Identification of Metabolism-Associated Biomarkers for Early and Precise Diagnosis of Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
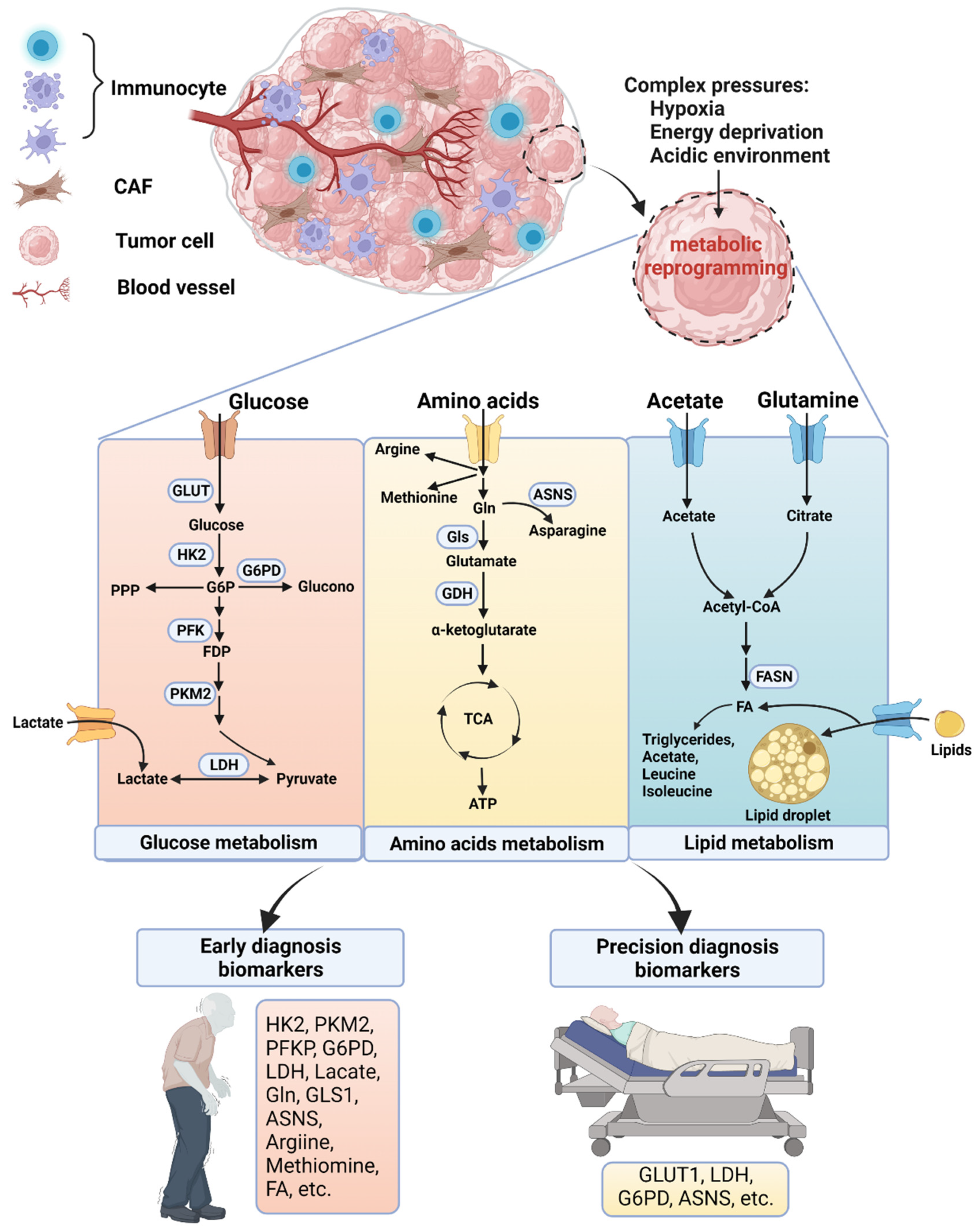
2. Altered Cellular Metabolism in OSCC
2.1. Glucose Metabolism
2.1.1. Glycolysis
2.1.2. Pentose Phosphate Pathway (PPP)
2.2. Amino Acid Metabolism—Gln and Methionine
2.3. Lipid Metabolism
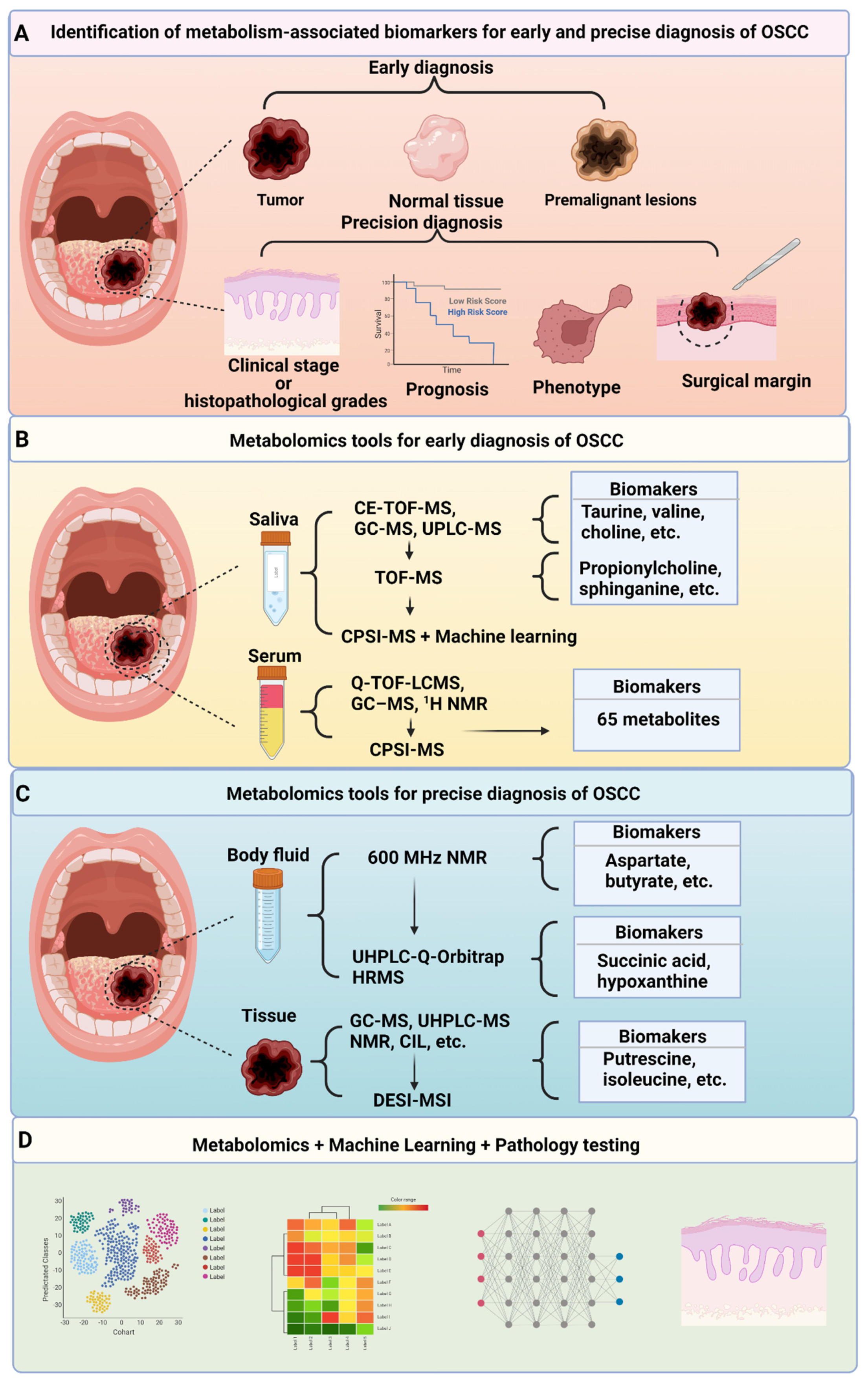
3. Clinical Applications of Metabolism-Targeted Diagnosis
3.1. Metabolism-Targeted Early Diagnosis
3.1.1. Metabolism-Targeted Early Diagnosis—Saliva
3.1.2. Metabolism-Targeted Early Diagnosis—Serum and Urine
3.2. Metabolism-Targeted Precision Diagnosis
3.2.1. Metabolism-Targeted Precision Diagnosis—Body Fluids
3.2.2. Metabolism-Targeted Precision Diagnosis—Tissue Specimens
4. Future Research Directions of Metabolomics Applied to OSCC Diagnosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Du, M.; Nair, R.; Jamieson, L.; Liu, Z.; Bi, P. Incidence Trends of Lip, Oral Cavity, and Pharyngeal Cancers: Global Burden of Disease 1990–2017. J. Dent. Res. 2019, 99, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Koyfman, S.A.; Ismaila, N.; Crook, D.; D’Cruz, A.; Rodriguez, C.P.; Sher, D.J.; Silbermins, D.; Sturgis, E.M.; Tsue, T.T.; Weiss, J.; et al. Management of the Neck in Squamous Cell Carcinoma of the Oral Cavity and Oropharynx: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1753–1774. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.-P.; Leemans, C.R.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Madhura, M.G.; Rao, R.S.; Patil, S.; Fageeh, H.N.; Alhazmi, A.; Awan, K.H. Advanced diagnostic aids for oral cancer. Disease-a-Month 2020, 66, 101034. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.; Kerr, A.R.; Epstein, J.B. Oral and Pharyngeal Cancer Control and Early Detection. J. Cancer Educ. 2010, 25, 279–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lajolo, C.; Gioco, G.; Rupe, C.; Patini, R.; Rizzo, I.; Romeo, U.; Contaldo, M.; Cordaro, M. Patient perception after oral biopsies: An observational outpatient study. Clin. Oral Investig. 2021, 25, 5687–5697. [Google Scholar] [CrossRef] [PubMed]
- Steigen, S.E.; Søland, T.M.; Nginamau, E.S.; Laurvik, H.; Costea, D.; Johannessen, A.C.; Jebsen, P.; Bjerkli, I.; Uhlin-Hansen, L.; Hadler-Olsen, E. Grading of oral squamous cell carcinomas – Intra and interrater agreeability: Simpler is better? J. Oral Pathol. Med. 2020, 49, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.R. The hematoxylin and eosin stain in anatomic pathology—An often-neglected focus of quality assurance in the laboratory. Semin. Diagn. Pathol. 2019, 36, 303–311. [Google Scholar] [CrossRef]
- Pu, Y.; Wang, Y.; Huang, X.; Chen, S.; Wang, Z.; Sun, G.; Tang, E.; Zhao, S.; Ni, Y.; Hu, Q. The influence of mild dysplasia at the surgical margin on the prognosis of oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2016, 45, 1372–1377. [Google Scholar] [CrossRef]
- Zheng, E.; Khariwala, S.S. Do All Patients With Head and Neck Cancer Require a Positron Emission Tomography Scan at Diagnosis? Laryngoscope 2019, 129, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Di Stasio, D.; Petruzzi, M.; Fiori, F.; Lajolo, C.; Santarelli, A.; Lucchese, A.; Serpico, R.; Contaldo, M. Noninvasive Imaging Methods to Improve the Diagnosis of Oral Carcinoma and Its Precursors: State of the Art and Proposal of a Three-Step Diagnostic Process. Cancers 2021, 13, 2864. [Google Scholar] [CrossRef] [PubMed]
- Vitório, J.G.; Duarte-Andrade, F.F.; Pereira, T.D.S.F.; Fonseca, F.P.; Amorim, L.S.D.; Martins-Chaves, R.R.; Gomes, C.C.; Canuto, G.A.B.; Gomez, R.S. Metabolic landscape of oral squamous cell carcinoma. Metabolomics 2020, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, D. Metabolomics study of oral cancers. Metabolomics 2019, 15, 22. [Google Scholar] [CrossRef]
- Guttikonda, V.R.; Harshani, J.M.; Yeluri, S. Glut-1 as a prognostic biomarker in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2014, 18, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Ayala, F.R.R.; Rocha, R.M.; Carvalho, K.C.; Carvalho, A.; Da Cunha, I.W.; Lourenço, S.V.; Soares, F.A. Glut1 and Glut3 as Potential Prognostic Markers for Oral Squamous Cell Carcinoma. Molecules 2010, 15, 2374–2387. [Google Scholar] [CrossRef]
- Eckert, A.W.; Lautner, M.H.W.; Schütze, A.; Taubert, H.; Schubert, J.; Bilkenroth, U. Coexpression of hypoxia-inducible factor-1α and glucose transporter-1 is associated with poor prognosis in oral squamous cell carcinoma patients. Histopathology 2011, 58, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.; Munz, A.; Teriete, P.; Nadtotschi, T.; Reinert, S. GLUT-1(+)/TKTL1(+) coexpression predicts poor outcome in oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, M.; Moergel, M.; Stockinger, M.; Jeong, J.H.; Fritz, G.; Lehr, H.A.; Whiteside, T.L. Overexpression of GLUT-1 is associated with resistance to radiotherapy and adverse prognosis in squa-mous cell carcinoma of the oral cavity. Oral Oncol. 2007, 43, 796–803. [Google Scholar] [CrossRef]
