Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes
Abstract
:1. Introduction
2. Overview of TGF-β Signaling
3. TGF-β Ligand
4. TGFBRs
5. Smad2/3
6. Smad4
7. Smad7
8. Other Possible Mechanisms Involved with TGF-β Signaling-Regulated β Cell Dysfunction and Loss
9. β Cell-Based Treatment of Diabetes by Targeting TGF-β Signaling
10. Conclusions, Perspectives, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Diabetes Factsheet N. 312. Available online: https://www.who.int/en/news-room/fact-sheets/detail/diabetes (accessed on 10 December 2021).
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Wang, P.; Fiaschi-Taesch, N.M.; Vasavada, R.C.; Scott, D.K.; García-Ocaña, A.; Stewart, A.F. Diabetes mellitus—Advances and challenges in human β-cell proliferation. Nat. Rev. Endocrinol. 2015, 11, 201–212. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Data Group. Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Szmuilowicz, E.D.; Josefson, J.L.; Metzger, B.E. Gestational Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2019, 48, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Papa, F.R. Endoplasmic Reticulum Stress, Pancreatic β-Cell Degeneration, and Diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic β-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Kajimoto, Y.; Fujitani, Y.; Watada, H.; Yamamoto, S.; Watarai, T.; Umayahara, Y.; Matsuhisa, M.; Gorogawa, S.-I.; Kuwayama, Y.; et al. PAX6 Mutation as a Genetic Factor Common to Aniridia and Glucose Intolerance. Diabetes 2002, 51, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhou, T.-C.; Liu, Y.-Y.; Li, X.; Wang, W.-X.; Irwin, D.; Zhang, Y.-P. Identification of HNF4A Mutation p.T130I and HNF1A Mutations p.I27L and p.S487N in a Han Chinese Family with Early-Onset Maternally Inherited Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 3582616. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.P.; Xu, X.H.; Fang, Y.L.; Jiang, L.; Chen, C.; Liang, L.; Wang, C.L. GCK mutations in Chinese MODY2 patients: A family pedigree report and review of Chinese literature. J. Pediatr. Endocrinol. Metab. 2016, 29, 959–964. [Google Scholar] [CrossRef]
- Staffers, D.A.; Ferrer, J.; Clarke, W.L.; Habener, J.F. Early-onset type-ll diabetes mellitus (MODY4) linked to IPF1. Nat. Genet. 1997, 17, 138–139. [Google Scholar] [CrossRef]
- Yan, J.; Peng, D.; Jiang, F.; Zhang, R.; Chen, M.; Wang, T.; Yan, D.; Wang, S.; Hou, X.; Bao, Y.; et al. Impaired pancreatic beta cell compensatory function is the main cause of type 2 diabetes in individuals with high genetic risk: A 9 year prospective cohort study in the Chinese population. Diabetologia 2016, 59, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poitout, V.; Robertson, R.P. Glucolipotoxicity: Fuel Excess and β-Cell Dysfunction. Endocr. Rev. 2008, 29, 351–366. [Google Scholar] [CrossRef] [PubMed]
- White, M.G.; Shaw, J.A.; Taylor, R. Type 2 Diabetes: The Pathologic Basis of Reversible β-Cell Dysfunction. Diabetes Care 2016, 39, 2080–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowluru, A. Oxidative Stress in Cytokine-Induced Dysfunction of the Pancreatic Beta Cell: Known Knowns and Known Unknowns. Metabolites 2020, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Rane, S.G. TGF-β Signaling in Pancreatic Islet β Cell Development and Function. Endocrinology 2021, 162, bqaa233. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Attisano, L. The TGFbeta Superfamily Signaling Pathway. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Meng, X.-M.; Tang, P.M.K.; Li, J.; Lan, H.Y.; Meng, X.-M.; Tang, P.M.K.; Li, J.; Lan, H.Y. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, H.-L.; Liu, T.-T.; Lan, H.-Y. TGF-β as a Master Regulator of Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 7881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Alexander, P.B.; Wang, X.-F. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; Meng, A. TGFβ family signaling and development. Development 2021, 148, dev188490. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Griffiths, M.J.; Dalton, S.L.; Wu, J.; Pittet, J.-F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. A Mechanism for Regulating Pulmonary Inflammation and Fibrosis: The Integrin αvβ6 Binds and Activates Latent TGF β1. Cell 1999, 96, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Lyons, R.M.; Gentry, L.E.; Purchio, A.F.; Moses, H.L. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J. Cell Biol. 1990, 110, 1361–1367. [Google Scholar] [CrossRef]
