.-P.; Xu, X.; He, J. Activation of Vago by Interferon Regulatory Factor (IRF) Suggests an Interferon System-like Antiviral Mechanism in Shrimp. Sci. Rep. 2015, 5, 15078. [Google Scholar] [CrossRef]
- Hégaret, H.; Wikfors, G.H.; Soudant, P. Flow Cytometric Analysis of Haemocytes from Eastern Oysters, Crassostrea virginica, Subjected to a Sudden Temperature Elevation. J. Exp. Mar. Biol. Ecol. 2003, 293, 249–265. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Guo, X.; Litman, G.W.; Dishaw, L.J.; Zhang, G. Massive Expansion and Functional Divergence of Innate Immune Genes in a Protostome. Sci. Rep. 2015, 5, 8693. [Google Scholar] [CrossRef]
- Schultz, J.H.; Adema, C.M. Comparative Immunogenomics of Molluscs. Dev. Comp. Immunol. 2017, 75, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Jonasz, M.; Fournier, G. Light Scattering by Particles in Water: Theoretical and Experimental Foundations; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780080548678. [Google Scholar]
- Fragkoudis, R.; Attarzadeh-Yazdi, G.; Nash, A.A.; Fazakerley, J.K.; Kohl, A. Advances in Dissecting Mosquito Innate Immune Responses to Arbovirus Infection. J. Gen. Virol. 2009, 90, 2061–2072. [Google Scholar] [CrossRef]
- Guo, X.; Ford, S.E. Infectious Diseases of Marine Molluscs and Host Responses as Revealed by Genomic Tools. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150206. [Google Scholar] [CrossRef] [PubMed]
- Deddouche, S.; Matt, N.; Budd, A.; Mueller, S.; Kemp, C.; Galiana-Arnoux, D.; Dostert, C.; Antoniewski, C.; Hoffmann, J.A.; Imler, J.-L. The DExD/H-Box Helicase Dicer-2 Mediates the Induction of Antiviral Activity in Drosophila. Nat. Immunol. 2008, 9, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Tenoever, B.R. RNA Viruses and the Host MicroRNA Machinery. Nat. Rev. Microbiol. 2013, 11, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.N.; Duchemin, J.-B.; Voysey, R.; Walker, P.J. Dicer-2-Dependent Activation of Culex Vago Occurs via the TRAF-Rel2 Signaling Pathway. PLoS Negl. Trop. Dis. 2014, 8, e2823. [Google Scholar] [CrossRef]
- Green, T.J.; Speck, P.; Geng, L.; Raftos, D.; Beard, M.R.; Helbig, K.J. Oyster Viperin Retains Direct Antiviral Activity and Its Transcription Occurs via a Signalling Pathway Involving a Heat-Stable Haemolymph Protein. J. Gen. Virol. 2015, 96, 3587–3597. [Google Scholar] [CrossRef]
- Qiao, X.; Zong, Y.; Liu, Z.; Wu, Z.; Li, Y.; Wang, L.; Song, L. The CGAS/STING-TBK1-IRF Regulatory Axis Orchestrates a Primitive Interferon-like Antiviral Mechanism in Oyster. Front. Immunol. 2021, 12, 689783. [Google Scholar] [CrossRef]
- He, Y.; Jouaux, A.; Ford, S.E.; Lelong, C.; Sourdaine, P.; Mathieu, M.; Guo, X. Transcriptome Analysis Reveals Strong and Complex Antiviral Response in a Mollusc. Fish Shellfish Immunol. 2015, 46, 131–144. [Google Scholar] [CrossRef]
- Green, T.J.; Raftos, D.; Speck, P.; Montagnani, C. Antiviral Immunity in Marine Molluscs. J. Gen. Virol. 2015, 96, 2471–2482. [Google Scholar] [CrossRef]
- Agius, J.R.; Corbeil, S.; Helbig, K.J. Immune Control of Herpesvirus Infection in Molluscs. Pathogens 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Tafesh-Edwards, G.; Eleftherianos, I. Drosophila Immunity against Natural and Nonnatural Viral Pathogens. Virology 2020, 540, 165–171. [Google Scholar] [CrossRef]