- Kunkel, M.; Reichert, T.E.; Benz, P.; Lehr, H.-A.; Jeong, J.-H.; Wieand, S.; Bartenstein, P.; Wagner, W.; Whiteside, T.L. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer 2003, 97, 1015–1024. [Google Scholar] [CrossRef]
- Nakazato, K.; Mogushi, K.; Kayamori, K.; Tsuchiya, M.; Takahashi, K.; Sumino, J.; Michi, Y.; Yoda, T.; Uzawa, N. Glucose metabolism changes during the development and progression of oral tongue squamous cell carcinomas. Oncol. Lett. 2019, 18, 1372–1380. [Google Scholar] [CrossRef] [Green Version]
- Kurihara-Shimomura, M.; Sasahira, T.; Nakashima, C.; Kuniyasu, H.; Shimomura, H.; Kirita, T. The Multifarious Functions of Pyruvate Kinase M2 in Oral Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2907. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.J.; Kim, J.Y.; Lee, D.Y.; Zhang, X.; Bazarsad, S.; Chung, W.Y.; Kim, J. PKM2 enhances cancer invasion via ETS-1-dependent induction of matrix metalloproteinase in oral squa-mous cell carcinoma cells. PLoS ONE 2019, 14, e0216661. [Google Scholar]
- Sun, X.; Zhang, L. MicroRNA-143 suppresses oral squamous cell carcinoma cell growth, invasion and glucose metabolism through targeting hexokinase 2. Biosci. Rep. 2017, 37, BSR20160404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, Q.; Niu, L.; Xu, L.; Guo, Y.; Wang, L.; Guo, C. Suppression of G6PD induces the expression and bisecting GlcNAc-branched N-glycosylation of E-Cadherin to block epithelial-mesenchymal transition and lymphatic metastasis. Br. J. Cancer 2020, 123, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Du, J.; Gu, Z. Circ-PVT1/miR-106a-5p/HK2 axis regulates cell growth, metastasis and glycolytic metabolism of oral squamous cell carcinoma. Mol. Cell. Biochem. 2020, 474, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Ramandi, M.A.; Motiee-Langroudi, M.; Jafari, M.; Sharouny, H.; Sheykhbahaei, N. Serum and salivary levels of lactate dehydrogenase in oral squamous cell carcinoma, oral lichen planus and oral lichenoid reaction. BMC Oral Heal. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.S.; Golgire, S. A study of salivary lactate dehydrogenase isoenzyme levels in patients with oral leukoplakia and squamous cell carcinoma by gel electrophoresis method. J. Oral Maxillofac. Pathol. 2014, 18, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokesh, K.; Kannabiran, J.; Rao, M.D. Salivary Lactate Dehydrogenase (LDH)—A Novel Technique in Oral Cancer Detection and Diagnosis. J. Clin. Diagn. Res. 2016, 10, ZC34–ZC37. [Google Scholar] [CrossRef]
- Saluja, T.S.; Spadigam, A.; Dhupar, A.; Syed, S. Equating salivary lactate dehydrogenase (LDH) with LDH-5 expression in patients with oral squamous cell carcinoma: An insight into metabolic reprogramming of cancer cell as a predictor of aggressive phenotype. Tumor Biol. 2015, 37, 5609–5620. [Google Scholar] [CrossRef]
- Cai, H.; Li, J.; Zhang, Y.; Liao, Y.; Zhu, Y.; Wang, C.; Hou, J. LDHA Promotes Oral Squamous Cell Carcinoma Progression Through Facilitating Glycolysis and Epitheli-al-Mesenchymal Transition. Front. Oncol. 2019, 9, 1446. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.; Alexander, D.; Munz, A.; Hoffmann, J.; Reinert, S. Increased LDH5 expression is associated with lymph node metastasis and outcome in oral squamous cell carcinoma. Clin. Exp. Metastasis 2012, 30, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, X.; Ding, X.; Li, H.; Geng, M.; Xie, Z.; Wu, H.; Huang, M. Lactate Dehydrogenase B Is Associated with the Response to Neoadjuvant Chemotherapy in Oral Squamous Cell Carcinoma. PLoS ONE 2015, 10, e0125976. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.; Xu, Z.; Wang, M.; Yan, T.; Huang, C.; Zhou, X.; Liu, Q.; Wang, L.; Chen, Y.; Wang, H.; et al. Tumoral microvesicle–activated glycometabolic reprogramming in fibroblasts promotes the progression of oral squamous cell carcinoma. FASEB J. 2019, 33, 5690–5703. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Z.; Rajthala, S.; Sapkota, D.; Dongre, H.; Parajuli, H.; Suliman, S.; Das, R.; Li, L.; Bindoff, L.A.; et al. Metabolic reprogramming of normal oral fibroblasts correlated with increased glycolytic metabolism of oral squamous cell carcinoma and precedes their activation into carcinoma associated fibroblasts. Cell. Mol. Life Sci. 2020, 77, 1115–1133. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, C.-Y.; Chen, W.-M.; Huang, P. Lactate Promotes Cancer Stem-like Property of Oral Sequamous Cell Carcinoma. Curr. Med Sci. 2019, 39, 403–409. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, E.; Shang, Z. 3D Co-culture of Cancer-Associated Fibroblast with Oral Cancer Organoids. J. Dent. Res. 2021, 100, 201–208. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Wu, M. Regulation of the pentose phosphate pathway in cancer. Protein Cell 2014, 5, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Mele, L.; Paino, F.; Papaccio, F.; Regad, T.; Boocock, D.; Stiuso, P.; Lombardi, A.; Liccardo, D.; Aquino, G.; Barbieri, A.; et al. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 2018, 9, 572. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.-C.; Hsiao, J.-R.; Jiang, S.-S.; Chang, J.-Y.; Chu, P.-Y.; Liu, K.-J.; Fang, H.-L.; Lin, L.-M.; Chen, H.-H.; Huang, Y.-W.; et al. c-MYC-directed NRF2 drives malignant progression of head and neck cancer via glucose-6-phosphate dehydrogenase and transketolase activation. Theranostics 2021, 11, 5232–5247. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.M.; Glazer, C.A.; Mithani, S.K.; Ochs, M.F.; Sun, W.; Bhan, S.; Vostrov, A.; Abdullaev, z.; Lobanenkov, V.; Gray, A.; et al. Coordinated activation of candidate proto-oncogenes and cancer testes antigens via promoter demethyla-tion in head and neck cancer and lung cancer. PLoS ONE 2009, 4, e4961. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, Y.; Glazer, C.A.; Shao, C.; Bhan, S.; Demokan, S.; Zhao, M.; Rudek, M.A.; Ha, P.K.; Califano, J.A. TKTL1 Is Activated by Promoter Hypomethylation and Contributes to Head and Neck Squamous Cell Carcinoma Carcinogenesis through Increased Aerobic Glycolysis and HIF1 Stabilization. Clin. Cancer Res. 2010, 16, 857–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Völker, H.-U.; Scheich, M.; Schmausser, B.; Kämmerer, U.; Eck, M. Overexpression of transketolase TKTL1 is associated with shorter survival in laryngeal squamous cell carcinomas. Eur. Arch. Otorhinolaryngol. 2007, 264, 1431–1436. [Google Scholar] [CrossRef]
- Mims, J.; Bansal, N.; Bharadwaj, M.S.; Chen, X.; Molina, A.J.; Tsang, A.W.; Furdui, C.M. Energy Metabolism in a Matched Model of Radiation Resistance for Head and Neck Squamous Cell Cancer. Radiat. Res. 2015, 183, 291–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivanand, S.M.; Vander Heiden, G. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Valter, K.; Maximchik, P.; Abdrakhmanov, A.; Senichkin, V.; Zhivotovsky, B.; Gogvadze, V. Distinct effects of etoposide on glutamine-addicted neuroblastoma. Cell Mol. Life Sci. 2020, 77, 1197–1207. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Xu, L.; Wang, Y.; Jiang, Q.; Liu, Z.; Zhang, J.; Zhou, Q.; Zeng, H.; Tong, S.; Wang, T.; et al. Tumor-associated Macrophage-derived Interleukin-23 Interlinks Kidney Cancer Glutamine Addiction with Immune Evasion. Eur. Urol. 2019, 75, 752–763. [Google Scholar] [CrossRef]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Ansari, R.; McIntyre, A.; Craze, M.L.; Ellis, I.; A Rakha, E.; Green, A.R. Altered glutamine metabolism in breast cancer; subtype dependencies and alternative adaptations. Histopathology 2017, 72, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Cetindis, M.; Biegner, T.; Munz, A.; Teriete, P.; Reinert, S.; Grimm, M. Glutaminolysis and carcinogenesis of oral squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2015, 273, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, W.; Ling, Z.; Hu, Q.; Fan, Z.; Cheng, B.; Tao, X. ASCT2 overexpression is associated with poor survival of OSCC patients and ASCT2 knockdown inhibited growth of glutamine-addicted OSCC cells. Cancer Med. 2020, 9, 3489–3499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Guo, Y.; Seo, W.; Zhang, R.; Lu, C.; Wang, Y.; Luo, L.; Paul, B.; Yan, W.; Saxena, D.; et al. Targeting cellular metabolism to reduce head and neck cancer growth. Sci. Rep. 2019, 9, 4995. [Google Scholar] [CrossRef] [Green Version]
- Kamarajan, P.; Rajendiran, T.M.; Kinchen, J.; Bermúdez, M.; Danciu, T.; Kapila, Y.L. Head and Neck Squamous Cell Carcinoma Metabolism Draws on Glutaminolysis, and Stemness Is Specifically Regulated by Glutaminolysis via Aldehyde Dehydrogenase. J. Proteome Res. 2017, 16, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Cai, B.; Ding, M.; Su, Z.; Liu, Y.; Shen, L. c-Myc Overexpression Promotes Oral Cancer Cell Proliferation and Migration by Enhancing Glutaminase and Glutamine Synthetase Activity. Am. J. Med. Sci. 2019, 358, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Lee, M.; Lee, Y.S.; Kim, S.H.; Lee, J.C.; Park, J.J.; Nam, H.Y.; Kim, M.R.; Han, M.W.; Kim, S.W.; et al. p53-dependent glutamine usage determines susceptibility to oxidative stress in radioresistant head and neck cancer cells. Cell. Signal. 2020, 77, 109820. [Google Scholar] [CrossRef] [PubMed]