- Hayashi, H.; Abdollah, S.; Qiu, Y.; Cai, J.; Xu, Y.-Y.; Grinnell, B.W.; Richardson, M.A., Jr.; Topper, J.N.; Gimbrone, M.A.; Wrana, J.L.; et al. The MAD-Related Protein Smad7 Associates with the TGFβ Receptor and Functions as an Antagonist of TGFβ Signaling. Cell 1997, 89, 1165–1173. [Google Scholar] [CrossRef] [Green Version]
- Kavsak, P.; Rasmussen, R.K.; Causing, C.G.; Bonni, S.; Zhu, H.; Thomsen, G.H.; Wrana, J.L. Smad7 Binds to Smurf2 to Form an E3 Ubiquitin Ligase that Targets the TGFβ Receptor for Degradation. Mol. Cell 2000, 6, 1365–1375. [Google Scholar] [CrossRef]
- Meurer, S.K.; Weiskirchen, R. Endoglin: An ‘Accessory’ Receptor Regulating Blood Cell Development and Inflammation. Int. J. Mol. Sci. 2020, 21, 9247. [Google Scholar] [CrossRef]
- Kim, S.K.; Henen, M.A.; Hinck, A.P. Structural biology of betaglycan and endoglin, membrane-bound co-receptors of the TGF-beta family. Exp. Biol. Med. 2019, 244, 1547–1558. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2017, 9, a022129. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, Y.; Tulachan, S.; Guo, P.; Welsh, C.; Wiersch, J.; Prasadan, K.; Paredes, J.; Shiota, C.; Xiao, X.; Wada, Y.; et al. Smad signaling pathways regulate pancreatic endocrine development. Dev. Biol. 2013, 378, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Tulachan, S.S.; Tei, E.; Hembree, M.; Crisera, C.; Prasadan, K.; Koizumi, M.; Shah, S.; Guo, P.; Bottinger, E.; Gittes, G.K. TGF-β isoform signaling regulates secondary transition and mesenchymal-induced endocrine development in the embryonic mouse pancreas. Dev. Biol. 2007, 305, 508–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gohary, Y.; Tulachan, S.; Wiersch, J.; Guo, P.; Welsh, C.; Prasadan, K.; Paredes, J.; Shiota, C.; Xiao, X.; Wada, Y.; et al. A Smad Signaling Network Regulates Islet Cell Proliferation. Diabetes 2014, 63, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, Y.; Friess, H.; Büchler, M.; Beger, H.G.; Gold, L.I.; Korc, M. Synthesis and Expression of Transforming Growth Factor β-1, β-2, and β-3 in the Endocrine and Exocrine Pancreas. Diabetes 1993, 42, 746–756. [Google Scholar] [CrossRef]
- Yadav, H.; Quijano, C.; Kamaraju, A.K.; Gavrilova, O.; Malek, R.; Chen, W.; Zerfas, P.; Zhigang, D.; Wright, E.C.; Stuelten, C.; et al. Protection from Obesity and Diabetes by Blockade of TGF-β/Smad3 Signaling. Cell Metab. 2011, 14, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.W.; Isono, M.; Chen, S.; la Cruz, M.C.I.-D.; Han, D.C.; Ziyadeh, F.N. Increased Glomerular and Tubular Expression of Transforming Growth Factor-β1, Its Type II Receptor, and Activation of the Smad Signaling Pathway in the db/db Mouse. Am. J. Pathol. 2001, 158, 1653–1663. [Google Scholar] [CrossRef]
- Dong, L.; Li, J.; Hu, Z.; Huang, X.; Wang, L.; Wang, H.; Ma, R.C.W.; Lan, H.; Yang, S. Deletion of Smad3 protects against diabetic myocardiopathy in db/db mice. J. Cell. Mol. Med. 2021, 25, 4860–4869. [Google Scholar] [CrossRef]
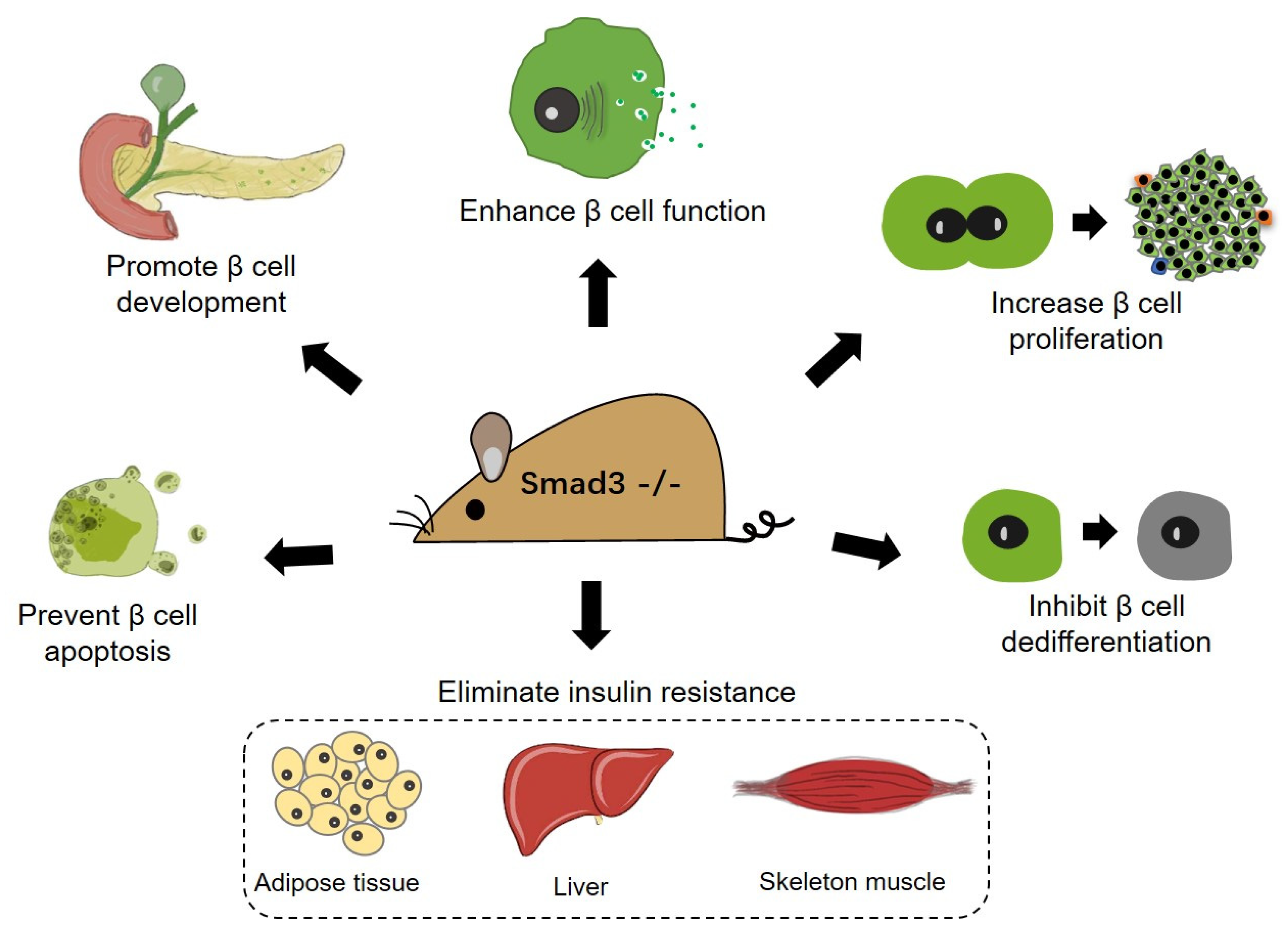