- Feng, M.; Fei, S.; Xia, J.; Labropoulou, V.; Swevers, L.; Sun, J. Antimicrobial Peptides as Potential Antiviral Factors in Insect Antiviral Immune Response. Front. Immunol. 2020, 11, 2030. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Schmitt, P.; Venier, P.; Rocha, G.; Rosa, R.D.; Destoumieux-Garzón, D. Functional Insights from the Evolutionary Diversification of Big Defensins. Front. Immunol. 2020, 11, 758. [Google Scholar] [CrossRef] [PubMed]
- Shafee, T.M.A.; Lay, F.T.; Hulett, M.D.; Anderson, M.A. The Defensins Consist of Two Independent, Convergent Protein Superfamilies. Mol. Biol. Evol. 2016, 33, 2345–2356. [Google Scholar] [CrossRef]
- Rosa, R.D.; Santini, A.; Fievet, J.; Bulet, P.; Destoumieux-Garzón, D.; Bachère, E. Big Defensins, a Diverse Family of Antimicrobial Peptides That Follows Different Patterns of Expression in Hemocytes of the Oyster Crassostrea gigas. PLoS ONE 2011, 6, e25594. [Google Scholar] [CrossRef]
- Salzet, M. Vertebrate Innate Immunity Resembles a Mosaic of Invertebrate Immune Responses. Trends Immunol. 2001, 22, 285–288. [Google Scholar] [CrossRef]
- Greco, S.; Gerdol, M.; Edomi, P.; Pallavicini, A. Molecular Diversity of Mytilin-like Defense Peptides in Mytilidae (Mollusca, Bivalvia). Antibiotics 2020, 9, 37. [Google Scholar] [CrossRef]
- Roch, P.; Yang, Y.; Toubiana, M.; Aumelas, A. NMR Structure of Mussel Mytilin, and Antiviral–Antibacterial Activities of Derived Synthetic Peptides. Dev. Comp. Immunol. 2008, 32, 227–238. [Google Scholar] [CrossRef]
- Burmester, T. Molecular Evolution of the Arthropod Hemocyanin Superfamily. Mol. Biol. Evol. 2001, 18, 184–195. [Google Scholar] [CrossRef]
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Hoffmann, J.A.; Imler, J.-L. The Jak-STAT Signaling Pathway Is Required but Not Sufficient for the Antiviral Response of Drosophila. Nat. Immunol. 2005, 6, 946–953. [Google Scholar] [CrossRef] [PubMed]
- van Holde, K.E.; Miller, K.I.; Decker, H. Hemocyanins and Invertebrate Evolution. J. Biol. Chem. 2001, 276, 15563–15566. [Google Scholar] [CrossRef]
- Hazes, B.; Magnus, K.A.; Bonaventura, C.; Bonaventura, J.; Dauter, Z.; Kalk, K.H.; Hol, W.G. Crystal Structure of Deoxygenated Limulus polyphemus Subunit II Hemocyanin at 2.18 Å Resolution: Clues for a Mechanism for Allosteric Regulation. Protein Sci. 1993, 2, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Parisi, M.G.; Parrinello, N.; Cammarata, M.; Roch, P. Molluscan Antimicrobial Peptides, a Review from Activity-Based Evidences to Computer-Assisted Sequences. ISJ-Invertebr. Surviv. J. 2011, 8, 85–97. [Google Scholar]
- De Zoysa, M.; Whang, I.; Lee, Y.; Lee, S.; Lee, J.-S.; Lee, J. Defensin from Disk Abalone Haliotis Discus Discus: Molecular Cloning, Sequence Characterization and Immune Response against Bacterial Infection. Fish Shellfish Immunol. 2010, 28, 261–266. [Google Scholar] [CrossRef]
- Mitta, G.; Vandenbulcke, F.; Hubert, F.; Roch, P. Mussel defensins are synthesised and processed in granulocytes then released into the plasma after bacterial challenge. J. Cell Sci. 1999, 112 Pt 23, 4233–4242. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Rosa, R.D.; Schmitt, P.; Barreto, C.; Vidal-Dupiol, J.; Mitta, G.; Gueguen, Y.; Bachère, E. Antimicrobial Peptides in Marine Invertebrate Health and Disease. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150300. [Google Scholar] [CrossRef]