- Wicker, C.A.; Hunt, B.G.; Krishnan, S.; Aziz, K.; Parajuli, S.; Palackdharry, S.; Elaban, W.R.; Wise-Draper, T.M.; Mills, G.B.; Waltz, S.E.; et al. Glutaminase inhibition with telaglenastat (CB-839) improves treatment response in combination with ionizing radiation in head and neck squamous cell carcinoma models. Cancer Lett. 2021, 502, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ding, L.; Yang, X.; Ding, Z.; Huang, X.; Zhang, L.; Chen, S.; Hu, Q.; Ni, Y. Asparagine Synthetase-Mediated l-Asparagine Metabolism Disorder Promotes the Perineural Invasion of Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 637226. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Fatema, C.N.; Kuroshima, T.; Asaka, T.; Abe, T.; Sato, J.; Shiga, T.; Kuge, Y.; Tamaki, N.; Kitagawa, Y. Prognostic value of MET-PET in oral cancer. J. Nucl. Med. 2017, 58, 285. [Google Scholar]
- Vuyyuri, S.B.; Hamstra, D.A.; Khanna, D.; Hamilton, C.A.; Markwart, S.M.; Campbell, K.C.; Sunkara, P.; Ross, B.D.; Rehemtulla, A. Evaluation of d-Methionine as a Novel Oral Radiation Protector for Prevention of Mucositis. Clin. Cancer Res. 2008, 14, 2161–2170. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-C.; Tsai, S.-T.; Kuo, C.-C.; Chang, J.S.; Jin, Y.-T.; Chang, J.-Y.; Hsiao, J.-R. Arginine deprivation as a new treatment strategy for head and neck cancer. Oral Oncol. 2012, 48, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.L.; Lau, N.C.H.; Liu, J.; Qu, X.; Tsui, S.K.-W.; Hou, J.; Law, C.T.-Y.; Ng, T.H.; Yam, J.W.P.; Chow, C.; et al. The Role of Arginine Metabolism in Oral Tongue Squamous Cell Carcinoma. Cancers 2021, 13, 6068. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [Green Version]
- Corbet, C.; Bastien, E.; Santiago de Jesus, J.P.; Dierge, E.; Martherus, R.; Vander Linden, C.; Doix, B.; Degavre, C.; Guilbaud, C.; Petit, L. TGFbeta2-induced formation of lipid droplets supports acidosis-driven EMT and the metastatic spreading of cancer cells. Nat. Commun. 2020, 11, 454. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Lu, M.; Shi, J.; Gong, Z.; Hua, L.; Li, Q.; Lim, B.; Zhang, X.H.-F.; Chen, X.; Li, S.; et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat. Immunol. 2020, 21, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- London, E. Membrane Structure–Function Insights from Asymmetric Lipid Vesicles. Accounts Chem. Res. 2019, 52, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Wierbowski, B.M.; Petrov, K.; Aravena, L.; Gu, G.; Xu, Y.; Salic, A. Hedgehog Pathway Activation Requires Coreceptor-Catalyzed, Lipid-Dependent Relay of the Sonic Hedgehog Ligand. Dev. Cell 2020, 55, 450–467.e8. [Google Scholar] [CrossRef] [PubMed]
- Borcik, C.G.; Versteeg, D.B.; Amani, R.; Yekefallah, M.; Khan, N.H.; Wylie, B.J. The Lipid Activation Mechanism of a Transmembrane Potassium Channel. J. Am. Chem. Soc. 2020, 142, 14102–14116. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Peng, J.; Chen, X.; Li, H.; Song, M.; Cheng, B.; Wu, T. Obesity and genes related to lipid metabolism predict poor survival in oral squamous cell carcinoma. Oral Oncol. 2019, 89, 14–22. [Google Scholar] [CrossRef]
- Gao, J.; Tian, G.; Han, X.; Zhu, Q. Twenty-four signature genes predict the prognosis of oral squamous cell carcinoma with high accuracy and repeatability. Mol. Med. Rep. 2017, 17, 2982–2990. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, V.; Subbannayya, Y.; Patil, S.; Puttamallesh, V.N.; Najar, M.; Datta, K.K.; Pinto, S.M.; Begum, S.; Mohanty, N.; Routray, S. Molecular alterations in oral cancer using high-throughput proteomic analysis of formalin-fixed paraf-fin-embedded tissue. J. Cell Commun. Signal. 2021, 15, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Haidari, S.; Tröltzsch, M.; Knösel, T.; Liokatis, P.; Kasintsova, A.; Eberl, M.; Ortner, F.; Otto, S.; Fegg, F.; Boskov, M.; et al. Fatty Acid Receptor CD36 Functions as a Surrogate Parameter for Lymph Node Metastasis in Oral Squa-mous Cell Carcinoma. Cancers 2021, 13, 4125. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xia, J.S.; Wu, J.H.; Chen, Y.G.; Qiu, C.J. Quercetin suppresses cell survival and invasion in oral squamous cell carcinoma via the miR-1254/CD36 cascade in vitro. Hum. Exp. Toxicol. 2021, 40, 1413–1421. [Google Scholar] [CrossRef]
- Sakurai, K.; Tomihara, K.; Yamazaki, M.; Heshiki, W.; Moniruzzaman, R.; Tachinami, H.; Ikeda, A.; Imaue, S.; Fujiwara, K. CD36 expression on oral squamous cell carcinoma cells correlates with enhanced proliferation and mi-gratory activity. Oral Dis. 2020, 26, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, Y.; Zhang, X. MiR-1254 Functions as a Tumor Suppressor in Oral Squamous Cell Carcinoma by Targeting CD36. Technol. Cancer Res. Treat. 2019, 18, 1533033819859447. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.-Y.; Wong, T.-Y.; Chiang, W.-F.; Chen, Y.-L. Fatty-acid-binding protein 5 promotes cell proliferation and invasion in oral squamous cell carcinoma. J. Oral Pathol. Med. 2010, 39, 342–348. [Google Scholar] [CrossRef]