- Sheng, J.; Wang, L.; Tang, P.M.-K.; Wang, H.-L.; Li, J.-C.; Xu, B.-H.; Xue, V.W.; Tan, R.-Z.; Jin, N.; Chan, T.-F.; et al. Smad3 deficiency promotes beta cell proliferation and function in db/db mice via restoring Pax6 expression. Theranostics 2021, 11, 2845–2859. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Mellado-Gil, J.M.; Bahn, Y.J.; Pathy, S.M.; Zhang, Y.E.; Rane, S.G. Protection from β-cell apoptosis by inhibition of TGF-β/Smad3 signaling. Cell Death Dis. 2020, 11, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanvito, F.; Herrera, P.; Huarte, J.; Nichols, A.; Montesano, R.; Orci, L.; Vassalli, J. TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development 1994, 120, 3451–3462. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, A.; Hellerstrom, C. TGF-beta stimulates insulin secretion and blocks mitogenic response of pancreatic beta-cells to glucose. Am. J. Physiol. Physiol. 1991, 260, C1046–C1051. [Google Scholar] [CrossRef]
- Lin, H.-M.; Lee, J.-H.; Yadav, H.; Kamaraju, A.K.; Liu, E.; Zhigang, D.; Vieira, A.; Kim, S.-J.; Collins, H.; Matschinsky, F.; et al. Transforming Growth Factor-β/Smad3 Signaling Regulates Insulin Gene Transcription and Pancreatic Islet β-Cell Function. J. Biol. Chem. 2009, 284, 12246–12257. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Gu, D.; Feng, L.; Curriden, S.; Arnush, M.; Krahl, T.; Gurushanthaiah, D.; Wilson, C.; Loskutoff, D.L.; Fox, H. Accumulation of extracellular matrix and developmental dysregulation in the pancreas by transgenic production of transforming growth factor-beta 1. Am. J. Pathol. 1995, 147, 42–52. [Google Scholar] [PubMed]
- Sanvito, F.; Nichols, A.; Herrera, P.L.; Huarte, J.; Wohlwend, A.I.; Vassalli, J.-D.; Orci, L. TGF-β1 Overexpression in Murine Pancreas Induces Chronic Pancreatitis and Together with TNF-α, Triggers Insulin-Dependent Diabetes. Biochem. Biophys. Res. Commun. 1995, 217, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Miralles, F.; Battelino, T.; Czernichow, P.; Scharfmann, R. TGF-β Plays a Key Role in Morphogenesis of the Pancreatic Islets of Langerhans by Controlling the Activity of the Matrix Metalloproteinase MMP-2. J. Cell Biol. 1998, 143, 827–836. [Google Scholar] [CrossRef] [Green Version]
- Moritani, M.; Yoshimoto, K.; Wong, F.S.; Tanaka, C.; Yamaoka, T.; Sano, T.; Komagata, Y.; Miyazaki, J.-I.; Kikutani, H.; Itakura, M. Abrogation of autoimmune diabetes in nonobese diabetic mice and protection against effector lymphocytes by transgenic paracrine TGF-beta1. J. Clin. Investig. 1998, 102, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Grewal, I.; Grewal, K.D.; Wong, F.S.; Wang, H.; Picarella, D.E.; Janeway, C.A.; Flavell, R.A. Expression of Transgene Encoded TGF-β in Islets Prevents Autoimmune Diabetes in NOD Mice by a Local Mechanism. J. Autoimmun. 2002, 19, 9–22. [Google Scholar] [CrossRef]
- Toren-Haritan, G.; Efrat, S. TGFβ Pathway Inhibition Redifferentiates Human Pancreatic Islet β Cells Expanded In Vitro. PLoS ONE 2015, 10, e0139168. [Google Scholar] [CrossRef]
- Blum, B.; Roose, A.N.; Barrandon, O.; Maehr, R.; Arvanites, A.C.; Davidow, L.S.; Davis, J.C.; Peterson, Q.; Rubin, L.; Melton, D.A. Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. eLife 2014, 3, e02809. [Google Scholar] [CrossRef]
- Dhawan, S.; Dirice, E.; Kulkarni, R.N.; Bhushan, A. Inhibition of TGF-beta Signaling Promotes Human Pancreatic beta-Cell Replication. Diabetes 2016, 65, 1208–1218. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Fischbach, S.; Song, Z.; Gaffar, I.; Zimmerman, R.; Wiersch, J.; Prasadan, K.; Shiota, C.; Guo, P.; Ramachandran, S.; et al. Transient Suppression of TGFβ Receptor Signaling Facilitates Human Islet Transplantation. Endocrinology 2016, 157, 1348–1356. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Karakose, E.; Liu, H.; Swartz, E.; Ackeifi, C.; Zlatanic, V.; Wilson, J.; González, B.J.; Bender, A.; Takane, K.K.; et al. Combined Inhibition of DYRK1A, SMAD, and Trithorax Pathways Synergizes to Induce Robust Replication in Adult Human Beta Cells. Cell Metab. 2019, 29, 638–652e5. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Gaffar, I.; Guo, P.; Wiersch, J.; Fischbach, S.; Peirish, L.; Song, Z.; El-Gohary, Y.; Prasadan, K.; Shiota, C.; et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc. Natl. Acad. Sci. USA 2014, 111, E1211–E1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Wiersch, J.; El-Gohary, Y.; Guo, P.; Prasadan, K.; Paredes, J.; Welsh, C.; Shiota, C.; Gittes, G.K. TGFβ Receptor Signaling Is Essential for Inflammation-Induced but Not β-Cell Workload–Induced β-Cell Proliferation. Diabetes 2013, 62, 1217–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkin, C.E.; Mahmoud, M.; Liu, B.; Sobamowo, E.O.; King, A.; Arthur, H.; Jones, P.M.; Wheeler-Jones, C.P. Modulation of endoglin expression in islets of langerhans by VEGF reveals a novel regulator of islet endothelial cell function. BMC Res. Notes 2016, 9, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, Y.; Nomura, M.; Tanaka, K.; Kondo, A.; Morinaga, H.; Okabe, T.; Yanase, T.; Nawata, H.; Takayanagi, R.; Li, E. Genetic interactions between activin type IIB receptor and Smad2 genes in asymmetrical patterning of the thoracic organs and the development of pancreas islets. Dev. Dyn. 2007, 236, 2865–2874. [Google Scholar] [CrossRef]
- Nomura, M.; Zhu, H.-L.; Wang, L.; Morinaga, H.; Takayanagi, R.; Teramoto, N. SMAD2 disruption in mouse pancreatic beta cells leads to islet hyperplasia and impaired insulin secretion due to the attenuation of ATP-sensitive K+ channel activity. Diabetologia 2014, 57, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Simeone, D.M.; Zhang, L.; Treutelaar, M.K.; Zhang, L.; Graziano, K.; Logsdon, C.D.; Burant, C.F. Islet hypertrophy following pancreatic disruption of Smad4 signaling. Am. J. Physiol. Metab. 2006, 291, E1305–E1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, N.G.; Apelqvist, A.A.; Gu, X.; Harmon, E.B.; Topper, J.N.; MacDonald, R.J.; Kim, S.K. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol. 2006, 4, e39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehrawat, A.; Shiota, C.; Mohamed, N.; DiNicola, J.; Saleh, M.; Kalsi, R.; Zhang, T.; Wang, Y.; Prasadan, K.; Gittes, G.K. SMAD7 enhances adult β-cell proliferation without significantly affecting β-cell function in mice. J. Biol. Chem. 2020, 295, 4858–4869. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Ponce, A.; Scheibner, K.; Lickert, H.; Bakhti, M. Cellular and molecular mechanisms coordinating pancreas development. Development 2017, 144, 2873–2888. [Google Scholar] [CrossRef] [Green Version]
- Guney, M.A.; Gannon, M. Pancreas cell fate. Birth Defects Res. Part C Embryo Today Rev. 2009, 87, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.B.; Huh, C.G.; Becker, D.; Geiser, A.; Lyght, M.; Flanders, K.C.; Roberts, A.B.; Sporn, M.B.; Ward, J.M.; Karlsson, S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 1993, 90, 770–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shull, M.M.; Ormsby, I.; Kier, A.B.; Pawlowski, S.; Diebold, R.J.; Yin, M.; Allen, R.; Sidman, C.; Proetzel, G.; Calvin, D.; et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 1992, 359, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Bottinger, E.P.; Jakubczak, J.L.; Roberts, I.S.; Mumy, M.; Hemmati, P.; Bagnall, K.; Merlino, G.; Wakefield, L.M. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997, 16, 2621–2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.N.; Bouwens, L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 1995, 38, 1405–1411. [Google Scholar] [CrossRef]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In Vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 2008, 455, 627–632. [Google Scholar] [CrossRef]