- Dupuy, J.W.; Bonami, J.R.; Roch, P. A Synthetic Antibacterial Peptide from Mytilus galloprovincialis Reduces Mortality Due to White Spot Syndrome Virus in Palaemonid Shrimp. J. Fish Dis. 2004, 27, 57–64. [Google Scholar] [CrossRef]
- Loth, K.; Vergnes, A.; Barreto, C.; Voisin, S.N.; Meudal, H.; Da Silva, J.; Bressan, A.; Belmadi, N.; Bachère, E.; Aucagne, V.; et al. The Ancestral N-Terminal Domain of Big Defensins Drives Bacterially Triggered Assembly into Antimicrobial Nanonets. mBio 2019, 10, e01821-19. [Google Scholar] [CrossRef]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20. [Google Scholar] [CrossRef]
- Zannella, C.; Mosca, F.; Mariani, F.; Franci, G.; Folliero, V.; Galdiero, M.; Tiscar, P.G.; Galdiero, M. Microbial Diseases of Bivalve Mollusks: Infections, Immunology and Antimicrobial Defense. Mar. Drugs 2017, 15, 182. [Google Scholar] [CrossRef]
- Rey-Campos, M.; Moreira, R.; Valenzuela-Muñoz, V.; Gallardo-Escárate, C.; Novoa, B.; Figueras, A. High Individual Variability in the Transcriptomic Response of Mediterranean Mussels to Vibrio Reveals the Involvement of Myticins in Tissue Injury. Sci. Rep. 2019, 9, 3569. [Google Scholar] [CrossRef]
- Costa, M.M.; Dios, S.; Alonso-Gutierrez, J.; Romero, A.; Novoa, B.; Figueras, A. Evidence of High Individual Diversity on Myticin C in Mussel (Mytilus Galloprovincialis). Dev. Comp. Immunol. 2009, 33, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Mitta, G.; Hubert, F.; Dyrynda, E.A.; Boudry, P.; Roch, P. Mytilin B and MGD2, Two Antimicrobial Peptides of Marine Mussels: Gene Structure and Expression Analysis. Dev. Comp. Immunol. 2000, 24, 381–393. [Google Scholar] [CrossRef]
- Carriel-Gomes, M.C.; Kratz, J.M.; Barracco, M.A.; Bachére, E.; Barardi, C.R.M.; Simões, C.M.O. In Vitro Antiviral Activity of Antimicrobial Peptides against Herpes Simplex Virus 1, Adenovirus, and Rotavirus. Mem. Inst. Oswaldo Cruz 2007, 102, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Tincu, J.A.; Taylor, S.W. Antimicrobial Peptides from Marine Invertebrates. Antimicrob. Agents Chemother. 2004, 48, 3645–3654. [Google Scholar] [CrossRef]
- Sperstad, S.V.; Haug, T.; Blencke, H.-M.; Styrvold, O.B.; Li, C.; Stensvåg, K. Antimicrobial Peptides from Marine Invertebrates: Challenges and Perspectives in Marine Antimicrobial Peptide Discovery. Biotechnol. Adv. 2011, 29, 519–530. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, W.; Yang, X.; Yan, X.; Liu, R. A Novel Cysteine-Rich Antimicrobial Peptide from the Mucus of the Snail of Achatina Fulica. Peptides 2013, 39, 1–5. [Google Scholar] [CrossRef]
- Coates, C.J.; Nairn, J. Diverse Immune Functions of Hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef]
- Dang, V.T.; Benkendorff, K.; Speck, P. In Vitro Antiviral Activity against Herpes Simplex Virus in the Abalone Haliotis Laevigata. J. Gen. Virol. 2011, 92, 627–637. [Google Scholar] [CrossRef]
- Talaei Zanjani, N.; Miranda-Saksena, M.; Valtchev, P.; Diefenbach, R.J.; Hueston, L.; Diefenbach, E.; Sairi, F.; Gomes, V.G.; Cunningham, A.L.; Dehghani, F. Abalone Hemocyanin Blocks the Entry of Herpes Simplex Virus 1 into Cells: A Potential New Antiviral Strategy. Antimicrob. Agents Chemother. 2016, 60, 1003–1012. [Google Scholar] [CrossRef]
- Green, T.J.; Robinson, N.; Chataway, T.; Benkendorff, K.; O’Connor, W.; Speck, P. Evidence That the Major Hemolymph Protein of the Pacific Oyster, Crassostrea Gigas, Has Antiviral Activity against Herpesviruses. Antivir. Res. 2014, 110, 168–174. [Google Scholar] [CrossRef]