- da Silva, S.D.; Cunha, I.W.; Nishimoto, I.N.; Soares, F.A.; Carraro, D.M.; Kowalski, L.P.; Graner, E. Clinicopathological significance of ubiquitin-specific protease 2a (USP2a), fatty acid synthase (FASN), and ErbB2 expression in oral squamous cell carcinomas. Oral Oncol. 2009, 45, e134–e139. [Google Scholar] [CrossRef]
- Silva, S.D.; Cunha, I.W.; Rangel, A.L.C.A.; Jorge, J.; Zecchin, K.G.; Agostini, M.; Kowalski, L.P.; Coletta, R.D.; Graner, E. Differential expression of fatty acid synthase (FAS) and ErbB2 in nonmalignant and malignant oral keratinocytes. Virchows Arch. 2008, 453, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Perez, D.; Nishimoto, I.; Alves, F.; Pinto, C.; Kowalski, L.; Graner, E. Fatty acid synthase expression in squamous cell carcinoma of the tongue: Clinicopathological findings. Oral Dis. 2008, 14, 376–382. [Google Scholar] [CrossRef]
- Almeida, L.Y.D.; Moreira, F.D.S.; Santos, G.A.S.D.; Cuadra Zelaya, F.J.M.; Ortiz, C.A.; Agostini, M.; Mariano, F.S.; Bastos, D.C.; Daher, U.R.N.; Kowalski, L.P.; et al. FASN inhibition sensitizes metastatic OSCC cells to cisplatin and paclitaxel by downregulating cyclin B1. Oral. Dis. 2021. [Google Scholar] [CrossRef]
- de Aquino, I.G.; Bastos, D.C.; Cuadra-Zelaya, F.J.M.; Teixeira, I.F.; Salo, T.; Della Coletta, R.; Graner, E. Anticancer properties of the fatty acid synthase inhibitor TVB-3166 on oral squamous cell carcinoma cell lines. Arch. Oral Biol. 2020, 113, 104707. [Google Scholar] [CrossRef]
- Wisniewski, D.J.; Ma, T.; Schneider, A. Nicotine induces oral dysplastic keratinocyte migration via fatty acid syn-thase-dependent epidermal growth factor receptor activation. Exp. Cell Res. 2018, 370, 343–352. [Google Scholar] [CrossRef]
- Hilvo, M.; Denkert, C.; Lehtinen, L.; Müller, B.; Brockmöller, S.; Seppänen-Laakso, T.; Budczies, J.; Bucher, E.; Yetukuri, L.; Castillo, S.; et al. Novel Theranostic Opportunities Offered by Characterization of Altered Membrane Lipid Metabolism in Breast Cancer Progression. Cancer Res. 2011, 71, 3236–3245. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, A.; Saraswat, M.; Joenväärä, S.; Agarwal, R.; Jyllikoski, D.; Wilkman, T.; Mäkitie, A.; Silén, S. Mass spectrometry–based lipidomics of oral squamous cell carcinoma tissue reveals aberrant cholesterol and glycerophospholipid metabolism—A Pilot study. Transl. Oncol. 2020, 13, 100807. [Google Scholar] [CrossRef]
- Gumus, R.; Capik, O.; Gundogdu, B.; Tatar, A.; Altinkaynak, K.; Ozdemir Tozlu, O.; Karatas, O.F. Low vitamin D and high cholesterol facilitate oral carcinogenesis in 4NQO-induced rat models via regu-lating glycolysis. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Jayakar, S.K.; Loudig, O.; Brandwein-Gensler, M.; Kim, R.S.; Ow, T.J.; Ustun, B.; Harris, T.M.; Prystowsky, M.B.; Childs, G.; Segall, J.E.; et al. Apolipoprotein E Promotes Invasion in Oral Squamous Cell Carcinoma. Am. J. Pathol. 2017, 187, 2259–2272. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.H.; Liu, H.; Chiang, W.F.; Chen, T.W.; Chu, L.J.; Yu, J.S.; Chen, S.J.; Chen, H.C.; Tan, B.C.M. MiR-31–5p-ACOX1 Axis Enhances Tumorigenic Fitness in Oral Squamous Cell Carcinoma Via the Promi-gratory Prostaglandin E2. Theranostics 2018, 8, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Husvik, C.; Khuu, C.; Bryne, M.; Halstensen, T.S. PGE2 Production in Oral Cancer Cell Lines is COX-2-dependent. J. Dent. Res. 2009, 88, 164–169. [Google Scholar] [CrossRef]
- Tang, D.-W.; Lin, S.-C.; Chang, K.-W.; Chi, C.-W.; Chang, C.-S.; Liu, T.-Y. Elevated expression of cyclooxygenase (COX)-2 in oral squamous cell carcinoma–evidence for COX-2 induction by areca quid ingredients in oral keratinocytes. J. Oral Pathol. Med. 2003, 32, 522–529. [Google Scholar] [CrossRef]
- Nystrom, M.L.; McCulloch, D.; Weinreb, P.H.; Violette, S.M.; Speight, P.M.; Marshall, J.F.; Hart, I.R.; Thomas, G.J. Cyclooxygenase-2 inhibition suppresses alphavbeta6 integrin-dependent oral squamous carcinoma invasion. Cancer Res. 2006, 66, 10833–10842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Wei, F.; Rao, S.L.; Kim, J.; Shin, H.; Cheng, J.; Tu, M.; Wong, D.T.; Kim, Y. Clinical validity of saliva and novel technology for cancer detection. Biochim. Biophys Acta. Rev. Cancer 2019, 1872, 49–59. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, P.; Wang, X.; Duan, Y. Investigation and identification of potential biomarkers in human saliva for the early diagnosis of oral squamous cell carcinoma. Clin. Chim. Acta 2014, 427, 79–85. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2009, 6, 78–95. [Google Scholar] [CrossRef] [Green Version]