- Dor, Y.; Brown, J.; Martinez, O.I.; Melton, D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004, 429, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Huang, X.; Liu, Z.; Pu, W.; Lv, Z.; He, L.; Li, Y.; Zhou, Q.; Lui, K.O.; Zhou, B. Pre-existing beta cells but not progenitors contribute to new beta cells in the adult pancreas. Nat. Metab. 2021, 3, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Cinti, F.; Bouchi, R.; Kim-Muller, J.Y.; Ohmura, Y.; Sandoval, P.R.; Masini, M.; Marselli, L.; Suleiman, M.; Ratner, L.E.; Marchetti, P.; et al. Evidence of β-Cell Dedifferentiation in Human Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1044–1054. [Google Scholar] [CrossRef] [Green Version]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic β Cell Dedifferentiation as a Mechanism of Diabetic β Cell Failure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russ, H.A.; Bar, Y.; Ravassard, P.; Efrat, S. In Vitro Proliferation of Cells Derived From Adult Human β-Cells Revealed By Cell-Lineage Tracing. Diabetes 2008, 57, 1575–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, N.; Ouziel-Yahalom, L.; Knoller, S.; Efrat, S.; Dor, Y. Lineage Tracing Evidence for In Vitro Dedifferentiation but Rare Proliferation of Mouse Pancreatic β-Cells. Diabetes 2007, 56, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.Y.; Sorensen, L.K.; Brooke, B.S.; Urness, L.D.; Davis, E.C.; Taylor, D.G.; Boak, B.B.; Wendel, D.P. Defective Angiogenesis in Mice Lacking Endoglin. Science 1999, 284, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Medela, A.M.; García-Ortiz, L.; Gómez-Marcos, M.A.; Recio-Rodríguez, J.I.; Sánchez-Rodríguez, A.; López-Novoa, J.M.; Martínez-Salgado, C. Increased plasma soluble endoglin levels as an indicator of cardiovascular alterations in hypertensive and diabetic patients. BMC Med. 2010, 8, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beiroa, D.; Romero-Picó, A.; Langa, C.; Bernabeu, C.; López, M.; López-Novoa, J.M.; Nogueiras, R.; Diéguez, C. Heterozygous Deficiency of Endoglin Decreases Insulin and Hepatic Triglyceride Levels during High Fat Diet. PLoS ONE 2013, 8, e54591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkin, C.E.; King, A.J.; Dhadda, P.; Chagastelles, P.; Nardi, N.; Wheeler-Jones, C.P.; Jones, P.M. Activin Receptor-Like Kinase 5 Inhibition Reverses Impairment of Endothelial Cell Viability by Endogenous Islet Mesenchymal Stromal Cells. Stem Cells 2013, 31, 547–559. [Google Scholar] [CrossRef]
- Konstantinova, I.; Lammert, E. Microvascular development: Learning from pancreatic islets. BioEssays 2004, 26, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, X.; Ren, X.; Tian, Y.; Chen, Z.; Xu, X.; Du, Y.; Jiang, C.; Fang, Y.; Liu, Z.; et al. Smad2 and Smad3 have differential sensitivity in relaying TGFβ signaling and inversely regulate early lineage specification. Sci. Rep. 2016, 6, 21602. [Google Scholar] [CrossRef] [Green Version]
- Nomura, M.; Li, E. Smad2 role in mesoderm formation, left–right patterning and craniofacial development. Nature 1998, 393, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Letterio, J.J.; Lechleider, R.J.; Chen, L.; Hayman, R.; Gu, H.; Roberts, A.B.; Deng, C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999, 18, 1280–1291. [Google Scholar] [CrossRef] [Green Version]
- Borton, A.J.; Frederick, J.P.; Datto, M.B.; Wang, X.-F.; Weinstein, R.S. The Loss of Smad3 Results in a Lower Rate of Bone Formation and Osteopenia Through Dysregulation of Osteoblast Differentiation and Apoptosis. J. Bone Miner. Res. 2001, 16, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, K.D.; Hoodless, P.; Bikoff, E.K.; Robertson, E.J. Formation of the definitive endoderm in mouse is a Smad2-dependent process. Development 2000, 127, 3079–3090. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-H.; Derynck, R. Specificity and versatility in TGF-β signaling through smads. Annu. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Wei, B.; He, H.-J.; Huang, X.-R.; Sheng, J.-Y.; Chen, X.-C.; Wang, L.; Tan, R.-Z.; Li, J.-C.; Liu, J.; et al. Smad3 deficiency improves islet-based therapy for diabetes and diabetic kidney injury by promoting β cell proliferation via the E2F3-dependent mechanism. Theranostics 2022, 12, 379–395. [Google Scholar] [CrossRef]