- Zhuang, J.; Coates, C.J.; Zhu, H.; Zhu, P.; Wu, Z.; Xie, L. Identification of Candidate Antimicrobial Peptides Derived from Abalone Hemocyanin. Dev. Comp. Immunol. 2015, 49, 96–102. [Google Scholar] [CrossRef] [PubMed]
- De Zoysa, M.; Nikapitiya, C.; Whang, I.; Lee, J.-S.; Lee, J. Abhisin: A Potential Antimicrobial Peptide Derived from Histone H2A of Disk Abalone (Haliotis Discus Discus). Fish Shellfish Immunol. 2009, 27, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; St-Pierre, C.; Bhaumik, P.; Nieminen, J. Galectins in Innate Immunity: Dual Functions of Host Soluble Beta-Galactoside-Binding Lectins as Damage-Associated Molecular Patterns (DAMPs) and as Receptors for Pathogen-Associated Molecular Patterns (PAMPs). Immunol. Rev. 2009, 230, 172–187. [Google Scholar] [CrossRef]
- Ertl, N.G.; O’Connor, W.A.; Papanicolaou, A.; Wiegand, A.N.; Elizur, A. Transcriptome Analysis of the Sydney Rock Oyster, Saccostrea Glomerata: Insights into Molluscan Immunity. PLOS ONE 2016, 11, e0156649. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R. Galectins as Pattern Recognition Receptors: Structure, Function, and Evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [PubMed]
- Mason, C.P.; Tarr, A.W. Human Lectins and Their Roles in Viral Infections. Molecules 2015, 20, 2229–2271. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, O.V.; Molchanova, V.I.; Chikalovets, I.V.; Kondrashina, A.S.; Li, W.; Lukyanov, P.A. Lectins of Marine Hydrobionts. Biochemistry 2013, 78, 760–770. [Google Scholar] [CrossRef]
- Vasta, G.R.; Feng, C.; Bianchet, M.A.; Bachvaroff, T.R.; Tasumi, S. Structural, Functional, and Evolutionary Aspects of Galectins in Aquatic Mollusks: From a Sweet Tooth to the Trojan Horse. Fish Shellfish Immunol. 2015, 46, 94–106. [Google Scholar] [CrossRef]
- Zelensky, A.N.; Gready, J.E. The C-Type Lectin-like Domain Superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, M.; Zhang, H.; Song, L. The Immune Role of C-Type Lectins in Molluscs. ISJ-Invertebr. Surviv. J. 2011, 8, 241–246. [Google Scholar]
- Pales Espinosa, E.; Perrigault, M.; Allam, B. Identification and Molecular Characterization of a Mucosal Lectin (MeML) from the Blue Mussel Mytilus Edulis and Its Potential Role in Particle Capture. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Pees, B.; Yang, W.; Zárate-Potes, A.; Schulenburg, H.; Dierking, K. High Innate Immune Specificity through Diversified C-Type Lectin-Like Domain Proteins in Invertebrates. J. Innate Immun. 2016, 8, 129–142. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Wang, L.; Song, L. Pathogen-Derived Carbohydrate Recognition in Molluscs Immune Defense. Int. J. Mol. Sci. 2018, 19, 721. [Google Scholar] [CrossRef]
- Tasumi, S.; Ohira, T.; Kawazoe, I.; Suetake, H.; Suzuki, Y.; Aida, K. Primary Structure and Characteristics of a Lectin from Skin Mucus of the Japanese Eel Anguilla Japonica. J. Biol. Chem. 2002, 277, 27305–27311. [Google Scholar] [CrossRef]
- Gourdine, J.-P.; Markiv, A.; Smith-Ravin, J. The Three-Dimensional Structure of Codakine and Related Marine C-Type Lectins. Fish Shellfish Immunol. 2007, 23, 831–839. [Google Scholar] [CrossRef]