- Ohshima, M.; Sugahara, K.; Kasahara, K.; Katakura, A. Metabolomic analysis of the saliva of Japanese patients with oral squamous cell carcinoma. Oncol. Rep. 2017, 37, 2727–2734. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Sugano, A.; Nakamura, M.; Kaneko, M.; Ota, S.; Hiwatari, K.; Enomoto, A.; Soga, T.; et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016, 6, 31520. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Gao, P.; Wang, X.; Duan, Y. The early diagnosis and monitoring of squamous cell carcinoma via saliva metabolomics. Sci. Rep. 2014, 4, 6802. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, X.; Narayanan, R.; Shankar, V.; Ethiraj, S.; Wang, X.; Duan, N.; Ni, Y.-H.; Hu, Q.; Zare, R.N. Oral squamous cell carcinoma diagnosed from saliva metabolic profiling. Proc. Natl. Acad. Sci. USA 2020, 117, 16167–16173. [Google Scholar] [CrossRef]
- Sridharan, G.; Ramani, P.; Patankar, S.; Vijayaraghavan, R. Evaluation of salivary metabolomics in oral leukoplakia and oral squamous cell carcinoma. J. Oral Pathol. Med. 2019, 48, 299–306. [Google Scholar] [CrossRef]
- Yang, X.-H.; Jing, Y.; Wang, S.; Ding, F.; Zhang, X.-X.; Chen, S.; Zhang, L.; Hu, Q.-G.; Ni, Y.-H. Integrated Non-targeted and Targeted Metabolomics Uncovers Amino Acid Markers of Oral Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 426. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Gupta, S.; Mahdi, A.A. 1H NMR-derived serum metabolomics of leukoplakia and squamous cell carcinoma. Clin. Chim. Acta 2015, 441, 47–55. [Google Scholar] [CrossRef] [PubMed]
- de Sá Alves, M.; de Sá Rodrigues, N.; Bandeira, C.M.; Chagas, J.F.S.; Pascoal, M.B.N.; Nepomuceno, G.L.J.T.; da Silva Martinho, H.; Alves, M.G.O.; Mendes, M.A.; Dias, M. Identification of Possible Salivary Metabolic Biomarkers and Altered Metabolic Pathways in South American Patients Diagnosed with Oral Squamous Cell Carcinoma. Metabolites 2021, 11, 650. [Google Scholar] [CrossRef]
- Wei, J.; Xie, G.; Zhou, Z.; Shi, P.; Qiu, Y.; Zheng, X.; Chen, T.; Su, M.; Zhao, A.; Jia, W. Salivary metabolite signatures of oral cancer and leukoplakia. Int. J. Cancer 2010, 129, 2207–2217. [Google Scholar] [CrossRef]
- Sridharan, G.; Ramani, P.; Patankar, S. Serum metabolomics in oral leukoplakia and oral squamous cell carcinoma. J. Cancer Res. Ther. 2017, 13, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, X.; Yang, X.; Han, W.; Fu, Y.; Wang, S.; Zhang, X.; Sun, G.; Lu, Y.; Wang, Z.; et al. Big cohort metabolomic profiling of serum for oral squamous cell carcinoma screening and diagnosis. Nat. Sci. 2022, 2, e20210071. [Google Scholar] [CrossRef]
- Acharya, S.; Rai, P.; Hallikeri, K.; Anehosur, V.; Kale, J. Serum lipid profile in oral squamous cell carcinoma: Alterations and association with some clinicopatho-logical parameters and tobacco use. Int. J. Oral Maxillofac. Surg. 2016, 45, 713–720. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Edamatsu, K.; Sugano, A.; Kitabatake, K.; Iino, M. Discrimination of oral squamous cell carcinoma from oral lichen planus by salivary metabolomics. Oral Dis. 2019, 26, 35–42. [Google Scholar] [CrossRef]
- Voelxen, N.F.; Blatt, S.; Knopf, P.; Henkel, M.; Appelhans, C.; Righesso, L.A.R.; Pabst, A.; Goldschmitt, J.; Walenta, S.; Neff, A.; et al. Comparative metabolic analysis in head and neck cancer and the normal gingiva. Clin. Oral Investig. 2017, 22, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-H.; Zhang, X.-X.; Jing, Y.; Ding, L.; Fu, Y.; Wang, S.; Hu, S.-Q.; Zhang, L.; Huang, X.-F.; Ni, Y.-H.; et al. Amino acids signatures of distance-related surgical margins of oral squamous cell carcinoma. EBioMedicine 2019, 48, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-K.; Lin, C.-Y.; Kang, C.-J.; Liao, C.-T.; Wang, W.-L.; Chiang, M.-H.; Yen, T.-C.; Lin, G. Nuclear Magnetic Resonance Metabolomics Biomarkers for Identifying High Risk Patients with Extranodal Extension in Oral Squamous Cell Carcinoma. J. Clin. Med. 2020, 9, 951. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-W.; Chen, Y.-T.; Hsieh, Y.-J.; Chang, K.-P.; Hsueh, P.-C.; Chen, T.; Yu, J.-S.; Chang, Y.-S.; Li, L.; Wu, C.-C. Integrated analyses utilizing metabolomics and transcriptomics reveal perturbation of the polyamine pathway in oral cavity squamous cell carcinoma. Anal. Chim. Acta 2019, 1050, 113–122. [Google Scholar] [CrossRef]
- Askari, M.; Darabi, M.; Mahmudabadi, R.Z.; Oboodiat, M.; Fayezi, S.; Hosseini, Z.M.; Pirzadeh, A. Tissue fatty acid composition and secretory phospholipase-A2 activity in oral squamous cell carcinoma. Clin. Transl. Oncol. 2014, 17, 378–383. [Google Scholar] [CrossRef]
- Xie, G.X.; Chen, T.L.; Qiu, Y.P.; Shi, P.; Zheng, X.J.; Su, M.M.; Zhao, A.H.; Zhou, Z.T.; Jia, W. Urine metabolite profiling offers potential early diagnosis of oral cancer. Metabolomics 2012, 8, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Yongkui, L.; Jian, L.; Wanghan; Jingui, L. 18FDG-PET/CT for the detection of regional nodal metastasis in patients with primary head and neck cancer before treatment: A meta-analysis. Surg. Oncol. 2013, 22, e11–e16. [Google Scholar] [CrossRef]