- Lytrivi, M.; Castell, A.-L.; Poitout, V.; Cnop, M. Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J. Mol. Biol. 2020, 432, 1514–1534. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell Deficit and Increased beta-Cell Apoptosis in Humans With Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.E.; Janson, J.; Soeller, W.C.; Butler, P.C. Increased β-Cell Apoptosis Prevents Adaptive Increase in β-Cell Mass in Mouse Model of Type 2 Diabetes: Evidence for Role of Islet Amyloid Formation Rather Than Direct Action of Amyloid. Diabetes 2003, 52, 2304–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blandino-Rosano, M.; Chen, A.Y.; Scheys, J.O.; Alejandro, E.U.; Gould, A.P.; Taranukha, T.; Elghazi, L.; Cras-Méneur, C.; Bernal-Mizrachi, E. mTORC1 signaling and regulation of pancreatic β-cell mass. Cell Cycle 2012, 11, 1892–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnemann, A.K.; Baan, M.; Davis, D.B. Pancreatic β-Cell Proliferation in Obesity1,2. Adv. Nutr. Int. Rev. J. 2014, 5, 278–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Wu, W.; Huang, C.; Cen, G.; Jiang, T.; Cao, J.; Huang, K.; Qiu, Z. SMAD4 and its role in pancreatic cancer. Tumor Biol. 2015, 36, 111–119. [Google Scholar] [CrossRef]
- Herrera, P. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 2000, 127, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Nakamura, T.; Hashimoto, N.; Matsuda, T.; Kotani, K.; Sakaue, H.; Kido, Y.; Hayashi, Y.; Nakayama, K.I.; White, M.F.; et al. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat. Med. 2005, 11, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.S.; Scacheri, P.C.; Ward, J.M.; McNally, S.R.; Swain, G.P.; Montagna, C.; Hager, J.H.; Hanahan, D.; Edlund, H.; Magnuson, M.; et al. Of Mice and MEN1: Insulinomas in a Conditional Mouse Knockout. Mol. Cell. Biol. 2003, 23, 6075–6085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnik, S.K.; Hughes, C.M.; Gu, X.; Rozenblatt-Rosen, O.; McLean, G.W.; Xiong, Y.; Meyerson, M.; Kim, S.K. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc. Natl. Acad. Sci. USA 2005, 102, 14659–14664. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Guo, M.; Matsuoka, T.-A.; Hagman, D.K.; Parazzoli, S.D.; Poitout, V.; Stein, R. The Islet β Cell-enriched MafA Activator Is a Key Regulator of Insulin Gene Transcription. J. Biol. Chem. 2005, 280, 11887–11894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, K.; Nagai, R. Islet inflammation in type 2 diabetes and physiology. J. Clin. Investig. 2017, 127, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2017, 127, 1589. [Google Scholar] [CrossRef]
- Sanjabi, S.; Zenewicz, L.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Sanjabi, S.; Flavell, R.A. Transforming Growth Factor-β Controls Development, Homeostasis, and Tolerance of T Cells by Regulatory T Cell-Dependent and -Independent Mechanisms. Immunity 2006, 25, 455–471. [Google Scholar] [CrossRef] [Green Version]
- Marie, J.; Liggitt, D.; Rudensky, A.Y. Cellular Mechanisms of Fatal Early-Onset Autoimmunity in Mice with the T Cell-Specific Targeting of Transforming Growth Factor-β Receptor. Immunity 2006, 25, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, A.; Houston, S.A.; Sherwood, E.; Casulli, J.; Travis, M.A. Regulation of Innate and Adaptive Immunity by TGFβ. Adv. Immunol. 2017, 134, 137–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.-H.; Sheng, J.; You, Y.-K.; Huang, X.-R.; Ma, R.C.; Wang, Q.; Lan, H.-Y. Deletion of Smad3 prevents renal fibrosis and inflammation in type 2 diabetic nephropathy. Metabolism 2020, 103, 154013. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.; Dafoe, D.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Liu, R.-M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, H.-O.; Jung, H.-J.; Seo, Y.-H.; Lee, Y.-K.; Hwang, S.-C.; Hwang, E.S.; Yoon, G. GSK3 inactivation is involved in mitochondrial complex IV defect in transforming growth factor (TGF) β1-induced senescence. Exp. Cell Res. 2012, 318, 1808–1819. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, G.; Wu, Q. Autophagic dysfunction of β cell dysfunction in type 2 diabetes, a double-edged sword. Genes Dis. 2021, 8, 438–447. [Google Scholar] [CrossRef]