- Zheng, P.; Wang, H.; Zhao, J.; Song, L.; Qiu, L.; Dong, C.; Wang, B.; Gai, Y.; Mu, C.; Li, C.; et al. A Lectin (CfLec-2) Aggregating Staphylococcus Haemolyticus from Scallop Chlamys Farreri. Fish Shellfish Immunol. 2008, 24, 286–293. [Google Scholar] [CrossRef]
- Bulgheresi, S.; Schabussova, I.; Chen, T.; Mullin, N.P.; Maizels, R.M.; Ott, J.A. A New C-Type Lectin Similar to the Human Immunoreceptor DC-SIGN Mediates Symbiont Acquisition by a Marine Nematode. Appl. Environ. Microbiol. 2006, 72, 2950–2956. [Google Scholar] [CrossRef]
- Weis, W.I.; Taylor, M.E.; Drickamer, K. The C-Type Lectin Superfamily in the Immune System. Immunol. Rev. 1998, 163, 19–34. [Google Scholar] [CrossRef]
- Mann, K.; Weiss, I.M.; André, S.; Gabius, H.J.; Fritz, M. The Amino-Acid Sequence of the Abalone (Haliotis Laevigata) Nacre Protein Perlucin. Detection of a Functional C-Type Lectin Domain with Galactose/Mannose Specificity. Eur. J. Biochem. 2000, 267, 5257–5264. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-S.; Kim, Y.-M.; Park, K.-I.; Kim Cho, S.; Choi, K.-S.; Cho, M. Analysis of EST and Lectin Expressions in Hemocytes of Manila Clams (Ruditapes Philippinarum) (Bivalvia: Mollusca) Infected with Perkinsus Olseni. Dev. Comp. Immunol. 2006, 30, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Song, L.; Xu, W.; Qian, P.-Y. Molecular Cloning and Immune Responsive Expression of a Novel C-Type Lectin Gene from Bay Scallop Argopecten Irradians. Fish Shellfish Immunol. 2008, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qiu, L.; Wei, X.; Wang, L.; Wang, L.; Zhou, Z.; Zhang, H.; Liu, L.; Song, L. An Ancient C-Type Lectin in Chlamys Farreri (CfLec-2) That Mediate Pathogen Recognition and Cellular Adhesion. Dev. Comp. Immunol. 2010, 34, 1274–1282. [Google Scholar] [CrossRef]
- Zhang, H.; Song, X.; Wang, L.; Kong, P.; Yang, J.; Liu, L.; Qiu, L.; Zhang, Y.; Qiu, L.; Song, L. AiCTL-6, a Novel C-Type Lectin from Bay Scallop Argopecten Irradians with a Long C-Type Lectin-like Domain. Fish Shellfish Immunol. 2011, 30, 17–26. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Zhang, H.; Qiu, L.; Wang, H.; Song, L. C-Type Lectin in Chlamys Farreri (CfLec-1) Mediating Immune Recognition and Opsonization. PLoS One 2011, 6, e17089. [Google Scholar] [CrossRef][Green Version]
- Jing, X.; Espinosa, E.P.; Perrigault, M.; Allam, B. Identification, Molecular Characterization and Expression Analysis of a Mucosal C-Type Lectin in the Eastern Oyster, Crassostrea Virginica. Fish Shellfish Immunol. 2011, 30, 851–858. [Google Scholar] [CrossRef]
- Wang, N.; Whang, I.; Lee, J. A Novel C-Type Lectin from Abalone, Haliotis Discus Discus, Agglutinates Vibrio Alginolyticus. Dev. Comp. Immunol. 2008, 32, 1034–1040. [Google Scholar] [CrossRef]
- Wang, H.; Song, L.; Li, C.; Zhao, J.; Zhang, H.; Ni, D.; Xu, W. Cloning and Characterization of a Novel C-Type Lectin from Zhikong Scallop Chlamys Farreri. Mol. Immunol. 2007, 44, 722–731. [Google Scholar] [CrossRef]
- Cummings, R.D.; McEver, R.P. C-Type Lectins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2010; ISBN 9780879697709. [Google Scholar]
- Huang, X.; Li, T.; Jin, M.; Yin, S.; Wang, W.; Ren, Q. Identification of a Macrobrachium Nipponense C-Type Lectin with a Close Evolutionary Relationship to Vertebrate Lectins. Mol. Immunol. 2017, 87, 141–151. [Google Scholar] [CrossRef]