- Lonneux, M.; Hamoir, M.; Reychler, H.; Maingon, P.; Duvillard, C.; Calais, G.; Bridji, B.; Digue, L.; Toubeau, M.; Grégoire, V. Positron Emission Tomography With [18F] Fluorodeoxyglucose Improves Staging and Patient Management in Patients With Head and Neck Squamous Cell Carcinoma: A Multicenter Prospective Study. J. Clin. Oncol. 2010, 28, 1190–1195. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Yang, X.; Tang, Q. 18FDG PET-CT for distant metastases in patients with recurrent head and neck cancer after definitive treatment. A meta-analysis. Oral Oncol. 2014, 50, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Guduguntla, P.V.; Guttikonda, R. Estimation of serum pyruvic acid levels in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2020, 24, 585. [Google Scholar]
- Zuo, L.; Chen, Z.; Chen, L.; Kang, J.; Shi, Y.; Liu, L.; Zhang, S.; Jia, Q.; Huang, Y.; Sun, Z. Integrative Analysis of Metabolomics and Transcriptomics Data Identifies Prognostic Biomarkers Associated With Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 750794. [Google Scholar] [CrossRef]
- Ye, G.; Liu, Y.; Yin, P.; Zeng, Z.; Huang, Q.; Kong, H.; Lu, X.; Zhong, L.; Zhang, Z.; Xu, G. Study of Induction Chemotherapy Efficacy in Oral Squamous Cell Carcinoma Using Pseudotargeted Metabolomics. J. Proteome Res. 2014, 13, 1994–2004. [Google Scholar] [CrossRef]
- Ruparel, S.; Bendele, M.; Wallace, A.; Green, D. Released Lipids Regulate Transient Receptor Potential Channel (TRP)-Dependent Oral Cancer Pain. Mol. Pain 2015, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Mignion, L.; Acciardo, S.; Gourgue, F.; Joudiou, N.; Caignet, X.; Goebbels, R.-M.; Corbet, C.; Feron, O.; Bouzin, C.; Cani, P.D.; et al. Metabolic Imaging Using Hyperpolarized Pyruvate–Lactate Exchange Assesses Response or Resistance to the EGFR Inhibitor Cetuximab in Patient-Derived HNSCC Xenografts. Clin. Cancer Res. 2019, 26, 1932–1943. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.M.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Metabolomics of Head and Neck Cancer: A Mini-Review. Front. Physiol. 2016, 7, 526. [Google Scholar] [CrossRef] [Green Version]
- Balog, J.; Sasi-Szabó, L.; Kinross, J.; Lewis, M.R.; Muirhead, L.J.; Veselkov, K.; Mirnezami, R.; Dezső, B.; Damjanovich, L.; Darzi, A.; et al. Intraoperative Tissue Identification Using Rapid Evaporative Ionization Mass Spectrometry. Sci. Transl. Med. 2013, 5, 194ra93. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Hayasaka, T.; Masaki, N.; Watanabe, Y.; Masumoto, K.; Nagata, T.; Katou, F.; Setou, M. Imaging mass spectrometry distinguished the cancer and stromal regions of oral squamous cell carci-noma by visualizing phosphatidylcholine (16:0/16:1) and phosphatidylcholine (18:1/20:4). Anal. Bioanal. Chem. 2014, 406, 1307–1316. [Google Scholar] [CrossRef]
- Yang, X.; Song, X.; Zhang, X.; Shankar, V.; Wang, S.; Yang, Y.; Chen, S.; Zhang, L.; Ni, Y.; Zare, R.N.; et al. In situ DESI-MSI lipidomic profiles of mucosal margin of oral squamous cell carcinoma. EBioMedicine 2021, 70, 103529. [Google Scholar] [CrossRef]
- Eberlin, L.S.; Tibshirani, R.J.; Zhang, J.; Longacre, T.A.; Berry, G.J.; Bingham, D.B.; Norton, J.A.; Zare, R.N.; Poultsides, G.A. Molecular assessment of surgical-resection margins of gastric cancer by mass-spectrometric imaging. Proc. Natl. Acad. Sci. USA 2014, 111, 2436–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerian, K.S.; Jarmusch, A.K.; Pirro, V.; Koch, M.O.; Masterson, T.A.; Cheng, L.; Cooks, R.G. Differentiation of prostate cancer from normal tissue in radical prostatectomy specimens by desorption electrospray ionization and touch spray ionization mass spectrometry. Analyst 2015, 140, 1090–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calligaris, D.; Caragacianu, D.; Liu, X.; Norton, I.; Thompson, C.J.; Richardson, A.L.; Golshan, M.; Easterling, M.L.; Santagata, S.; Dillon, D.A.; et al. Application of desorption electrospray ionization mass spectrometry imaging in breast cancer margin analysis. Proc. Natl. Acad. Sci. USA 2014, 111, 15184–15189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Comparison | Upregulated Metabolites | Downregulated Metabolites | Metabolite Analysis Technique | References |
|---|---|---|---|---|
| OSCC patients versus healthy individuals | Choline, betaine, pipecolinic acid | l-carnitine | UPLC-MS | [98] |
| Choline, p-hydroxyphenylacetic acid, 2-hydroxy-4-methylvaleric acid, valine, 3-phenyllactic acid, leucine, hexanoic acid, octanoic acid, terephthalic acid, γ-butyrobetaine, 3-(4-hydroxyphenyl) propionic acid, isoleucine, tryptophan, 3-phenylpropionic acid, 2-hydroxyvaleric acid, butyric acid, cadaverine, 2-oxoisovaleric acid, N6,N6,N6-trimethyllysine, taurine, glycolic acid, 3-hydroxybutyric acid, heptanoic acid, alanine | Urea | Capillary electrophoresis-MS (CE-MS) | [100] | |