- Ebato, C.; Uchida, T.; Arakawa, M.; Komatsu, M.; Ueno, T.; Komiya, K.; Azuma, K.; Hirose, T.; Tanaka, K.; Kominami, E.; et al. Autophagy Is Important in Islet Homeostasis and Compensatory Increase of Beta Cell Mass in Response to High-Fat Diet. Cell Metab. 2008, 8, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Choi, M.E. Regulation of Autophagy by TGF-β: Emerging Role in Kidney Fibrosis. Semin. Nephrol. 2014, 34, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Chen, X.-C.; Li, Z.-H.; Wu, H.-L.; Jing, K.-P.; Huang, X.-R.; Ye, L.; Wei, B.; Lan, H.-Y.; Liu, H.-F. SMAD3 promotes autophagy dysregulation by triggering lysosome depletion in tubular epithelial cells in diabetic nephropathy. Autophagy 2021, 17, 2325–2344. [Google Scholar] [CrossRef]
- Lavallee, T.M.; Zhan, X.H.; Herbstritt, C.J.; Kough, E.C.; Green, S.J.; Pribluda, V.S. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002, 62, 3691–3697. [Google Scholar]
- Yorifuji, T.; Uchida, T.; Abe, H.; Toyofuku, Y.; Tamaki, M.; Fujitani, Y.; Hirose, T.; Kawamori, R.; Takeda, S.; Watada, H. 2-Methoxyestradiol ameliorates glucose tolerance with the increase in β-cell mass in db/db mice. J. Diabetes Investig. 2011, 2, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Salama, S.A.; Diaz-Arrastia, C.R.; Kilic, G.S.; Kamel, M.W. 2-Methoxyestradiol causes functional repression of transforming growth factor β3 signaling by ameliorating Smad and non-Smad signaling pathways in immortalized uterine fibroid cells. Fertil. Steril. 2012, 98, 178–184.e1. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naim, A.B.; Neamatallah, T.; Eid, B.G.; Esmat, A.; AlAmoudi, A.J.; Aziz, G.A.; Ashour, O.M. 2-Methoxyestradiol Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats through Inhibition of HIF-1α/TGF-β/Smad2 Axis. Oxidative Med. Cell. Longev. 2018, 2018, 4389484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Yan, T.; Chen, B.; Liu, F.; Liu, X.; Xie, Y. SIS3, a good candidate for the reverse of type 2 diabetes mellitus in mice. Fundam. Clin. Pharmacol. 2021, 35, 389–396. [Google Scholar] [CrossRef]
- Ämmälä, C.; Drury, W.J.; Knerr, L.; Ahlstedt, I.; Stillemark-Billton, P.; Wennberg-Huldt, C.; Andersson, E.-M.; Valeur, E.; Jansson-Löfmark, R.; Janzén, D.; et al. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci. Adv. 2018, 4, eaat3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushawah, G.; Hernandez-Huertas, L.; del Prado, J.A.-N.; Martinez-Morales, J.R.; DeVore, M.L.; Hassan, H.; Moreno-Sanchez, I.; Tomas-Gallardo, L.; Díaz-Moscoso, A.; Monges, D.E.; et al. CRISPR-Cas13d Induces Efficient mRNA Knockdown in Animal Embryos. Dev. Cell 2020, 54, 805–817.e7. [Google Scholar] [CrossRef]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
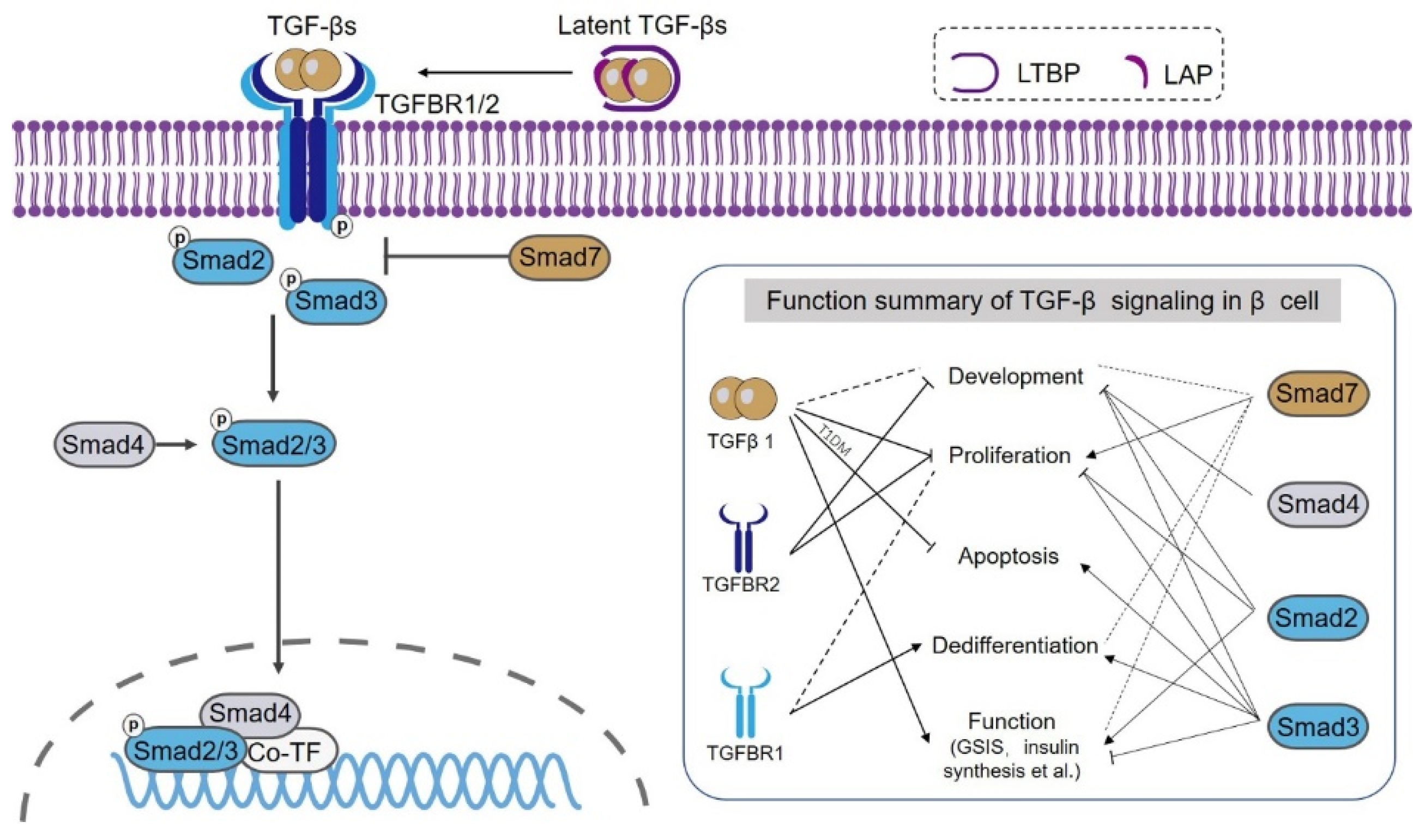
| Component | Biological Function in β Cell/Diabetes | Experimental Model and Parameter | Reference |
|---|---|---|---|
| TGF-β ligand | Promote/suppress β cell development. | Cultured pancreatic rudiment treated with TGF-β1. | [31,39] |
| Promote insulin secretion in low glucose condition (11.1 mM). | Cultured rat islets treated with TGF-β1. | [40] | |
| Suppress transcription of genes related to insulin synthesis, processing, GSIS, and β cell maturation. | Cultured mouse islets treated with TGF-β1. | [41] | |
| Disorganized (smaller) islet but with normal β cell development, β cell mass, and insulin secretion. | Mice with transgenic expression of Tgfb1 in β cell. | [42,43] | |
| No influence on β cell development but with perturbed islet structure. | Cultured rat pancreatic rudiment treated with TGF-β1 or its neutralizing antibody. | [44] | |
| Protect against autoimmune T1DM. | NOD mice with Tgfb1 overexpression in β or α cell. | [45,46] | |
| TGFBR1 | Promote redifferentiation but suppress proliferation of dedifferentiated β cell upon knockdown or pharmacological inhibition. | Long-termed (3 weeks) culture of human and rodent islet cells. | [47,48] |