- Feng, C.; Ghosh, A.; Amin, M.N.; Giomarelli, B.; Shridhar, S.; Banerjee, A.; Fernández-Robledo, J.A.; Bianchet, M.A.; Wang, L.-X.; Wilson, I.B.H.; et al. The Galectin CvGal1 from the Eastern Oyster (Crassostrea Virginica) Binds to Blood Group A Oligosaccharides on the Hemocyte Surface. J. Biol. Chem. 2013, 288, 24394–24409. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-L.; Chen, Y.-H.; Wang, S.-W.; Huang, Y.-J.; Leu, C.-H.; Yeh, N.-C.; Chu, C.-Y.; Lin, C.-C.; Shieh, G.-S.; Chen, Y.-L.; et al. Galectin-1 Binds to Influenza Virus and Ameliorates Influenza Virus Pathogenesis. J. Virol. 2011, 85, 10010–10020. [Google Scholar] [CrossRef]
- Vasta, G.R. Roles of Galectins in Infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Lin, C.-Y.; Chang, M.R.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Chen, Y.-H.; Liu, F.-T.; Wang, S.-F. The Role of Galectins in Virus Infection - A Systemic Literature Review. J. Microbiol. Immunol. Infect. 2020, 53, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.P.; Bickham, U.; Bayne, C.J. Molluscan Cells in Culture: Primary Cell Cultures and Cell Lines. Can. J. Zool. 2013, 91, 391–404. [Google Scholar] [CrossRef]
- Willer, D.F.; Nicholls, R.J.; Aldridge, D.C. Opportunities and Challenges for Upscaled Global Bivalve Seafood Production. Nat. Food 2021, 2, 935–943. [Google Scholar] [CrossRef]
- Vasta, G.R.; Wang, J.-X. Galectin-Mediated Immune Recognition: Opsonic Roles with Contrasting Outcomes in Selected Shrimp and Bivalve Mollusk Species. Dev. Comp. Immunol. 2020, 110, 103721. [Google Scholar] [CrossRef]
- Green, T.J.; Vergnes, A.; Montagnani, C.; de Lorgeril, J. Distinct Immune Responses of Juvenile and Adult Oysters (Crassostrea Gigas) to Viral and Bacterial Infections. Vet. Res. 2016, 47, 72. [Google Scholar] [CrossRef]
- Gayashani Sandamalika, W.M.; Lee, J. Quadruple Domain-Containing Galectin from Marine Invertebrate Disk Abalone (Haliotis Discus Discus): Molecular Perspectives in Early Development, Immune Expression, and Potent Antiviral Responses. Fish Shellfish Immunol. 2020, 106, 920–929. [Google Scholar] [CrossRef]
- Xiang, Z.; Qu, F.; Wang, F.; Li, J.; Zhang, Y.; Yu, Z. Characteristic and Functional Analysis of a Ficolin-like Protein from the Oyster Crassostrea Hongkongensis. Fish Shellfish Immunol. 2014, 40, 514–523. [Google Scholar] [CrossRef]
- Neave, M.J.; Corbeil, S.; McColl, K.A.; Crane, M.S.J. Investigating the Natural Resistance of Blackfoot Pāua Haliotis Iris to Abalone Viral Ganglioneuritis Using Whole Transcriptome Analysis. Dis. Aquat. Organ. 2019, 135, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Thakur, N.; Tandon, H.; Kumar, M. AVPdb: A Database of Experimentally Validated Antiviral Peptides Targeting Medically Important Viruses. Nucleic Acids Res. 2014, 42, D1147–D1153. [Google Scholar] [CrossRef] [PubMed]
- Fernández Robledo, J.A.; Yadavalli, R.; Allam, B.; Pales Espinosa, E.; Gerdol, M.; Greco, S.; Stevick, R.J.; Gómez-Chiarri, M.; Zhang, Y.; Heil, C.A.; et al. From the Raw Bar to the Bench: Bivalves as Models for Human Health. Dev. Comp. Immunol. 2019, 92, 260–282. [Google Scholar] [CrossRef] [PubMed]

| Soluble Mediator | Protein Class | Example Species | General Protein Physical Characteristics | References | |
|---|---|---|---|---|---|
| Defensins | AMPs | Molluscs | M. galloprovincialis (MGD-1 and MGD-2) | Size: 18–60aa C-Domains: 6, hydrophobic, 3–4 disulfide bridges, α-helix linked to two stranded β-sheets. | [39,40,41,42] |