| Lactic acid, hydroxyphenyllactic acid, N-nonanoylglycine, 5-hydroxymethyluracil, succinic acid, ornithine, hexanoylcarnitine, propionylcholine, carnitine | 4-Hydroxy-L-glutamic acid, acetylphenylalanine, sphinganine, phytosphingosine, S-carboxymethyl-L-cysteine | Reversed phase liquid chromatography and hydrophilic interaction chromatography | [102] | |
| Putrescine, cadaverine, thymidine, adenosine, 5-aminopentoate | Hippuric acid, phosphocholine, glucose, serine, adrenic acid | Conductive polymer spray ionization mass spectrometry (CPSI-MS) and desorption electrospray ionization MS imaging (DESI-MSI) | [103] | |
| 1-methylhistidine, pseudouridine, inositol 1,3,4-triphosphate, d-glycerate-2-phosphate, 4-nitroquinoline-1-oxide, 2-oxoarginine, norcocaine nitroxide, sphinganine-1-phosphate | l-homocysteic acid, ubiquinone, neuraminic acid, estradiol valerate | Q-TOF-liquid chromatography-MS (Q-TOF-LC-MS) | [104] | |
| Glutamate, aspartic acid, proline | GC-MS and ultrahigh-performance liquid chromatography-tandem MS (UHPLC-MS/MS) | [105] | ||
| Propionate, acetone, acetate, choline | Valine, threonine, Gln, creatinine | 1H NMR | [106] | |
| Malic acid, maltose, methionine, inosine | GC-MS | [107] | ||
| Lactic acid, eicosanoic acid | Valine, γ-aminobutyric acid | Ultraperformance liquid chromatography and Q-TOF-MS | [108] | |
| Estradiol-17-β-3-sulfate, L-carnitine, 5-methylthioadenosine, 8-hydroxyadenine, 2-methylcitric acid, putrescine, estrone-3-sulfate | Q-TOF-LC-MS | [109] | ||
| PC, DG, sphingosine-1-phosphate, oleamide | LysoPC (18:3), lysoPC (20:4), lysoPE (20:3/0:0), lysoSM (d18:1), erythritol, nonanovlcamitine | CPSI-MS | [110] | |
| TC, HDL, LDL | Automated biochemistry analyser | [111] | ||
| OSCC patients versus premalignant lesions individuals | Putrescine, cadaverine, thymidine, adenosine, 5-aminopentoate | Hippuric acid, phosphocholine, glucose, serine, adrenic acid, | CPSI-MS and DESI-MSI | [103] |
| lactic acid | valine, phenylalanine | UPLC | [108] | |
| 5,6-Dihydrouridine, 4-hydroxypenbutolol glucuronide, 8-hydroxyadenine, putrescine | Q-TOF-LC-MS | [109] | ||
| Trimethylamine N-oxide, putrescine, creatinine, 5-aminovalerate, pipecolate, N-acetylputrescine, γ-butyrobetaine, indole-3-acetate, N1-acetylspermine, 2’-deoxyinosine, ethanolamine phosphate, N-acetylglucosamine | N-acetylhistidine, o-acetylcarnitine | CE-MS | [112] | |
| Acetone, acetate, choline | Valine, Gln, creatinine | 1H NMR | [106] | |
| OSCC tissue versus adjacent normal tissue | Lactate | Glucose | Metabolic bioluminescence imaging | [113] |
| Aspartic, asparagin | GC-MS and UHPLC-MS/MS | [114] | ||
| Carnitine, | Alanine, pyruvate | NMR | [115] | |
| putrescine, glycyl-leucine, phenylalanine, | Chemical isotope labeling | [116] | ||
| stearic acid (18:0), sPLA2 | Oleic acid (18:1n-9), linoleic acid (18:2n-6) | Gas liquid chromatograpy | [117] | |
| OSCC tissue versus margin-2 (0.5–1 cm) | Aspartic acid, glutamate, proline, valine | GC-MS and UHPLC-MS/MS | [114] | |
| margin-1 (0–0.5 cm) versus margin-2 | Proline, alanine, serine, aspartic acid, glutamate, Gln, ornithine, histidine, asparagine | GC-MS and UHPLC-MS/MS | [114] | |
| Extranodal extension (ENE)-positive versus ENE-negative | Aspartate, butyrate, carnitine, glutamate, glutathione, glycine, glycolate, guanosine, sucrose | Alanine, choline, glucose, isoleucine, lactate, leucine, myo-inositol, O-acetylcholine, oxypurinol, phenylalanine, pyruvate, succinate, tyrosine, valine, xanthine | 600-MHz NMR | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, X.; Wang, S.; Li, Z.; Hu, X.; Yang, X.; Song, Y.; Jing, Y.; Hu, Q.; Ni, Y. Identification of Metabolism-Associated Biomarkers for Early and Precise Diagnosis of Oral Squamous Cell Carcinoma. Biomolecules 2022, 12, 400. https://doi.org/10.3390/biom12030400
Wang Y, Zhang X, Wang S, Li Z, Hu X, Yang X, Song Y, Jing Y, Hu Q, Ni Y. Identification of Metabolism-Associated Biomarkers for Early and Precise Diagnosis of Oral Squamous Cell Carcinoma. Biomolecules. 2022; 12(3):400. https://doi.org/10.3390/biom12030400
Chicago/Turabian StyleWang, Yuhan, Xiaoxin Zhang, Shuai Wang, Zihui Li, Xinyang Hu, Xihu Yang, Yuxian Song, Yue Jing, Qingang Hu, and Yanhong Ni. 2022. "Identification of Metabolism-Associated Biomarkers for Early and Precise Diagnosis of Oral Squamous Cell Carcinoma" Biomolecules 12, no. 3: 400. https://doi.org/10.3390/biom12030400
APA StyleWang, Y., Zhang, X., Wang, S., Li, Z., Hu, X., Yang, X., Song, Y., Jing, Y., Hu, Q., & Ni, Y. (2022). Identification of Metabolism-Associated Biomarkers for Early and Precise Diagnosis of Oral Squamous Cell Carcinoma. Biomolecules, 12(3), 400. https://doi.org/10.3390/biom12030400