| Promote β cell proliferation upon pharmacological inhibition. | Cultured mouse and human β cell; mice treated with chemical inhibitor; human islet allograft. | [49,50,51] | |
| Suppress β cell proliferation upon pharmacological inhibition or combinational deletion with Tgfbr2. | Mice with pancreatic duct ligation; β cell cocultured with M2 macrophage. | [52,53] | |
| No influence on β cell proliferation. | Mice with partial pancreatectomy and conditional knockout under Pdx1-Cre-ERT or Ptf1a-Cre. | [32] | |
| TGFBR2 | Promote β cell proliferation upon deletion. | Mice with partial pancreatectomy and conditional knockout under Pdx1-Cre-ERT or Ptf1a-Cre. | [32] |
| Suppress β cell proliferation upon combinational deletion with Tgfbr1. | Mice with pancreatic duct ligation. | [53] | |
| endoglin | Suppress the formation of intra-islet blood vessels upon deficiency. | Mice with inducible deletion of endoglin in endothelial cells | [54] |
| Smad2 | Islet hypoplasia upon heterogeneous deletion. | Heterogeneous Smad2-null mice. | [55] |
| Promote β cell proliferation upon deletion. | Mice with partial pancreatectomy and conditional knockout under Pdx1-Cre-ERT or Ptf1a-Cre. | [32] | |
| Cause islet/β cell hyperplasia but β cell dysfunction with impaired insulin secretion and glucose homeostasis upon conditional deletion in β cell. | Mice with conditional Smad2 knockout under RIP. | [56] | |
| Smad3 | Directly repression of insulin transcription by promoter binding; repress insulin synthesis and secretion; attenuate GSIS and glucose tolerance | β cell line INS-1E; Smad3-deficient mice and corresponding islets | [41] |
| Promote β cell proliferation in response to systemic insulin demand upon deletion. | Mice with partial pancreatectomy; db/db mice. | [32,37] | |
| Promote β cell apoptosis. | HFD-fed mice | [38] | |
| Smad4 | Induce islet hyperplasia upon deletion. | Mice overexpressing dominant-negative Smad4 under elastase promoter. | [57] |
| Smad7 | Suppress β cell lineage development upon overexpression at the gestational stage. | Doxycycline-inducible (Tet-Off) β cell-specific Smad7 overexpression under the regulation of Pdx-1 promoter during gestation. | [58] |
| Promote β cell proliferation in response to systemic insulin demand and local pancreatic inflammation, and also in physiological conditions. | Mice with partial pancreatectomy and conditional Smad7 knockout under Pdx1-Cre-ERT or Ptf1a-Cre or Ngn3-Cre; mice with β cell-specific Smad7 overexpression by AAV-mediated gene delivery; mice with pancreatic duct ligation; β cell-specific Smad7 overexpression in adult mice. | [32,52,59] | |
| Involved with β cell dedifferentiation but does not influence β cell function and glucose homeostasis upon overexpression. | Doxycycline-inducible (Tet-On) β cell-specific Smad7 overexpression under the regulation of Ins1 promoter in adult mice. | [59] | |
| Induce reversible diabetes with β cell dysfunction but no significant dedifferentiation. | Doxycycline-inducible (Tet-Off) β cell-specific Smad7 overexpression under the regulation of Pdx-1 promoter in adult mice. | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-L.; Wang, L.; Zhao, C.-Y.; Lan, H.-Y. Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes. Biomolecules 2022, 12, 373. https://doi.org/10.3390/biom12030373
Wang H-L, Wang L, Zhao C-Y, Lan H-Y. Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes. Biomolecules. 2022; 12(3):373. https://doi.org/10.3390/biom12030373
Chicago/Turabian StyleWang, Hong-Lian, Li Wang, Chang-Ying Zhao, and Hui-Yao Lan. 2022. "Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes" Biomolecules 12, no. 3: 373. https://doi.org/10.3390/biom12030373
APA StyleWang, H.-L., Wang, L., Zhao, C.-Y., & Lan, H.-Y. (2022). Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes. Biomolecules, 12(3), 373. https://doi.org/10.3390/biom12030373