| Invertebrates | D. melanogaster (Drosomycin) | Size: 20–70aa C-Domains: 6, hydrophobic, 3–4 disulfide bridges, α-helix linked to two stranded β-sheets. | [39] | ||
| Vertebrates | Humans (HBD-2 and HBD-3) | Size: 70–120aa C-Domains: 6, hydrophobic, 3 disulfide bridges, α-helix linked to three stranded β-sheets. | [39,40,41] | ||
| Big Defensins | AMPs | Molluscs | C. gigas (BigDef1-3) | Size: 70–180aa C-Domains: 6, hydrophobic, 3 disulfide bridges, α-helix linked to two stranded β-sheets. * Additional hydrophobic N-domain. | [6,39,40,41] |
| Invertebrates | Tachypleus tridentatus (Big Defensin) | Size: 70–120aa C-Domains: 6, 3 disulfide bridges, α-helix linked to two stranded β-sheets. * Additional hydrophobic N-domain. | [6,39,41] | ||
| Vertebrates | N/A | N/A | |||
| Myticins | AMPs | Molluscs | Only Mytilus genus; M. galloprovincialis, M. edublis (myticin-C) | Size: 30–100aa C-Domains: 8, hydrophilic, 4 disulfide bridges α-helix linked to two stranded β-sheets. | [6,39,43] |
| Mytilins | AMPs | Molluscs | Only Mytilus genus; M. galloprovincialis, M. edublis (mytilin-B) | Size: 30–100aa C-Domains: 8, hydrophilic, 4 disulfide bridges α-helix linked to two stranded β-sheets. | [6,42,43,44] |
| Hemocyanin | AMPs | Molluscs | Haliotis discus discus | Size: 350–450aa C-domains: 7–8 C, hydrophobic, 3 disulfide bridges, α-helix linked to two stranded β-sheets. * Additional hydrophobic N-domain. | [35,45,46,47,48] |
| Invertebrates | Mostly arthropods; Limulus polyphemus | Size: 350–450aa C-domains: 7–8 C, hydrophobic, 3 disulfide bridges, α-helix linked to two stranded β-sheets. * Additional hydrophobic N-domain. | [45] | ||
| Vertebrates | N/A | N/A | |||
| Motif | Example CTLs | Mollusc Species | Reference |
|---|---|---|---|
| EPN | Codakine Cflecs AiCTLs | Codakia orbicularis Chlamys farreri Argopecten irradians | [78,79,80,81,82,83,84,85] |
| QPD | MCL-3 AiCTLs | Ruditapes philippinarum Argopecten irradians | [78,86,87,88] |
| EPD | Clfecs AiCTLs | Chlamys farreri Argopecten irradians | [78,83,89,90,91] |
| QPG | CLHd MeML CvML | Haliotis discus discus Mytilus edulis Crassostrea virginica | [78,92,93] |
| QPS | CLHd MeML CvML | Haliotis discus discus Mytilus edulis Crassostrea virginica | [78,92,93] |
| YPG | CLHd MeML CvML | Haliotis discus discus Mytilus edulis Crassostrea virginica | [77,78,92,93] |
| ENC | MeML | Mytilus edulis | [77,78] |
| YPT | Cflecs AiCTLs | Chlamys farreri Argopecten irradians | [77,83,94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, A.; Agius, J.; Ackerly, D.; Beddoe, T.; Helbig, K. The Role of Anti-Viral Effector Molecules in Mollusc Hemolymph. Biomolecules 2022, 12, 345. https://doi.org/10.3390/biom12030345
Watson A, Agius J, Ackerly D, Beddoe T, Helbig K. The Role of Anti-Viral Effector Molecules in Mollusc Hemolymph. Biomolecules. 2022; 12(3):345. https://doi.org/10.3390/biom12030345
Chicago/Turabian StyleWatson, Angus, Jacinta Agius, Danielle Ackerly, Travis Beddoe, and Karla Helbig. 2022. "The Role of Anti-Viral Effector Molecules in Mollusc Hemolymph" Biomolecules 12, no. 3: 345. https://doi.org/10.3390/biom12030345
APA StyleWatson, A., Agius, J., Ackerly, D., Beddoe, T., & Helbig, K. (2022). The Role of Anti-Viral Effector Molecules in Mollusc Hemolymph. Biomolecules, 12(3), 345. https://doi.org/10.3390/biom12030345









